Abstract
Short tandem repeats (STRs) are hypervariable genetic elements that occur frequently in coding regions. Their high mutation rate readily generates genetic variation, contributing to adaptive evolution and human diseases. We previously reported that natural ELF3 polyglutamine variants cause reciprocal genetic incompatibilities in two divergent Arabidopsis thaliana backgrounds. Here, we dissect the genetic architecture of this incompatibility, revealing as many as four loci putatively interacting with ELF3. We were able to specifically identify one such ELF3-interacting gene, LSH9. We further used a yeast two-hybrid strategy to identify proteins whose physical interactions with ELF3 were affected by polyglutamine tract length. We found two proteins for which this was the case, ELF4 and AtGLDP1. Using these two approaches, we identify specific genetic interactions and physical mechanisms by which the ELF3 polyglutamine tract may mediate the observed genetic incompatibilities. Our work elucidates how STR variation, which is generally underascertained in population-scale sequencing, can contribute to phenotypic variation. Furthermore, our results support our proposal that highly variable STR loci can contribute to the epistatic component of heritability.
Keywords: polyglutamine, short tandem repeat, microsatellite, epistasis, robustness
EVOLUTION is a tinkerer rather than a designer (Jacob 1977; Alon 2003); that is, adaptations are generally short-term, incremental fixes rather than alterations in fundamental biological plans. This principle is believed to underlie many design properties of biological systems. Thus, many (or most) genetic adaptations may be compensations for other genetic variants in a given background (Szamecz et al. 2014). One abundant source of genetic variation for such tinkering lies in short tandem repeats (STRs), genetic elements with high mutation rates. Due to these high mutation rates, STRs may be more likely than substitutions to contribute adaptive variants on a per-locus basis (Kashi et al. 1997; Gemayel et al. 2010; Hannan 2010). If compensation plays an important role in adaptation as predicted, we may expect that STRs also contribute compensatory variants at a higher rate (on average) than other loci. Although it may be difficult to establish directionality of compensation in any given case, for instance by ordering mutations chronologically, overall STRs should act as compensatory mutations more often than less mutable loci. To sum up, if “tinkering” is a dominant mode of adaptation, variable STRs affecting phenotype are then expected to show frequent epistasis with other loci. Indeed, this expectation is borne out in the handful of well-characterized STRs (Press et al. 2014).
One such STR resides in the Arabidopsis thaliana gene ELF3, where it encodes a polyglutamine tract that varies in length across different natural strains (Tajima et al. 2007; Undurraga et al. 2012). We have previously shown that these ELF3–STR variants have strong effects on phenotype, and that these effects differ depending on the genetic background expressing a particular variant (Undurraga et al. 2012). These observations suggest that background-specific variants are modifying the effect of STR alleles through epistasis. The high variability of the ELF3–STR relative to expectations suggests that this STR may compensate for many background-specific polymorphisms across globally distributed strains of A. thaliana.
ELF3 has been previously identified as a plausible candidate gene underlying a QTL for trait variance (i.e., noise) in the phenotypes of A. thaliana recombinant inbred lines (Jimenez-Gomez et al. 2011; Lachowiec et al. 2015). These results invite comparison to known “robustness genes” such as HSP90 (Sangster et al. 2007, 2008a,b), which can reveal or conceal the phenotypic consequences of many other genetic variants. A mechanistic explanation of this robustness phenomenon is epistasis, in which a robustness gene interacts with many other loci (Queitsch et al. 2012; Lachowiec et al. 2015), as for the promiscuous chaperone HSP90 (Taipale et al. 2010). Our previous findings and the many studies describing ELF3’s crucial functions in plant development lead us to hypothesize that ELF3 lies at the center of an epistatic network and that the ELF3 polyglutamine tract modifies these interactions.
It is well-established that ELF3 functions promiscuously as an adaptor protein in multiple protein complexes that are involved in a variety of developmental pathways (Liu et al. 2001; Yu et al. 2008; Yoshida et al. 2009; Nusinow et al. 2011; Chow et al. 2012; Huang et al. 2016). Polyglutamine tracts such as the one encoded by the ELF3–STR often mediate protein interactions (Perutz et al. 1994; Stott et al. 1995; Schaefer et al. 2012). Therefore, it is plausible that variation in the ELF3 polyglutamine tract affects ELF3’s interactions with its partner proteins. The ELF3 C terminus, which contains the STR-encoded polyglutamine tract, is necessary for nuclear localization (Herrero et al. 2012) and ELF3 homodimerization (Liu et al. 2001), but thus far relatively few proteins (PIF4 and TOC1) have been shown to interact with this domain (Nieto et al. 2014; Huang et al. 2016). Thus, the phenotypic and epistatic effects of ELF3–polyQ variation may arise from altered protein interactions, altered ELF3 nuclear localization, altered regulation of the PIF4 developmental integrator, or a combination thereof. In consequence, there are a variety of potential mechanisms for epistatic interactions of ELF3 and other loci, and thus we consider the broadest interpretation of epistasis: the simple nonadditivity of effects of genetic variants.
Here, we use genetic analysis of the ELF3–STR-based incompatibility to identify ELF3–STR-interacting loci, and describe how ELF3–polyQ variation affects ELF3 protein interactions. This work presents evidence that this STR anchors a complex network of epistasis, potentially due to its role as a compensatory modifier of several other loci.
Materials and Methods
Plant material and growth conditions
We generated a Columbia (Col) × Wassilewskija (Ws) F2 population by crossing to genetically map loci interacting with ELF3 in contributing to hypocotyl length. Seedling hypocotyl length was assayed in seedlings grown for 15 days in incubators set to short days (8 hr light:16 hr dark) at 22° on vertical plates as described previously (Undurraga et al. 2012). The elf3-200 (Undurraga et al. 2012) and elf3-4 (Hicks et al. 1996) mutants have been previously described. Transfer DNA (T-DNA) lines (Alonso et al. 2003; Kleinboelting et al. 2012) were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus, OH). After phenotypic analysis, we stored seedlings at 4° before genotyping.
Genotyping
We genotyped the ELF3–STR across 720 F2 segregants to identify ELF3 homozygotes for further genomic analysis. The STR differs by 27 bp and is thus readily distinguishable by agarose gel electrophoresis. We used one to two true leaves from each seedling for DNA extraction. PCR was performed in 10-µl volume containing 0.5 µM primers (Supplemental Material, Table S1), 0.2 µM each deoxynucleotide triphosphate (dNTP), 1 µl 10× ExTaq buffer, and 0.1 units ExTaq (Takara, Tokyo, Japan); with initial denaturation step of 95° for 5 minutes, followed by 40 cycles of 95° 30 seconds, 49° 20 seconds, 72° 10 seconds, with a final extension step at 72° for 5 minutes. For PCR analysis of other loci, PCR was performed in 20 µl volume containing 0.5 µM primers (Table S1), 0.2 µM each dNTP, 2 µl 10× ExTaq buffer, and 0.25 units Taq polymerase (NEB, Ipswich, MA); with initial denaturation step of 95° for 5 minutes, followed by 35 cycles of 95° 30 seconds, 55° 30 seconds, 72° 1 minute, with a final extension step at 72° for 5 minutes.
Genome resequencing
We selected ELF3 homozygotes among the F2’s for genotyping by sequencing according to their phenotypes. For each ELF3–STR allele, we selected 24 ELF3 homozygotes (Ws/Ws and Col/Col) at each phenotypic extreme (the shortest and longest hypocotyls). The sampling of extremes is an effective and statistically justified method for genetic mapping (Lander and Botstein 1989). These 96 individuals were analyzed in a genotyping-by-sequencing approach (Figure S1 and Table S3). These seedlings were transplanted to soil and grown under long days for 2–3 weeks. They were then stored at 4° until DNA extraction was performed. One late rosette-stage Ws individual was used for Ws whole-genome resequencing. DNA extraction was performed using the DNeasy Plant Mini kit (QIAGEN, Valencia, CA) according to the kit protocol. This DNA was quantified using high-sensitivity Qubit fluorescence analysis (ThermoFisher Scientific, Waltham, MA) and regenotyped with ELF3–STR primers (Table S1). We used 10 ng DNA from each F2 segregant in NextEra transposase library preparations (Illumina, San Diego, CA), or a standard 50-ng preparation for the Ws library. Library quality was assessed on a BioAnalyzer (Agilent, Santa Clara, CA) or agarose gels. The Ws individual was sequenced in one 300-cycle MiSeq v2 run (300 bp single-end reads) to ∼12× coverage. The F2 segregant libraries were pooled and sequenced in one 200-cycle HiSeq v3 run to ∼2× average coverage (100-bp paired-end reads, Table S3).
Sequence analysis
We aligned reads to the Col reference genome using BWA v0.7.5 MEM (Li 2013), and variants were called using SAMtools v0.1.19 (Li et al. 2009). We identified high-quality Ws variants (Q > 40) from Ws parent data and compared these with variants in previously sequenced related strains (Gan et al. 2011). We combined all F2 segregant genotype calls into a single variant call format (VCF) file and filtered these for loci with such Ws variants. We used SNPtools (Wang et al. 2013) to perform haplotype and genotype imputation for each locus in F2 segregants. For workflows employed in sequence analysis, see File S1. Following sequence analysis, we found one individual to be a heterozygote at the ELF3 locus. We omitted this individual from all following analyses requiring ELF3 homozygotes.
QTL analysis
We reduced F2 genotypes to a set of 500 loci randomly sampled from the imputed genotypes, plus a single nucleotide variant (SNV) marking the ELF3 locus. We used these genotypes to estimate a genetic map and perform QTL analysis using the R/qtl package (Broman et al. 2003). We genotyped 30 additional F2’s (further ELF3 homozygotes with extreme phenotypes) with PCR markers (Table S1) to increase QTL accuracy. To directly test for epistasis with ELF3, we adapted a previously described method for evaluating interactions with prespecified loci (Sangster et al. 2008a). We defined an empirical null distribution for the difference of LOD scores (“delta LOD”) expected between random samples of genotyped F2’s, comparing this to the actual difference observed between ELF3–Col and ELF3–Ws homozygotes. We implemented this custom nonparametric epistasis test using R/qtl functions. For a more detailed description of commands and the epistasis test, see File S1.
Candidate gene analysis
We assessed candidate gene function and interaction with ELF3 using homozygous T-DNA insertion lines (Alonso et al. 2003; Kleinboelting et al. 2012) obtained from the Arabidopsis Biological Resource Center. We phenotyped these lines for hypocotyl length under SD at 15 days as for F2’s. All such experiments were performed at least twice. We obtained elf3 lsh9 and elf3 nup98 double mutants by crossing relevant lines and genotyping (primers in Table S1). Expression analysis confirmed no detectable LSH9 expression in the lsh9 mutant, suggesting that it is a null mutant. Mutant lines are listed in Table S2.
Yeast two hybrid
We used yeast two hybrid (Y2H) to assess different proteins for interactions with ELF3 and specifically to test the hypothesis that ELF3–polyQ variation affects these interactions. We PCR cloned ELF3 variants with different STR lengths out of complementary DNAs (cDNAs) of previously described A. thaliana carrying ELF3 transgenes (Undurraga et al. 2012) into the XmaI/BamHI sites of pGBKT7. These included synthetic ELF3s with 0Q (no polyQ), 7Q (endogenous variant in Col), 16Q (endogenous variant in Ws), and 23Q (endogenous to strains Br-0 and Bur-0). We PCR cloned genes to be tested for ELF3 interactions into the EcoRI/XhoI sites of pGADT7 from cDNAs of indicated strains (Table S1 for primers). Clones were confirmed by restriction digest and sequencing. We performed a Y2H screen of the ELF3–7Q variant against the Arabidopsis Mate and Plate cDNA library (Clontech, Madison, WI), essentially according to the manufacturer’s instructions, with selections performed on Synthetic Complete (C) −Leu −Trp −His plates incubated at 23°. We tested clones, which also showed activation of the ADE2 reporter gene (and did not autoactivate) against the various ELF3–polyQ constructs (see File S1 for details, full details on clones given in File S2).
As a more quantitative assay of Y2H interaction, we assayed LacZ activity through X-gal cleavage essentially as previously described (Möckli and Auerbach 2004), again in strains using PJ69-4α as MATα parent. LacZ expression is, like HIS3 and ADE2, also driven by Y2H in this system. For weakly activating constructs (GLDP1 and ELF4), we used 0.2 absorbance units of yeast in each assay to reduce background and assessed color development at points between 16 and 72 hr of incubation at room temperature.
Quantitative PCR
For measuring ELF3 and LSH9 transcript levels, we collected ∼30 mg pooled aerial tissue from short-day-grown seedlings of each relevant genotype at Zeitgeber time (ZT) 8 at 7 days postgermination. RNA preparation, reverse transcription, and quantitative PCR (qPCR) was performed as described previously (Undurraga et al. 2012), using primers in Table S1.
Statistical analysis
All statistical analyses and plotting was performed using R 3.2.1 (R Development Core Team 2016). Analysis scripts are provided with data as detailed below.
Data availability
High-throughput sequencing data are available in BAM format at National Center for Biotechnology Information Sequence Read Archive accession no. SRP077615. Processed genotype data, phenotype data, and analysis code are available at https://figshare.com/articles/Variable_ELF3_polyQ_modulates_complex_epistasis_data_and_code/3467717.
Results
Genetic analysis of ELF3–STR effects on hypocotyl length
To investigate the genetic architecture of epistasis for the ELF3–STR, we used hypocotyl length under short days, a trait controlled by ELF3–STR variation (Undurraga et al. 2012). We previously reported a mutual incompatibility of these ELF3–STR variants between the Col and Ws strains using transgenic analysis. Here, we phenotyped a Col × Ws F2 population for this trait, which we expected to show substantial variation according to the segregation of the ELF3–STR variants.
The Col and Ws strains did not substantially differ in this trait (P = 0.16, Kolmogorov–Smirnov test, Figure 1A). Although most F2 seedlings showed phenotypes within the range of the two parental lines, the F2 phenotypic distribution showed a long upper tail of transgressive variation and consequently a different distribution from either parent (P = 0.0039 against Ws, P = 0.055 against Col, Kolmogorov–Smirnov tests). As longer hypocotyls in light conditions indicate ELF3 dysfunction in the circadian clock (Liu et al. 2001), this observation agrees with the hypothesized incompatibility of Col and Ws alleles (which include the ELF3–STR). We replicated this observation in a much larger population (1106 seedlings), which was used for further genetic analysis.
Figure 1.
Phenotypic transgression in Col × Ws F2 segregants. (A) F2 segregant phenotypes compared to parents and an elf3 null mutant in the Col background; n ≈ 50 for each homozygous line and n ≈ 100 for F2’s. Colors indicate genotypes as indicated. Hypocotyl length was determined at 15 days under short days. (B) Phenotypic distributions of a large population of Col × Ws F2 segregants, for 720 extreme individuals genotyped at the ELF3 locus. N = 1106 total seedlings. A total of 386 seedlings with intermediate phenotypes were not genotyped (indicated by the black box).
To investigate the genetic basis of the phenotypic transgression in hypocotyl length, we harvested approximately two of three seedlings representing the longest and shortest hypocotyls (Figure S1). We genotyped the ELF3–STR in these seedlings and observed a strong main effect of ELF3 on phenotype (Figure 1B), in which the Col allele of ELF3 frequently showed transgressive phenotypes, although some individuals homozygous for the Ws allele also showed transgressive phenotypes. We cannot exclude any effect from ELF3-linked variants other than the STR, but given the known effects of ELF3–STR variation in these backgrounds (Undurraga et al. 2012), we consider the STR as the most plausible Chr2 variant underlying the observed transgression. Specifically, a naïve regression analysis of the data in Figure 1B indicated that each ELF3–Col allele increased hypocotyl length by 0.87 ± 0.077 mm, and that the ELF3 locus thereby explained 15% of phenotypic variation. This analysis is of course misleading, because it implies that Col seedlings should show longer hypocotyls than Ws seedlings due to ELF3 genotype, which is not the case (Figure 1A). Moreover, this observation suggests that the relevant epistatic model under which ELF3 operates with other loci is reminiscent of capacitance (Bergman and Siegal 2003), in which mutational effects are revealed by ELF3 dysfunction.
Among these seedlings, the individuals with extreme phenotypes and individuals homozygous at the ELF3 locus are expected to be most informative about ELF3–STR effects on phenotype. Furthermore, ELF3 genetic interactions are expected to be most apparent in ELF3 homozygotes. Therefore, we focused our analysis on these homozygotes, which we analyzed using a genotyping-by-sequencing approach (Figure S1). For details of our approach, see Materials and Methods and Figure S2, Figure S3).
With these data, we performed a one-locus QTL scan to identify chromosomal regions contributing to hypocotyl length (Figure 2A). This analysis indicated a QTL on Chr2 corresponding to ELF3 as expected, but also significant QTL on Chr1, Chr4, Chr5, and possibly one or more additional QTL on Chr2 affecting the phenotype. A two-dimensional QTL scan suggested that at least some of these QTL interact epistatically with the ELF3 locus (Figure S4).
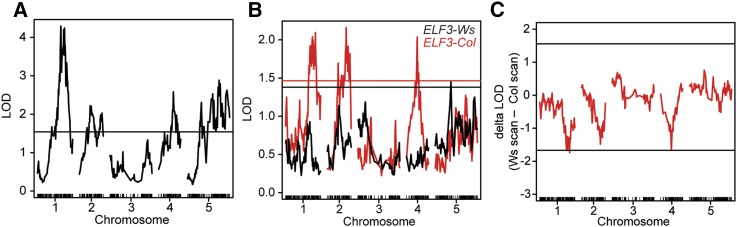
Figure 2.
QTL analysis identifies interactions of ELF3 with multiple loci. (A) One-dimensional QTL scan including all sequenced F2’s. Horizontal line indicates 99% significance threshold based on permutations. (B) QTL scan stratified by ELF3–STR genotype (all genotypes but those of indicated F2’s masked in each analysis). Horizontal lines of each color indicate 99% significance threshold based on permutations for each scan. (C) A nonparametric test of epistasis between ELF3 and other loci, using the independent QTL scans shown in B, ELF3 is located on chromosome 2. Horizontal lines indicate 95% significance threshold based on permutations.
We investigated potential epistatic effects of ELF3 on other loci, performing one-dimensional QTL scans on each ELF3 homozygote background separately (masking the genotypes of all individuals with other ELF3 genotypes). This analysis identified loci whose effects are potentially contingent on one ELF3 allele or the other. We observed that the same LOD peaks were replicated well in ELF3–Col homozygotes, but poorly in ELF3–Ws homozygotes (Figure 2B). Notably, a second Chr2 QTL was thus made obvious in this analysis, indicating that loci other than ELF3 on Chr2 are relevant to the phenotype (at least in ELF3–Col plants). These results suggested that the ELF3–STR genotype is epistatic to at least three other loci controlling hypocotyl elongation, whose effects are masked in ELF3–Ws plants. To formally test for epistasis with ELF3, we compared the difference in LOD scores between the two QTL scans at all loci to a permutation-based null distribution, and found that the peaks on Chr1 and Chr4 (and to a lesser extent Chr2) were all stronger in the ELF3-Col background (Figure 2C). Consequently, each of these QTL likely contains one or more background-specific ELF3 interactors.
We considered the genetic contribution of these loci to the phenotype using a multiple QTL mapping approach, using both the independently estimated QTL locations and a refined model reestimating QTL positions based on information from all QTL (Table S4). In each case, loci on Chr1, Chr2, Chr4, and Chr5 were supported, along with interactions between Chr2 (ELF3) and the other two loci on Chr1 and Chr4. In the refined model, the ELF3 locus showed a dramatically higher LOD score and other loci generally decreased in importance (especially the second Chr2 locus), again suggesting epistatic masking by ELF3. We conclude that although ELF3 interacts epistatically with a variety of other loci in determining hypocotyl length, the principal contributors to ELF3-mediated effects on the trait appear to be on Chr1 and Chr4. Moreover, direct inspection of phenotypic effects of ELF3 in interaction with each putative locus among F2 segregants supported the hypothesis of epistasis with ELF3 most clearly for the Chr1 and Chr4 loci (Figure S5).
Candidate gene analysis identifies LSH9 as a genetic interactor of ELF3
The chromosome intervals identified by our QTL analysis encompassed a large number of genes and partially overlapped with an inversion between these backgrounds on Chr4 (Rowan et al. 2015), although the QTL peak was >1 Mb outside the mapped inversion. Previous work using a multiparent A. thaliana mapping population (Kover et al. 2009) also identified possible candidate genes affecting hypocotyl length in these regions (Khattak 2014).
We phenotyped mutants of several candidate genes in the Col background under the conditions of our intercross experiment (15-day SD hypocotyl length, Figure S6). We observed small phenotypic effects of the T-DNA insertion mutants lsh9 and nup98. However, these small effects on their own cannot explain the transgressive phenotypic variation in F2’s (Figure 1A).
The secondary Chr2 peak (other than ELF3) included the known ELF3 interactor PIF4. pif4 null mutants are known to partially suppress the long hypocotyl defect of elf3 mutants, and thus PIF4 is a candidate gene with prior support. However, our genetic data showed comparatively weak signal at the Chr2 peak when accounting for the ELF3 QTL, and its interval was very wide (18Mb, Table S4). When examining the actual phenotypic effects of this QTL, the interaction appeared ambiguous compared to the Chr1 and Chr4 signals (Figure S5). We therefore focused on the novel candidate genes NUP98 on Chr1 and LSH9 on Chr4, given the strong support for their interaction with ELF3 in this population (Figure 2, Figure S5, and Table S4).
We generated double mutants between mutants of these genes and the elf3 null mutant to determine whether these genes interacted epistatically with ELF3. We found little evidence for an interaction between nup98 and elf3 mutations (Figure S6C). However, we detected a significant interaction between ELF3 and LSH9, in the form of reciprocal sign epistasis between the two null mutants affecting hypocotyl length (Figure 3A). Although lsh9 single mutants had significantly shorter hypocotyls than WT, lsh9 elf3 double mutant hypocotyls were substantially longer than in elf3 single mutants. LIGHT-DEPENDENT SHORT HYPOCOTYLS 9 (LSH9) is an uncharacterized gene belonging to a gene family named for LSH1, which is known to act in hypocotyl elongation (Zhao et al. 2004). Publicly available expression data indicated that LSH9 transcript levels are highest in the hypocotyl and root and show light-dependent circadian fluctuations (Winter et al. 2007). Like other genes in this family, LSH9 encodes a putative nuclear localization sequence but has no other distinguishing features.
Figure 3.
ELF3 interacts genetically with LSH9, which shows background-specific expression. (A) Double mutant analysis of elf3 and lsh9 seedlings (in the Col background) grown for 15 days under short days. ANOVA analysis of the interaction between the mutant effects on phenotype is displayed. Error bars indicate SD, n > 35 for each genotype. Experiments were repeated with similar results. (B) qRT-PCR analysis of LSH9 transcript levels across genotypes. Seedlings were grown under SD and harvested at ZT8 (dusk). LSH9 expression is expressed as a proportion of Ws expression, normalized relative to UBC21, error bars are SE from four biological replicates.
To test our hypothesis that ELF3–STR-mediated epistasis may be due to altered protein interactions, we investigated whether LSH9 and ELF3 interacted physically using Y2H. However, we were unable to detect a physical interaction between the Col or Ws variants of LSH9 and ELF3 (Figure S7), suggesting a different mechanistic basis for the observed genetic interaction. For example, LSH9 expression may depend on ELF3 function as a transcriptional regulator. Alternatively, ELF3 expression may depend on LSH9 function. We tested both hypotheses by measuring expression levels of LSH9 and ELF3 in, respectively, elf3 or lsh9 mutant backgrounds (elf3 mutants were available in both Col and Ws backgrounds, but lsh9 mutants were available only in Col). ELF3 expression levels were unchanged in lsh9 mutants (Figure S8). Moreover, levels of LSH9 transcript did not significantly differ between WT and elf3 mutants in either strain background. However, LSH9 expression was reduced in the Ws background relative to Col independently of ELF3 genotype (Figure 3B). This result is consistent with the observed phenotypic interaction in F2’s, which showed elongated hypocotyls when Col alleles at the ELF3 locus cosegregated with Ws alleles at the LSH9 locus (Figure S5), thereby pairing poorly functioning ELF3 alleles with potentially lower LSH9 expression levels.
Taken together, ELF3–LSH9 epistasis between Col and Ws may be due to regulatory changes between these two backgrounds altering LSH9 transcript levels. For example, genomic data indicate that the LSH9 promoter contains an STR polymorphism in the Ws background that may alter LSH9 expression (Alonso-Blanco et al. 2016).
ELF3–polyQ tract variation affects known protein interactions
While our QTL analysis identified several potential interactors of the ELF3 locus, there was no obvious mechanistic interpretation of ELF3 polyQ variation’s effect on protein function. Based on prior knowledge on ELF3 and polyQ tract function (Schaefer et al. 2012; Huang et al. 2016), we hypothesized that polyQ variation may affect ELF3’s interactions with other proteins. Thus, in parallel with our genetic analysis, we used Y2H to directly identify A. thaliana proteins whose physical interactions with ELF3 are polyQ modulated. We first explored whether several variants of ELF3 (including 7Q and 16Q) show Y2H interactions with the well-described ELF3 interactors PHYB (Liu et al. 2001), ELF4 (Nusinow et al. 2011; Herrero et al. 2012) (Figure 4), and PIF4 (Nieto et al. 2014). None of the ELF3 constructs showed autoactivation in yeast when paired with an empty vector (Figure S9). The ELF3-interacting domain of PHYB has two coding variants between Col and Ws, and we thus tested both Ws and Col variants of this domain. We found that both forms showed apparently equal affinity with all polyQ variants of ELF3. ELF4, which has no coding variants between Col and Ws, also interacted with all polyglutamine variants of ELF3, although rather weakly compared to PHYB. Under these conditions, a subtle preference of ELF4 for some polyQ variants was apparent (e.g., ELF3–16Q vs. ELF3–0Q). We confirmed this preference in a quantitative Y2H LacZ assay (Figure 4B). We were not able to replicate the previously reported ELF3–PIF4 interaction (Nieto et al. 2014) for any ELF3–polyQ variant in our Y2H system (Figure S10) and were thus unable to evaluate effects of polyQ variation on ELF3–PIF4 interactions.
Figure 4.
Y2H interaction of ELF3 with known protein interactors can be modulated by polyQ variation. (A) Yeast carrying indicated vectors were spotted in fivefold dilutions onto C −Leu −Trp (C−LT) or C −Leu −Trp −His −Ade (C−LTHA) media. PHYB-Cterm, previously defined C-terminal truncations of PHYB sufficient for ELF3 interaction (Liu et al. 2001) from the Col and Ws backgrounds. For each protein X, experiments were repeated with independent PJ69-4α + pGADT7-X transformants with similar results. (B) LacZ assays support polyQ effects on ELF3–ELF4 interaction. The strains shown in A also express LacZ from the Y2H promoter, whose activity was assayed in yeast cell lysates (see Materials and Methods). In each assay, all observations are expressed relative to the activity of the empty vector, whose mean is set to 0. Error bars indicate SD across three technical replicates. This experiment was repeated with similar results.
Together, our data suggest that ELF3–polyQ tract variation can affect ELF3 protein interactions, in particular if these interactions are weaker (as for ELF4) and presumably more sensitive to structural variation in ELF3.
Y2H screen identifies three novel ELF3 interactors, one of which is polyQ modulated
None of the known ELF3 interactors were encoded by genes located in the major Chr1 and Chr4 QTL identified by our genetic screen. If the ELF3–polyQ tract mediates protein interactions, these regions should contain additional, previously undescribed polyQ-modulated ELF3 interactors. We screened the ELF3–7Q protein for interactions with proteins from a commercially available library derived from Col, to detect ELF3–protein interactions within the Col background.
We subjected Y2H positives to several rounds of confirmation (Supplementary Material), yielding a total of three novel proteins that robustly interacted with ELF3: PLAC8 domain-containing protein AT4G23470, LUL4, and AtGLDP1 (Figure 5). AT4G23470 was recovered in two independent clones, and LUL4 was recovered in three independent clones. The PLAC8 domain protein AT4G23470 is encoded by a gene within the QTL interval on chromosome 4, but this protein showed no variation in affinity among the various ELF3–polyQs. LUL4, a putative ubiquitin ligase, is not encoded in any of the mapped QTL and also shows no variation in affinity among the various ELF3–polyQs. Thus, differential interaction with these proteins is unlikely to underlie the observed epistasis.
Figure 5.
Y2H screen identifies new interactors of ELF3. (A) Yeast carrying indicated vectors were spotted in fivefold dilutions onto C −Leu −Trp (C−LT) or C −Leu −Trp −His −Ade (C−LTHA) media. For each protein X, experiments were repeated with at least two independent PJ69-4α + pGADT7-X transformants with similar results. (B) LacZ assays support polyQ effects on ELF3–AtGLDP1 interaction. The strains shown in A also express LacZ from the Y2H promoter, whose activity was assayed in cell lysates (see Materials and Methods). In each assay, all observations are expressed relative to the activity of the empty vector, whose mean is set to 0. Error bars indicate SD across three technical replicates. This experiment was repeated with similar results.
In contrast, the AtGLDP1 protein, which is encoded on Chr4 but not within the QTL interval, appeared to show a subtle preference for the synthetic ELF3–0Q construct over longer polyQ tracts. We confirmed this preference in a quantitative LacZ assay (Figure 5B). Although our screen is unlikely to exhaust hitherto-unknown ELF3 interactors, our data suggest that the ELF3–polyQ tract can affect ELF3’s interactions with other proteins. Moreover, polyQ variation appears to affect weaker ELF3–protein interactions; strong protein interactions (for example ELF3–LUL4 and ELF3–PHYB) are robust to polyQ variation.
Discussion
The contribution of STR variation to complex traits is thought to be considerable (Kashi et al. 1997; Press et al. 2014). Specifically, it has been proposed that STR variation contributes disproportionately to the epistatic term of genetic variance, due to its potential to contribute compensatory mutations. However, the molecular mechanisms by which different STRs contribute to genetic variance should derive from their particular features. For instance, polyQ variation may be expected to affect protein interactions (Perutz et al. 1994; Schaefer et al. 2012) or the transactivation activity of affected proteins (Escher et al. 2000). In this study, we considered the case of the previously described ELF3–STR (Undurraga et al. 2012).
We found that the genetic architecture of ELF3-dependent phenotypes is highly epistatic between the divergent Col and Ws strains, leading to substantial phenotypic transgression in the well-studied hypocotyl length trait (Zagotta et al. 1996; Dowson-Day and Millar 1999; Nusinow et al. 2011). We identified at least three QTL showing genetic interactions with the ELF3–STR in a Col × Ws cross. These QTL generally did not coincide with obvious candidate genes known to affect ELF3 function (with the potential exception of PIF4). Our confirmation of one genetic interaction (LSH9) in the Col background suggests that these QTL encompass variants affecting hypocotyl length in tandem with ELF3–STR variation. We cannot formally exclude the hypothesis that variants linked to ELF3 (other than the Col and Ws ELF3–STR variants) may contribute to the observed phenotypic variation. For example, the ELF3–A362V substitution in the A. thaliana strain Sha affects ELF3 function in the circadian clock (this site is invariant between Col and Ws) (Anwer et al. 2014). However, our previous work demonstrated that ELF3–STR variation suffices to produce strong phenotypic incompatibility between the Col and Ws background (Undurraga et al. 2012). Therefore, we reason that ELF3–STR variation is the most parsimonious explanation for the phenotypic variation, and in particular the observed transgression in the Col × Ws cross.
We further used Y2H screening to explore whether ELF3 polyQ tract variation affects protein interactions. ELF3’s promiscuous physical associations with other proteins are essential to its many functions in plant development (Liu et al. 2001; Kolmos et al. 2011; Nusinow et al. 2011; Herrero et al. 2012). Disruption of these interactions suggested an attractive mechanism by which ELF3–polyQ tract variation might affect ELF3 function. Assaying several known and novel ELF3-interacting proteins yielded evidence for a modest effect of polyQ variation on weaker protein interactions. However, there was no generic requirement for specific ELF3 polyQ tract lengths across all interactors. Indeed, the modest effects that we found were interactor specific and thus not likely to generalize.
We did find that the ELF3–ELF4 interaction, which is crucial for circadian function and thus hypocotyl length (Nusinow et al. 2011; Herrero et al. 2012), demonstrates a subtle preference for the Ws 16Q–ELF3 variant. However, there is no sequence variation in ELF4 between Col and Ws and we did not detect the ELF4 locus by QTL analysis, suggesting that this binding preference does not explain the transgressive phenotypes revealed by ELF3–STR variation (Figure 1A). However, the subtle polyQ dependence of the ELF3–ELF4 interaction may play a role through indirect interactions.
Alternatively, rather than modulating ELF3 function as an encoded polyQ tract, the ELF3–STR may affect ELF3 transcription or processing. A previous study of an intronic STR in A. thaliana demonstrated that certain hyperexpanded STR alleles led to dysregulation of the IIL1 gene (Sureshkumar et al. 2009), presumably due to aberrant processing of IIL1 transcripts. Others have previously argued that such “informational” (as opposed to “operational”) processes are more likely to have genetic or physical interactions (Jain et al. 1999). We have not tested this hypothesis, although our previous studies found no correlation between ELF3–STR variation and ELF3 expression across many natural strains (Undurraga et al. 2012).
Taken together, our findings support a model in which highly variable STRs can contribute to the epistatic component of heritability through both direct and indirect functional interactions with other loci. One potential concern with our model is that one might expect highly epistatic loci to be more constrained than other loci, and thus unlikely to accumulate more compensatory mutations. Nonetheless, the existence of the ELF3–STR epistatic network suggests that the constraints are not insurmountable, potentially as a result of a cascade of compensations. The underlying evolutionary dynamics of this process, and of high mutation rates in general, deserve more study.
ELF3 is a model for subexpansion polyQ variation
PolyQ variation is best known from hyperexpansions that are associated with several incurable human neurological disorders (Orr and Zoghbi 2007; Fondon et al. 2008; Usdin 2008; Hannan 2010), in which CAG (although generally not CAA) repeats dramatically elongate to lengths >50 units, reaching over 200 units in some patients. We argue that these hyperexpansion disorders are poor models for the functional impact of subexpansion variation of polyQ tracts and propose instead that ELF3–polyQ might serve as a more appropriate model.
Although ELF3–polyQ tract variation can reach a length associated with disease in humans, it differs qualitatively from the well-studied human polyQ tract hyperexpansions. Human polyQ hyperexpansions are associated with protein aggregation or plaque formation, a phenomenon that requires a sufficiently long uninterrupted polyglutamine domain (Sharma et al. 1999; Lu and Murphy 2015). These previous in vitro studies suggest that although the ELF3–polyQ variants in Col and Ws are sufficiently different to alter phenotype, neither is long enough to lead to aggregation by these mechanisms (although the ELF3–23Q variant may be within this range).
Next, the effects of the disease-associated polyQ hyperexpansions are generally dominant, due to the nature of their molecular effects, which are generally thought to show a protein gain of function (Orr and Zoghbi 2007; Fondon et al. 2008). We observed no evidence that ELF3–polyQ variation behaves in a dominant fashion, but rather that Col × Ws F1’s show approximately WT phenotypes. The effects of ELF3–polyQ variation manifest only when separated from a favorably interacting genetic context (as in segregating F2’s).
Last, the (sometimes implicit) expectation from polyQ hyperexpansion phenotypes is that there is a linear, or at least monotonic, association between phenotypes and polyQ length. For instance, the degree of huntingtin polyQ expansion is strongly correlated with Huntington’s disease severity (Andrew et al. 1993). In contrast, there is little evidence for any monotonic relationship between a ELF3–polyQ-related phenotype and ELF3–polyQ length (Undurraga et al. 2012; Press et al. 2014, 2016). Indeed, all indications are that the mapping between ELF3–polyQ tract length and phenotype is nonmonotonic and strongly contingent on genetic background, unlike the classic polyQ disease models. Our results suggest that subexpansion polyQ tract variation can engage in multiple genetic interactions and at least in some cases modulate protein interactions. More work is needed to evaluate the generality of our findings and determine the breadth of molecular mechanisms by which modest, subexpansion polyQ variation can affect phenotype.
ELF3 as a robustness gene
Here, we operated under the assumption that a few strong polyQ-modulated interactions with ELF3 explain the polyQ-dependent genetic architectures. Alternatively, the ELF3 polyQ tract may modulate many transient interactions that are perturbed by hypomorphic ELF3 activity. In this interpretation, ELF3 acts as a “robustness gene” (Lempe et al. 2013). The best-described example of such is the protein chaperone HSP90 (Rutherford and Lindquist 1998), whose multiple transient interactions with many proteins—∼10% of the yeast proteome (Zhao et al. 2005)—lead to pleiotropic effects upon HSP90 inhibition or dysregulation (Sangster et al. 2007). ELF3 has been previously proposed as a robustness gene (Jimenez-Gomez et al. 2011), consistent with its promiscuity in protein complexes and the pleiotropic nature of elf3 mutant phenotypes. Our finding that functional modulation of ELF3 by polyQ variation reveals several genetic interactors is consistent with this interpretation.
A similar hypothesis is that ELF3 “gates” robustness effects from robustness genes with which it interacts. For instance, we have recently shown that ELF3 function is epistatic to some of HSP90’s pleiotropic phenotypic effects (M. Zisong, P. Rival, M. Press, C. Queitsch, and S. Davis, unpublished data), and ELF4 has also been proposed as a robustness gene governing circadian rhythms and flowering (Lempe et al. 2013). Here, we show that polyQ variation affects ELF3–ELF4 binding, which would provide a mechanistic link between ELF3–polyQ effects and a known robustness gene.
These hypotheses remain speculative in the absence of more explicit tests. Nonetheless, we suggest that the pleiotropic effects of polyQ variation in ELF3 (or similar cases) may be better understood by considering ELF3 as a robustness gene, in which phenotypic effects are determined by a variety of important but individually small interactions of this highly connected epistatic hub.
Acknowledgments
We thank Karla Schultz and Katie Uckele for technical assistance, Choli Lee and Jay Shendure for assistance with high-throughput sequencing of the Col × Ws F2 population, members of the Shendure laboratory for advice regarding library preparation, Amy Lanctot for generating the pGBK–ELF3–0Q and pGBK–ELF3–23Q constructs, Daniel Melamed and Stanley Fields for guidance in carrying out Y2H experiments and the generous gift of yeast strains, Giang Ong and Maitreya Dunham for access to the MiSeq instrument for resequencing the Ws genome, Stanley Fields and Evan Eichler for access to LightCycler instruments, Elhanan Borenstein and members of the Queitsch and Borenstein laboratories for helpful conversations, and two anonymous reviewers for helpful suggestions toward improving the text. M.O.P. was supported in part by National Human Genome Research Institute Interdisciplinary Training in Genome Sciences grant 2T32HG35-16. C.Q. is supported by National Institutes of Health New Innovator Award DP2OD008371.
Footnotes
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.193359/-/DC1.
Communicating editor: K. Bomblies
Literature Cited
- Alon U., 2003. Biological networks: the tinkerer as an engineer. Science 301: 1866–1867. [DOI] [PubMed] [Google Scholar]
- Alonso J. M., Stepanova A. N., Leisse T. J., Kim C. J., Chen H., et al. , 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657. [DOI] [PubMed] [Google Scholar]
- Andrew S. E., Goldberg Y. P., Kremer B., Telenius H., Theilmann J., et al. , 1993. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet. 4: 398–403. [DOI] [PubMed] [Google Scholar]
- Anwer M. U., Boikoglou E., Herrero E., Hallstein M., Davis A. M., et al. , 2014. Natural variation reveals that intracellular distribution of ELF3 protein is associated with function in the circadian clock. eLife 3: e02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman A., Siegal M. L., 2003. Evolutionary capacitance as a general feature of complex gene networks. Nature 424: 549–552. [DOI] [PubMed] [Google Scholar]
- Broman K. W., Wu H., Sen S., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19: 889–890. [DOI] [PubMed] [Google Scholar]
- Chow B. Y., Helfer A., Nusinow D. A., Kay S. A., 2012. ELF3 recruitment to the PRR9 promoter requires other evening complex members in the Arabidopsis circadian clock. Plant Signal. Behav. 7: 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowson-Day M. J., Millar A. J., 1999. Circadian dysfunction causes aberrant hypocotyl elongation patterns in Arabidopsis. Plant J. 17: 63–71. [DOI] [PubMed] [Google Scholar]
- Escher D., Bodmer-Glavas M., Barberis A., Schaffner W., 2000. Conservation of glutamine-rich transactivation function between yeast and humans. Mol. Cell. Biol. 20: 2774–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondon J. W., Hammock E. A. D., Hannan A. J., King D. G., 2008. Simple sequence repeats: genetic modulators of brain function and behavior. Trends Neurosci. 31: 328–334. [DOI] [PubMed] [Google Scholar]
- Gan X., Stegle O., Behr J., Steffen J. G., Drewe P., et al. , 2011. Multiple reference genomes and transcriptomes for Arabidopsis thaliana. Nature 477: 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemayel R., Vinces M. D., Legendre M., Verstrepen K. J., 2010. Variable tandem repeats accelerate evolution of coding and regulatory sequences. Annu. Rev. Genet. 44: 445–477. [DOI] [PubMed] [Google Scholar]
- Hannan A. J., 2010. Tandem repeat polymorphisms: modulators of disease susceptibility and candidates for “missing heritability”. Trends Genet. 26: 59–65. [DOI] [PubMed] [Google Scholar]
- Herrero E., Kolmos E., Bujdoso N., Yuan Y., Wang M., et al. , 2012. EARLY FLOWERING4 recruitment of EARLY FLOWERING3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 24: 428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks K. A., Millar A. J., Carre I. A., Somers D. E., Straume M., et al. , 1996. Conditional circadian dysfunction of the Arabidopsis early-flowering 3 mutant. Science 274: 790–792. [DOI] [PubMed] [Google Scholar]
- Huang H., Alvarez S., Bindbeutel R., Shen Z., Naldrett M. J., et al. , 2016. Identification of evening complex associated proteins in Arabidopsis by affinity purification and mass spectrometry. Mol. Cell. Proteomics 15: 201–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob F., 1977. Evolution and tinkering. Science 196: 1161–1166. [DOI] [PubMed] [Google Scholar]
- Jain R., Rivera M. C., Lake J. A., 1999. Horizontal gene transfer among genomes: the complexity hypothesis. Proc. Natl. Acad. Sci. USA 96: 3801–3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gomez J. M., Corwin J. A., Joseph B., Maloof J. N., Kliebenstein D. J., 2011. Genomic analysis of QTLs and genes altering natural variation in stochastic noise. PLoS Genet. 7: e1002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashi Y., King D., Soller M., 1997. Simple sequence repeats as a source of quantitative genetic variation. Trends Genet. 13: 74–78. [DOI] [PubMed] [Google Scholar]
- Khattak, A. K., 2014 Natural variation in Arabidopsis thaliana growth in response to ambient temperatures. Ph.D. Thesis, University of East Anglia, Norwich, England. [Google Scholar]
- Kleinboelting N., Huep G., Kloetgen A., Viehoever P., Weisshaar B., 2012. GABI-Kat SimpleSearch: new features of the Arabidopsis thaliana T-DNA mutant database. Nucleic Acids Res. 40: D1211–D1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolmos E., Herrero E., Bujdoso N., Millar A. J., Tóth R., et al. , 2011. A reduced-function allele reveals that EARLY FLOWERING3 repressive action on the circadian clock is modulated by phytochrome signals in Arabidopsis. Plant Cell Online 23: 3230–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kover P. X., Valdar W., Trakalo J., Scarcelli N., Ehrenreich I. M., et al. , 2009. A multiparent advanced generation inter-cross to fine-map quantitative traits in Arabidopsis thaliana. PLoS Genet. 5: e1000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowiec J., Queitsch C., Kliebenstein D. J., 2016. Molecular mechanisms governing differential robustness of development and environmental responses in plants. Ann. Bot. 117:795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E. S., Botstein D., 1989. Mapping Mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempe J., Lachowiec J., Sullivan A. M., Queitsch C., 2013. Molecular mechanisms of robustness in plants. Curr. Opin. Plant Biol. 16: 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., 2013 Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. ArXiv13033997 Q-Bio.
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., et al. , 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25: 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. L., Covington M. F., Fankhauser C., Chory J., Wagner D. R., 2001. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13: 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Murphy R. M., 2015. Asparagine repeat peptides: aggregation kinetics and comparison with glutamine repeats. Biochemistry 54: 4784–4794. [DOI] [PubMed] [Google Scholar]
- Möckli N., Auerbach D., 2004. Quantitative β-galactosidase assay suitable for high-throughput applications in the yeast two-hybrid system. Biotechniques 36: 872–876. [DOI] [PubMed] [Google Scholar]
- Nieto C., López-Salmerón V., Davière J.-M., Prat S., 2014. ELF3–PIF4 interaction regulates plant growth independently of the evening complex. Curr. Biol. 25: 187–193. [DOI] [PubMed] [Google Scholar]
- Nusinow D. A., Helfer A., Hamilton E. E., King J. J., Imaizumi T., et al. , 2011. The ELF4–ELF3–LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Zoghbi H. Y., 2007. Trinucleotide repeat disorders. Annu. Rev. Neurosci. 30: 575–621. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Johnson T., Suzuki M., Finch J. T., 1994. Glutamine repeats as polar zippers: their possible role in inherited neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 91: 5355–5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press M. O., Carlson K. D., Queitsch C., 2014. The overdue promise of short tandem repeat variation for heritability. Trends Genet. 30: 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press M. O., Lanctot A., Queitsch C., 2016. PIF4 and ELF3 act independently in Arabidopsis thaliana thermoresponsive flowering. PLoS One 11: e0161791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queitsch C., Carlson K. D., Girirajan S., 2012. Lessons from model organisms: phenotypic robustness and missing heritability in complex disease. PLoS Genet. 8: e1003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team , 2016. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Rowan B. A., Patel V., Weigel D., Schneeberger K., 2015. Rapid and inexpensive whole-genome genotyping-by-sequencing for crossover localization and fine-scale genetic mapping. G3 5: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford S. L., Lindquist S., 1998. Hsp90 as a capacitor for morphological evolution. Nature 396: 336–342. [DOI] [PubMed] [Google Scholar]
- Sangster T. A., Bahrami A., Wilczek A., Watanabe E., Schellenberg K., et al. , 2007. Phenotypic diversity and altered environmental plasticity in Arabidopsis thaliana with reduced Hsp90 levels. PLoS One 2: e648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster T. A., Salathia N., Lee H. N., Watanabe E., Schellenberg K., et al. , 2008a HSP90-buffered genetic variation is common in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 2969–2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster T. A, Salathia N., Undurraga S., Milo R., Schellenberg K., et al. , 2008b HSP90 affects the expression of genetic variation and developmental stability in quantitative traits. Proc. Natl. Acad. Sci. USA 105: 2963–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M. H., Wanker E. E., Andrade-Navarro M. A., 2012. Evolution and function of CAG/polyglutamine repeats in protein-protein interaction networks. Nucleic Acids Res. 40: 4273–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D., Sharma S., Pasha S., Brahmachari S. K., 1999. Peptide models for inherited neurodegenerative disorders: conformation and aggregation properties of long polyglutamine peptides with and without interruptions. FEBS Lett. 456: 181–185. [DOI] [PubMed] [Google Scholar]
- Stott K., Blackburn J. M., Butler P. J., Perutz M., 1995. Incorporation of glutamine repeats makes protein oligomerize: implications for neurodegenerative diseases. Proc. Natl. Acad. Sci. USA 92: 6509–6513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sureshkumar S., Todesco M., Schneeberger K., Harilal R., Balasubramanian S., et al. , 2009. A genetic defect caused by a triplet repeat expansion in Arabidopsis thaliana. Science 323: 1060–1063. [DOI] [PubMed] [Google Scholar]
- Szamecz B., Boross G., Kalapis D., Kovács K., Fekete G., et al. , 2014. The genomic landscape of compensatory evolution. PLoS Biol. 12: e1001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale M., Jarosz D. F., Lindquist S., 2010. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 11: 515–528. [DOI] [PubMed] [Google Scholar]
- Tajima T., Oda A., Nakagawa M., Kamada H., Mizoguchi T., 2007. Natural variation of polyglutamine repeats of a circadian clock gene ELF3 in Arabidopsis. Plant Biotechnol. 24: 237–240. [Google Scholar]
- Undurraga S. F., Press M. O., Legendre M., Bujdoso N., Bale J., et al. , 2012. Background-dependent effects of polyglutamine variation in the Arabidopsis thaliana gene ELF3. Proc. Natl. Acad. Sci. USA 109: 19363–19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usdin K., 2008. The biological effects of simple tandem repeats: lessons from the repeat expansion diseases. Genome Res. 18: 1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu J., Yu J., Gibbs R. A., Yu F., 2013. An integrative variant analysis pipeline for accurate genotype/haplotype inference in population NGS data. Genome Res. 23: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D., Vinegar B., Nahal H., Ammar R., Wilson G. V., et al. , 2007. An “electronic fluorescent pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS One 2: e718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R., Fekih R., Fujiwara S., Oda A., Miyata K., et al. , 2009. Possible role of early flowering 3 (ELF3) in clock-dependent floral regulation by short vegetative phase (SVP) in Arabidopsis thaliana. New Phytol. 182: 838–850. [DOI] [PubMed] [Google Scholar]
- Yu J.-W., Rubio V., Lee N.-Y., Bai S., Lee S.-Y., et al. , 2008. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol. Cell 32: 617–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta M. T., Hicks K. A., Jacobs C. I., Young J. C., Hangarter R. P., et al. , 1996. The Arabidopsis ELF3 gene regulates vegetative photomorphogenesis and the photoperiodic induction of flowering. Plant J. 10: 691–702. [DOI] [PubMed] [Google Scholar]
- Zhao L., Nakazawa M., Takase T., Manabe K., Kobayashi M., et al. , 2004. Overexpression of LSH1, a member of an uncharacterised gene family, causes enhanced light regulation of seedling development. Plant J. 37: 694–706. [DOI] [PubMed] [Google Scholar]
- Zhao R., Davey M., Hsu Y.-C., Kaplanek P., Tong A., et al. , 2005. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell 120: 715–727. [DOI] [PubMed] [Google Scholar]
- 1001 Genomes Consortium , 2016. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 166: 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
High-throughput sequencing data are available in BAM format at National Center for Biotechnology Information Sequence Read Archive accession no. SRP077615. Processed genotype data, phenotype data, and analysis code are available at https://figshare.com/articles/Variable_ELF3_polyQ_modulates_complex_epistasis_data_and_code/3467717.