Abstract
We report results of an immunoassay for Blastomyces dermatitidis antigenuria. Sensitivity was 92.9%, and specificity was 79.3%. Cross-reactions occurred in 96.3% of patients with histoplasmosis, 100% of patients with paracoccidioidomycosis, 70% of patients with penicilliosis marneffei, 2.9% of patients with cryptococcosis, and 1.1% of patients with aspergillosis. Reproducibility was 96.3%. These findings support a potential role for antigen testing in blastomycosis.
Most patients with blastomycosis exhibit progressive illnesses that require antifungal therapy. In one study, diagnosis was delayed for more than 1 month in nearly half of the cases (3). Blastomycosis was correctly suspected in only 20% of patients, resulting in unnecessary surgeries and treatment delays (7). In two-thirds of patients who died of acute respiratory distress syndrome caused by blastomycosis, the diagnosis was either not suspected or considered only after the patient became moribund (10).
At the University of Mississippi Medical Center, the first testing method for diagnosis was cytology in 58% of cases, KOH in 28% of cases, and histology in 12% of cases (8). The overall sensitivities were 93% for cytology, 85.1% for histology, 66.4% for culture, and 48.4% for KOH preparation. Pathologists who are less experienced with morphological identification of fungal organisms may not achieve these levels of sensitivity. Furthermore, Blastomyces dermatitidis may be confused with other fungi, including Histoplasma capsulatum (6). Commercially available serological tests are not believed to play a major role for diagnosis of blastomycosis (2). In this report, we assess the feasibility of antigen detection for diagnosis of blastomycosis.
Specimens from patients with blastomycosis or other fungal infections were tested. The diagnosis of blastomycosis was based upon clinical findings and supported by positive cultures for B. dermatitidis or histopathology studies (12). Control subjects were described in earlier reports (9, 11, 12), and those with histoplasmosis had high levels of Histoplasma antigenuria. Control subjects with aspergillosis included patients who were enrolled in an Institutional Review Board-approved protocol to evaluate antigen detection for diagnosis in patients with positive Aspergillus galactomannan antigenemia as identified during clinical testing. Specimens from patients with cryptococcal infection included those with positive results for cryptococcal antigen in blood or cerebrospinal fluid (CSF) as identified during clinical testing; specimens from AIDS patients with cryptococcal meningitis enrolled in an antifungal prophylaxis trial were also used. Urine specimens from healthy volunteers were obtained from laboratory workers.
The B. dermatitidis antigen assay used rabbit antibodies to formalin-killed B. dermatitidis mold, prepared as described previously (13). The immunoassay uses microtiter wells coated with the immunoglobulin G anti-B. dermatitidis antibodies (4). Antigen that bound to the antibody-coated wells was detected with biotinylated anti-B. dermatitidis immunoglobulin G. The cutoff for positivity was determined by receiver operator characteristic (ROC) analysis, evaluating urine specimens from patients with blastomycosis versus those from healthy volunteers. Enzyme immunoassay (EIA) units were obtained by dividing the optical density at 450 nm of the specimen by that of the ROC cutoff. Assay reproducibility was assessed by estimating the Bland and Altman limits of agreement (1).
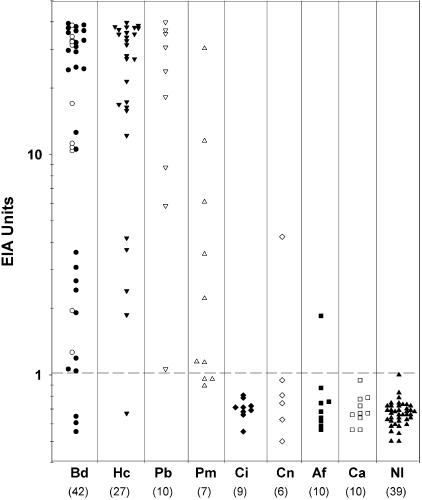
ROC analysis demonstrated excellent differentiation between results from the 42 patients with blastomycosis and the 39 healthy volunteers, with an area under the curve of 0.942, a 95% confidence interval of 0.868 to 0.981, and a standard error of 0.026. An optical density of 0.094 was identified as the optimal cutoff by ROC analysis. The results for patients and controls are shown in Fig. 1. Antigen was detected in the urine of 92.9% of patients with blastomycosis, including 25 of 28 with disseminated blastomycosis (89.3%) and all 14 cases of pulmonary blastomycosis (100%) (Table 1). Cross-reactive antigen was detected in the urine from 96.3% of patients with histoplasmosis, all patients with paracoccidioidomycosis, and 7 of 10 patients with penicilliosis marneffei. Cross-reactive antigen was detected for a single patient with cryptococcal meningitis (4.23 U) and another with aspergillosis (1.85 U) but was not detected for those with candidiasis or coccidioidomycosis or for healthy volunteers.
FIG. 1.
The broken line drawn at 1 EIA unit differentiates positive from negative results. For the blastomycosis cases, open circles indicate pulmonary blastomycosis and filled circles indicate disseminated blastomycosis. The numbers in parentheses beneath the abbreviations indicate the numbers of patients. Bd, B. dermatitidis; Hc, H. capsulatum; Pb, P. brasiliensis; Pm, P. marneffei; Ci, C. immitis; Af, A. fumigatus; Ca, C. albicans; N1, normal controls.
TABLE 1.
Blastomyces antigen positivity blastomycosis cases and controls
| Patient group | No. positive/no. tested | % positive (95% confidence interval) |
|---|---|---|
| Blastomycosis | ||
| All | 39/42 | 92.9 (80.5-98.5) |
| Pulmonary | 14/14 | |
| Disseminated | 25/28 | |
| Histoplasmosis | 26/27 | 96.3 (81.0-99.9) |
| Paracoccidioidomycosis | 10/10 | 100 (69.1-100.0) |
| Penicilliosis marneffei | 7/10 | 70 (34.7-93.3) |
| Cryptococcosis | ||
| All | 2/68 | 2.9 (0.4-10.2) |
| Urine, initial cases | 1/6 | |
| Urine, additional cases | 0/19 | |
| Serum or CSF | 1/43 | |
| Aspergillosis | ||
| All | 1/88 | 1.1 (0.0-6.2) |
| Urine, initial cases | 1/9 | |
| Urine, additional cases | 0/12 | |
| Serum, additional cases | 0/8 | |
| Galactomannan positive serum | 0/59 | |
| Coccidioidomycosis | 0/9 | 0 (0.0-33.6) |
| Candidiasis | 0/10 | 0 (0.0-30.8) |
| Healthy volunteers | 0/39 | 0 (0.0-9.0) |
To further assess the occurrence of cross-reactions in aspergillosis and cryptococcosis, additional testing was performed. Of 43 CSF or serum specimens containing cryptococcal polysaccharide antigen and 19 urine specimens from patients with cryptococcal meningitis, 1 CSF specimen (1.61%) was positive, at 10.40 U. Cryptococcus neoformans polysaccharide antigen, which was used as a positive control in the latex agglutination assay, was positive at 5.58 U. Urine or serum specimens from 79 additional patients with proven or probable invasive aspergillosis were tested, and none were positive. Furthermore, the A. fumigatus antigen, which was used as a positive control in the Platelia galactomannan assay, was negative in the Blastomyces antigen assay.
Reproducibility was evaluated by comparing results of two different assays. Antigen results were reproducibly positive or negative in 96.27% of specimens. The reproducibility of antigen levels was evaluated by analysis of difference between results of the first and second experiments. While the mean difference between test 1 and 2 was −0.2 EIA units (EU) (P = 0.281), and antigen levels below 6 EUs agreed closely, those above 6 EUs varied more widely.
This is the first report of antigen detection for diagnosis of blastomycosis. The sensitivity was excellent, comparable to that described for cytology of blastomycosis (8) and antigen detection of histoplasmosis (4), and the reproducibility was high. Cross-reactions were seen in patients with histoplasmosis, paracoccidioidomycosis, and penicilliosis marneffei.
The impact of cross-reactivity with other mycoses, particularly histoplasmosis, requires further investigation. While an assay that distinguishes between blastomycosis and histoplasmosis may be desirable, it may not be feasible since the epitope detected appears to be a shared glycoprotein antigen (5). The importance of early diagnosis may outweigh the inability to differentiate histoplasmosis and blastomycosis, however.
Cross-reactions were not seen for coccidioidomycosis or candidiasis, but were noted for a few patients with aspergillosis or cryptococcosis. The antigen levels in the two patients with cryptococcal meningitis were relatively high, as was that of the purified cryptococcal polysaccharide antigen. The detection of cross-reactive antigen in only 2.9% of specimens from patients, however, suggests a unique epitope different from that identified for blastomycosis. Cross-reactivity in aspergillosis is less certain, as the single positive result was only slightly above the cutoff for positivity, and Aspergillus galactomannan was not cross-reactive in the assay.
While the clinical use of this test will require additional experience to define its role relative to that of cytology and the impact of cross-reactivity in other endemic mycoses, the following diagnostic algorithm is proposed. The approach may include cytology and/or histopathology of body fluids and tissues and antigen detection in urine or other body fluids suspected to be infected. Until a more specific antigen detection assay or serological test is available, definitive diagnosis will require demonstration of the organism by fungal culture.
Acknowledgments
We thank L. Joseph Wheat for guidance in the conduct of the project and assistance in the preparation of the manuscript.
REFERENCES
- 1.Bland, J. M., and D. G. Altman. 1986. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307-310. [PubMed] [Google Scholar]
- 2.Bradsher, R. W., S. W. Chapman, and P. G. Pappas. 2003. Blastomycosis. Infect. Dis. Clin. N. Am. 17:21-40, vii. [DOI] [PubMed] [Google Scholar]
- 3.Chapman, S. W., A. C. Lin, K. A. Hendricks, R. L. Nolan, M. M. Currier, K. R. Morris, and H. R. Turner. 1997. Endemic blastomycosis in Mississippi: epidemiological and clinical studies. Semin. Respir. Infect. 12:219-228. [PubMed] [Google Scholar]
- 4.Durkin, M. M., P. A. Connolly, and L. J. Wheat. 1997. Comparison of radioimmunoassay and enzyme-linked immunoassay methods for detection of Histoplasma capsulatum var. capsulatum antigen. J. Clin. Microbiol. 35:2252-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher, M. A., A. M. Legendre, and G. M. Scalarone. 1997. Immunological and chemical characterization of glycoproteins in IEF fractions of Blastomyces dermatitidis yeast lysate antigen. Mycoses 40:83-90. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman, L. 1992. Laboratory methods for the diagnosis and confirmation of systemic mycoses. Clin. Infect. Dis. 14(Suppl. 1):S23-S29. [DOI] [PubMed] [Google Scholar]
- 7.Lemos, L. B., M. Baliga, and M. Guo. 2002. Blastomycosis: the great pretender can also be an opportunist. Initial clinical diagnosis and underlying diseases in 123 patients. Ann. Diagn. Pathol. 6:194-203. [DOI] [PubMed] [Google Scholar]
- 8.Lemos, L. B., M. Guo, and M. Baliga. 2000. Blastomycosis: organ involvement and etiologic diagnosis. A review of 123 patients from Mississippi. Ann. Diagn. Pathol. 4:391-406. [DOI] [PubMed] [Google Scholar]
- 9.McKinsey, D. S., L. J. Wheat, G. A. Cloud, M. Pierce, J. R. Black, D. M. Bamberger, M. Goldman, C. J. Thomas, H. M. Gutsch, B. Moskovitz, W. E. Dismukes, C. A. Kauffman, et al. 1999. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo-controlled, double-blind study. Clin. Infect. Dis. 28:1049-1056. [DOI] [PubMed] [Google Scholar]
- 10.Vasquez, J., J. B. Mehta, R. Agarwal, and F. Sarubbi. 1998. Blastomycosis in northeast Tennessee. Chest 114:436-443. [DOI] [PubMed] [Google Scholar]
- 11.Wheat, J., S. MaWhinney, R. Hafner, D. McKinsey, D. F. Chen, A. Korzun, K. J. Skahan, P. Johnson, R. Hamill, D. Bamberger, P. Pappas, J. Stansell, S. Koletar, K. Squires, R. A. Larsen, T. Cheung, N. Hyslop, K. K. Lai, D. Schneider, C. Kauffman, M. Saag, W. Dismukes, and W. Powderly. 1997. Treatment of histoplasmosis with fluconazole in patients with acquired immunodeficiency syndrome. Am. J. Med. 103:223-232. [DOI] [PubMed] [Google Scholar]
- 12.Wheat, J., H. Wheat, P. Connolly, M. Kleiman, K. Supparatpinyo, K. Nelson, R. Bradsher, and A. Restrepo. 1997. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin. Infect. Dis. 24:1169-1171. [DOI] [PubMed] [Google Scholar]
- 13.Wheat, L. J., R. B. Kohler, and R. P. Tewari. 1986. Diagnosis of disseminated histoplasmosis by detection of Histoplasma capsulatum antigen in serum and urine specimens. N. Engl. J. Med. 314:83-88. [DOI] [PubMed] [Google Scholar]