Abstract
The occurrence of extended-spectrum β-lactamase (ESBL)-producing isolates has increased worldwide. Fecal carriage of ESBL-producing isolates has mainly been detected in nosocomial outbreaks, and few studies have evaluated fecal carriage during nonoutbreak situations and among patients in the community. We have studied the prevalence of ESBLs in 1,239 fecal samples from 849 patients (64.1% of whom were ambulatory) in 1991 and have compared the prevalence data with those obtained in 2003 for 400 fecal samples from 386 patients (75.9% of whom were ambulatory) and 108 samples from independent healthy volunteers. Samples were diluted in saline and cultured in two MacConkey agar plates supplemented with ceftazidime (1 μg/ml) and cefotaxime (1 μg/ml), respectively. Colonies were screened (by the double-disk synergy test) for ESBL production. The clonal relatedness of all ESBL-producing isolates was determined by pulsed-field gel electrophoresis with XbaI digestion; and the ESBLs of all ESBL-producing isolates were characterized by isoelectric focusing, PCR, and sequencing. The rates of fecal carriage of ESBL-producing isolates increased significantly (P < 0.001) in both hospitalized patients and outpatients, from 0.3 and 0.7%, respectively, in 1991, to 11.8 and 5.5%, respectively, in 2003. The rate of occurrence of ESBL-producing isolates among healthy volunteers was 3.7%. All ESBL-producing isolates recovered in 2003 were nonepidemic clones of Escherichia coli. ESBL characterization revealed an increasing diversity of ESBL types: TEM-4 and CTX-M-10 were the only enzymes detected in 1991, whereas TEM-4, TEM-52, SHV-12, CTX-M-9, CTX-M-10, CTX-M-14, and a CTX-M-2-like enzyme were recovered in 2003. The ESBL-producing isolates recovered from outpatients in 2003 corresponded to a CTX-M-9-type cluster (62.5%) and SHV-12 (31.2%), whereas TEM-4 was detected only in hospitalized patients. The frequencies of coresistance in isolates recovered in 2003 were as follows: sulfonamide, 75%; tetracycline, 64.3%; streptomycin, 57.1%; quinolones, 53.5%; and trimethoprim, 50%. The increased prevalence of fecal carriage of ESBL-producing isolates during nonoutbreak situations in hospitalized patients and the establishment of these isolates in the community with coresistance to non-β-lactam antibiotics, including quinolones, represent an opportunity for these isolates to become endemic.
β-Lactamase production is the most common mechanism of bacterial resistance to β-lactam antibiotics. Many new β-lactam antibiotics have been developed in the last few decades. However, with the introduction of each new β-lactam antibiotic a new β-lactamase class causing resistance to that drug has emerged (27). One of these new classes is the extended-spectrum β-lactamases (ESBLs), which hydrolyze oximino-β-lactams, such as the expanded-spectrum cephalosporins, but not the carbapenems, and which are highly susceptible to inhibition by clavulanic acid and tazobactam (24). ESBLs were first reported in the mid-1980s, and most of them have been found in Klebsiella pneumoniae and Escherichia coli (7).
Many ESBLs arise from simple point mutations in existing plasmid-mediated β-lactamase genes, like TEM- and SHV-derived ESBLs; however, members of a new group of ESBLs (the CTX-M type), derived from chromosomal class A β-lactamases, have been identified in the past 10 years. The CTX-M enzymes are not related to TEM or SHV enzymes, as they share only 40% identity with these ESBLs. The first CTX-M enzyme was described in Germany in 1989 and nearly simultaneously in Argentina (4). They have been detected in a variety of species of the family Enterobacteriaceae in very distant regions, such as Europe, the Near and Far East, South America, and, more recently, North America (4, 7, 30). Although one specific enzyme could be detected in different regions, it seems that there are predominant enzymes in each region (4). It has been suggested that these ESBLs are responsible for the recent huge increment in ESBLs throughout the world.
As ESBLs are frequently encoded by genes located on different transferable genetic elements, a variety of epidemiological situations have been identified, ranging from sporadic cases to large outbreaks (10). Whereas ESBLs were initially associated with nosocomial outbreaks caused by single enzyme-producing strains, recent studies have revealed more complex situations, with a significant increase in community isolates (11, 13, 14, 20, 36, 39).
Patients with fecal carriage of ESBL-producing bacterial isolates have been investigated previously during nosocomial outbreaks (12, 17, 38), but the number of prospective longitudinal studies conducted in the hospital setting during nonoutbreak situations or in the community remains scarce (5, 28, 29). Moreover, to the best of our knowledge, the rate of fecal carriage of ESBL-producing bacterial isolates during different and distant periods of time in the same location has not been investigated previously.
The aim of the present study was to investigate the prevalence of Enterobacteriaceae producing different types of ESBLs in the human fecal flora in both hospitalized and nonhospitalized patients in a nonoutbreak scenario and during two different periods of times more than a decade apart, 1991 and 2003. A supplementary group with healthy volunteers was included in the study in 2003.
MATERIALS AND METHODS
Detection of ESBL-producing isolates in fecal samples.
A total of 1,239 fecal samples from 849 patients (64.1% of whom were ambulatory) prospectively collected during 1991 and 400 fecal samples from 386 patients (75.9% of whom were ambulatory) prospectively collected during 2003 were screened for the presence of ESBL-producing Enterobacteriaceae. Sampling was carried out during nonoutbreak periods that were at least 1 year from the time of a recorded epidemic. None of the outpatients studied were residents of a skilled care facility or lived in a nursing home or a health care center. In 2003, 108 fecal samples from an equivalent number of healthy volunteers without recent hospitalization and/or without recent exposure to antibiotics (within the previous 3 months) were studied.
The procedure used to screen isolates for ESBL production was identical during both experimental periods. Samples were collected and processed within 4 h of sampling. A total of 0.5 g of each fecal sample was suspended in 5 ml of saline, and aliquots of 200 μl were seeded into two MacConkey agar plates (Oxoid Ltd., Basingstoke, England), one supplemented with 1 μg of cefotaxime per ml and one supplemented with 1 μg of ceftazidime per ml, and incubated for 48 h. One colony representing each distinct colonial morphotype was regrown in the same selective plate and further analyzed. All isolates that grew were screened for ESBL production by using both the resistance phenotype and the double-disk synergy test (19, 24). One isolate of each morphotype from each patient was selected for ESBL characterization. Different isolates from the same patient were also studied in case of different susceptibilities or isoelectric focusing patterns.
Identification and antimicrobial susceptibility testing.
Bacterial identification and initial antibiotic susceptibility testing were performed by using the semiautomated PASCO system (Difco, Detroit, Mich.) or the WIDER system (Soria-Melguizo, Madrid, Spain). The susceptibility patterns were confirmed by the standard NCCLS microdilution method (32). The double-disk synergy test was performed with conventional amoxicillin-clavulanate, cefotaxime, ceftazidime, and cefepime disks that were applied 20 and 30 mm apart (19). The standard disk diffusion method (31) was used to investigate the ESBL-associated resistance profiles. Disks were purchased from Oxoid.
Isoelectric focusing.
Bacteria exponentially growing at 37°C in Luria-Bertani medium were harvested, and cell-free lysates were prepared by sonication. Isoelectric focusing was performed by applying the crude sonic extract to Phast gels (pH 3 to 9) in a Phastsystem apparatus (Pharmacia AB, Uppsala, Sweden) (18). β-Lactamases with known pIs (pIs 5.9, 5.4, 7.6, and 8.1) were used in parallel with the controls. Gels were stained with 500 μg of nitrocefin (Oxoid) per ml to identify β-lactamase bands.
PCR detection and sequencing of ESBLs.
Genomic DNA from wild-type isolates was used as the template for PCR. ESBL amplification was performed with the appropriate primers and cycling conditions for the TEM, SHV, CTX-M-2, CTX-M-10, CTX-M-9, OXA-1, OXA-2, and OXA-10 ESBL types, as described previously (1, 11, 25, 33, 34). The PCR products were separated in 0.8% agarose gels and visualized under UV light after the gels were stained with ethidium bromide. The PCR products were purified with a QIAquick PCR purification kit (Qiagen, Hilden, Germany) and were sequenced on an ABI Prism 377 automated sequencer (Perkin-Elmer, Norwalk, Conn.).
Pulsed-field gel electrophoresis (PFGE).
Bacterial DNA was prepared as described previously (21), and XbaI (Roche GmbH, Mannheim, Germany) was used as the restriction enzyme. Digested DNA was separated in a CHEF-DRIII system (Bio-Rad, La Jolla, Calif.), and the conditions were as follows: 14°C, 6 V/cm, 10 to 40 s, 27 h. The patterns obtained were interpreted according to the criteria established by Tenover et al. (42).
Statistical analysis.
Statistical significance for comparison of proportions was calculated by the chi-square test (a P value <0.05 was considered statistically significant).
RESULTS
The rates of patient fecal carriage of ESBL-producing strains of the family Enterobacteriaceae recovered during two nonoutbreak periods separated by 12 years increased in Madrid, Spain, from 0.6% (5 of 849 patients) in 1991 to 7.0% (27 of 386 patients) in 2003 (P < 0.001). The corresponding figures when hospitalized and ambulatory patients were considered separately were 0.3% (1 of 305 patients) and 0.7% (4 of 544 patients), respectively, in 1991 and 11.8% (16 of 293 patients) and 5.5% (11 of 93 patients), respectively, in 2003 (P < 0.001). The rate of fecal carriage of ESBL-producing isolates of the Enterobacteriaceae was slightly lower in healthy volunteers (3.7%; 4 of 108 volunteers), who were enrolled during 2003.
The characterization of the ESBLs, the identities of the ESBL-producing isolates of the Enterobacteriaceae, and the patients' hospital locations are shown in Table 1. All 32 isolates recovered during 2003 (12 from hospitalized patients, 16 from outpatients, and 4 from healthy volunteers) were E. coli, whereas isolates recovered in 1991 were E. coli (n = 4), Klebsiella pneumoniae (n = 1), and Citrobacter freundii (n = 1). In 2003 the distribution of ESBL-positive isolates by patient gender was similar among both males and females, whereas in 1991 a higher proportion of males harbored ESBL-producing isolates. In addition, the mean ages of the patients were higher in 1991 (73 ± 28.7 years) than in 2003 (32.5 ± 28.7 years) (Table 1).
TABLE 1.
Characteristics of ESBL-producing isolates from fecal samples of hospitalized and nonhospitalized patients in 1991 and 2003 at the University Hospital Ramón y Cajal
| Yr and strain | Organism | PFGE pattern | Patient gender (age [yr])c | Ward of origin | pI(s) | ESBL sequence |
|---|---|---|---|---|---|---|
| 1991 | ||||||
| Ec13 | E. coli | EC6T | F (78) | Pneumology | 5.9 | TEM-4 |
| Ec14 | E. coli | DEGd | M (77) | Outpatient | 5.9 + 5.4 | TEM-4 |
| Ec15 | E. coli | DEG | M (75) | Outpatient | 5.9 + 5.4 | TEM-4 |
| Ec16 | E. coli | EC7C | M (64) | Outpatient | 8.1 | CTX-M-10 |
| Kp30a | K. pneumoniae | Kp30C | M (71) | Outpatient | 8.1 | CTX-M-10 |
| Cf1a | C. freundii | CF1C | Outpatient | 8.1 | CTX-M-10 | |
| 2003 | ||||||
| Fec 231 | E. coli | ECF231T | M (67) | Gastroenterology | 5.9 | TEM-4 |
| Fec 355 | E. coli | ECF355T | F (80) | Gastroenterology | 5.9 | TEM-4 |
| Fec 295 | E. coli | ECF295T | F (71) | Gastroenterology | 5.9 + 5.4 | TEM-52 |
| Fec 394 | E. coli | ECF394S | M (2) | Pediatric | 7.6 + 5.4 | SHV-2 |
| Fec 284Zb | E. coli | ECF284S | F (58) | Infectious Diseases | 8.2 | SHV-12 |
| Fec 335 | E. coli | ECF335 | F (63) | Gastroenterology | 8.2 | SHV-12 |
| Fec 222 | E. coli | ECF222S | F (38) | Outpatient | 8.2 | SHV-12 |
| Fec 250 | E. coli | ECF250S | M (13) | Outpatient | 8.2 | SHV-12 |
| Fec 251 | E. coli | ECF250S | M (12) | Outpatient | 8.2 | SHV-12 |
| Fec 310 | E. coli | ECF310S | F (2) | Outpatient | 8.2 + 5.4 | SHV-12 |
| Fec 340 | E. coli | DEG | F (8) | Outpatient | 8.2 + 5.4 | SHV-12 |
| Fec 147 | E. coli | ECF147C | M (UK) | Outpatient | 8.1 + 5.4 | CTX-M-10 |
| Fec 284Xb | E. coli | ECF284C | Infectious Diseases | 8.1 | CTX-M-9 | |
| Fec 395 | E. coli | DEG | F (75) | Cardiology | 8.1 + 5.4 | CTX-M-9 |
| Fec 5 | E. coli | ECF5C | M (2) | Outpatient | 8.1 | CTX-M-9 |
| Fec 87 | E. coli | ECF87C | F (4) | Outpatient | 8.1 | CTX-M-9 |
| Fec 311 | E. coli | ECF311C | F (UK) | Outpatient | 8.1 + 5.4 | CTX-M-9 |
| Fec 386 | E. coli | ECF386C | F (43) | Outpatient | 8.1 + 5.4 | CTX-M-9 |
| Fec 325 | E. coli | ECF325C | M (31) | Infectious Diseases | 8.1 + 5.4 | CTX-M-14 |
| Fec 327 | E. coli | ECF327C | M (6) | Infectious Diseases | 8.1 + 5.4 | CTX-M-14 |
| Fec 397 | E. coli | ECF397C | F (18) | Nephrology | 8.1 | CTX-M-14 |
| Fec 166 | E. coli | DEG | M (UK) | Outpatient | 8.1 | CTX-M-14 |
| Fec 216 | E. coli | ECF216C | M (2) | Outpatient | 8.1 | CTX-M-14 |
| Fec 245 | E. coli | ECF245C | F (5) | Outpatient | 8.1 | CTX-M-14 |
| Fec 268 | E. coli | ECF245C | F (UK) | Outpatient | 8.1 | CTX-M-14 |
| Fec 336 | E. coli | ECF336C | F (41) | Outpatient | 8.1 + 5.4 | CTX-M-14 |
| Fec 383 | E. coli | ECF383C | M (8) | Outpatient | 8.1 + 5.4 | CTX-M-14 |
| Fec 38 | E. coli | ECF38 | M (73) | Hematology | 7.6 | Unknown |
Isolated from one patient.
Isolated from one patient.
F, female; M, male; UK, unknown.
DEG, degrade digested DNA.
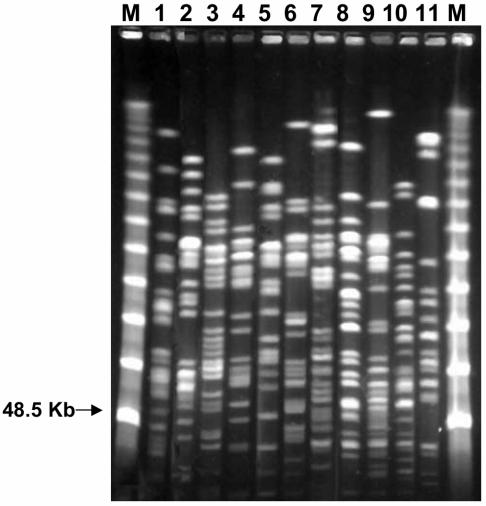
A high degree of diversity in the PFGE patterns of the E. coli isolates was found during both periods (Fig. 1). None of the hospitalized patients shared an identical ESBL-producing E. coli PFGE type, denoting the absence of epidemic isolates within the population studied. In contrast, four outpatients in 2003 shared two clones (one clone each in two patients): clone ECF250S produced an SHV-12 ESBL and clone ECF245C produced a CTX-M-14 ESBL. The clone that produced SHV-12 was isolated from two outpatients from the same family (two brothers), whereas the clone that produced CTX-M-14 was isolated from two outpatients with no apparent epidemiological link.
FIG. 1.
Diversity of ESBL-producing E. coli isolates recovered from fecal samples (lanes 1 to 11, different E. coli isolates). Lanes M, molecular marker (λ phage 48.5 concatemers).
Two different E. coli clones (clones ECF284C and ECF284S) producing two different ESBLs (CTX-M-9 and SHV-12) were recovered from a fecal sample from a patient hospitalized in the Infectious Diseases ward of the Hospital Universitario Ramón y Cajal during 2003. An outpatient from the 1991 period was simultaneously colonized with K. pneumoniae and E. coli clones that produced the same ESBL (CTX-M-10). Interestingly, no evidence of any invasive infection in patients harboring ESBL-producing isolates was found in any of our series.
The ESBL characterization revealed an increasing diversity of enzyme types: TEM-4 (50%) and CTX-M-10 (50%) were the only ESBLs detected in 1991, whereas TEM-4 (7.1%), TEM-52 (3.6%), CTX-M-10 (3.6%), SHV-2 (3.6%), SHV-12 (25.0%), CTX-M-9 (21.4%), CTX-M-14 (32.1%), and an unknown ESBL type of pI 7.6 (3.6%) were recovered in 2003 (Table 1). Outpatient fecal carriage during 2003 was mainly due to organisms producing the CTX-M-9-type enzyme cluster (62.5%) and the SHV-12 enzyme (31.2%). TEM ESBL types were mainly detected in hospitalized patients (Table 1). The ESBL-producing isolates obtained from healthy volunteers are described in Table 2. SHV-12 was detected in two volunteers, and CTX-M-14 and a CTX-2-like enzyme were each detected in one volunteer (Table 2).
TABLE 2.
Characteristics of ESBL-producing isolates from fecal samples from healthy volunteers in 2003
| Straina | PFGE pattern | Patient gender (age [yr])b | pI | ESBL sequence |
|---|---|---|---|---|
| VS27 | ECVS27C | M (24) | 8.1 | CTX-M-2 like |
| VS41 | ECVS41S | F (23) | 8.2 | SVH-12 |
| VS85 | ECVS85S | F (25) | 8.2 | SHV-12 |
| VS62 | ECVS62C | F (25) | 8.1 | CTX-M-14 |
All isolates were E. coli.
M, male; F, female.
The β-lactam susceptibility profiles and the associated antimicrobial resistance of the ESBL-producing isolates are shown in Table 3. When NCCLS breakpoints are considered, all isolates were susceptible to imipenem, whereas all of them were resistant to cefotaxime. Tazobactam restored the piperacillin susceptibilities (piperacillin-tazobactam MIC range, ≤16/4 to 32 μg/ml) of all except one of the isolates (piperacillin-tazobactam MIC, >64/4 μg/ml). The frequencies of resistance among the isolates recovered in 2003 were as follows: streptomycin, 57.1%; nalidixic acid, 53.5%; tetracycline, 64.3%; sulfonamide, 75%, and trimethoprim, 50%. The frequencies were lower among the small number of ESBL-producing isolates recovered in 1991 (Table 3), which can be related to the shift in the types of enzymes. Isolates recovered from outpatients were mainly responsible for the increased frequency of the associated resistance, particularly isolates producing the SHV-12, CTX-M-9, and CTX-M-14 ESBLs.
TABLE 3.
Antimicrobial susceptibility profiles of ESBL-producing isolates of the family Enterobacteriaceae from fecal samples and associated resistance patterns
| Yr and isolate | ESBL | MIC (μg/ml)a
|
Resistance phenotype | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMC | PIP | P/T | FOX | CTX | CTX-C | CAZ | CAZ-C | CPM | ATM | IMP | |||
| 1991 | |||||||||||||
| Ec13 | TEM-4 | ≤4/2 | >64 | ≤16/4 | ≤2 | >8 | ≤0.12/4 | 4 | ≤0.12/4 | 16 | 8 | ≤0.5 | |
| Ec14 | TEM-4 | 8/4 | >64 | 32/4 | 4 | >8 | ≤0.12/4 | 16 | ≤0.12/4 | 8 | 8 | ≤0.5 | St Sp Gm Tb Net Nm Nal Cip Tet Sul Tp |
| Ec15 | TEM-4 | 8/4 | >64 | ≤16/4 | ≤2 | >8 | ≤0.12/4 | 16 | ≤0.12/4 | 32 | 16 | ≤0.5 | St Gm Tb Nal Cip Tet Sul |
| Ec16 | CTX-M-10 | 8/4 | >64 | ≤16/4 | ≤2 | >8 | ≤0.12/4 | 2 | ≤0.12/4 | 32 | 64 | ≤0.5 | |
| Kp30 | CTX-M-10 | 8/4 | >64 | ≤16/4 | ≤2 | >8 | ≤0.12/4 | 8 | ≤0.12/4 | >32 | >64 | ≤0.5 | |
| CF | CTX-M-10 | 8/4 | >64 | ≤16/4 | >16 | >8 | ≤0.12/4 | 8 | ≤0.12/4 | 32 | 64 | ≤0.5 | |
| 2003 | |||||||||||||
| Fec 231 | TEM-4 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 16 | ≤0.12/4 | 32 | 8 | ≤0.5 | |
| Fec 355 | TEM-4 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 8 | ≤0.12/4 | 8 | 16 | ≤0.5 | St |
| Fec 295 | TEM-52 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 32 | ≤0.12/4 | >32 | 8 | ≤0.5 | St Nal Tet Sul Tp Clo |
| Fec 394 | SHV-2 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 8 | ≤0.12/4 | 32 | 16 | ≤0.5 | Tet Sul Tp Clo |
| Fec 284Z | SHV-12 | ≤4/2 | >64 | ≤16/4 | ≤2 | 128 | ≤0.12/4 | 128 | ≤0.12/4 | 32 | 64 | ≤0.5 | Tet Sul Clo |
| Fec 335 | SHV-12 | ≤4/2 | >64 | ≤16/4 | ≤2 | 64 | ≤0.12/4 | 64 | ≤0.12/4 | 32 | 32 | ≤0.5 | Sul Clo |
| Fec 222 | SHV-12 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | >128 | ≤0.12/4 | 32 | ≤0.5 | ||
| Fec 250 | SHV-12 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | >128 | ≤0.12/4 | >32 | 64 | ≤0.5 | Nal Sul Clo |
| Fec 251 | SHV-12 | ≤4/2 | >64 | ≤16/4 | 4 | 128 | ≤0.12/4 | 128 | ≤0.12/4 | 32 | 64 | ≤0.5 | Nal Sul Clo |
| Fec 310 | SHV-12 | ≤4/2 | >64 | ≤16/4 | 4 | 128 | ≤0.12/4 | 128 | ≤0.12/4 | 32 | 32 | ≤0.5 | Nal Cip Tet Sul Clo |
| Fec 340 | SHV-12 | ≤4/2 | >64 | ≤16/4 | ≤2 | 128 | ≤0.12/4 | 128 | ≤0.12/4 | 32 | 64 | ≤0.5 | St Nal Cip Tet Sul Tp |
| Fec 147 | CTX-M-10 | 8/4 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 2 | ≤0.12/4 | >32 | >64 | ≤0.5 | St Sp Nal Sul Tp |
| Fec 284X | CTX-M-9 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 1 | ≤0.12/4 | >32 | >64 | ≤0.5 | Nal |
| Fec 395 | CTX-M-9 | ≤4/2 | >64 | ≤16/4 | 4 | 128 | ≤0.12/4 | 4 | ≤0.12/4 | >32 | >64 | ≤0.5 | St Tet Sul |
| Fec 5 | CTX-M-9 | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | 1 | ≤0.12/4 | >32 | 32 | ≤0.5 | St Sp Tet Sul Tp |
| Fec 87 | CTX-M-9 | ≤4/2 | >64 | ≤16/4 | 8 | >128 | ≤0.12/4 | 1 | ≤0.12/4 | >32 | 64 | ≤0.5 | St Sp Nal Cip Tet Sul Tp |
| Fec 311 | CTX-M-9 | 8/4 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 1 | ≤0.12/4 | >32 | >64 | ≤0.5 | St Sp Tet Sul Tp |
| Fec 386 | CTX-M-9 | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | ≤0.5 | ≤0.12/4 | >32 | 4 | ≤0.5 | St Sp Nal Cip Tet Sul Tp |
| Fec 325 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | ≤2 | 128 | ≤0.12/4 | ≤0.5 | ≤0.12/4 | 32 | 2 | ≤0.5 | St Nal Tet Sul Tp |
| Fec 327 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | 4 | 128 | ≤0.12/4 | 1 | ≤0.12/4 | 16 | 4 | ≤0.5 | St Nal Cip Tet Sul Tp Clo |
| Fec 397 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | 1 | ≤0.12/4 | >32 | 32 | ≤0.5 | Nal |
| Fec 166 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | ≤0.5 | ≤0.12/4 | 8 | 4 | ≤0.5 | Nal Tet Sul |
| Fec 216 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | ≤0.5 | ≤0.12/4 | >32 | 4 | ≤0.5 | St Sp Nm Km Nal Cip Tet Sul Tp |
| Fec 245 | CTX-M-14 | 8/4 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 2 | 0.25/4 | >32 | 8 | ≤0.5 | St Tet |
| Fec 268 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 2 | ≤0.12/4 | >32 | >64 | ≤0.5 | St Tet |
| Fec 336 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 1 | ≤0.12/4 | >32 | >64 | ≤0.5 | Nal Cip Tet Sul Tp Clo |
| Fec 383 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | 2 | ≤0.12/4 | >32 | >64 | ≤0.5 | St Nm Sul Tp |
| Fec 38 | Unknown | >16/8 | >64 | >64/4 | 8 | 128 | 4/4 | 1 | 0.25/4 | >32 | ≤0.5 | ≤0.5 | St Sp Tet Sul Tp Clo |
| 2003, healthy volunteers | |||||||||||||
| VS 27 | CTX-M-2 like | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | 8 | ≤0.12/4 | >32 | >64 | ≤0.5 | Nal Cip Tet Sul |
| VS 41 | SHV-12 | ≤4/2 | >64 | ≤16/4 | ≤2 | >128 | ≤0.12/4 | >128 | ≤0.12/4 | >32 | >64 | ≤0.5 | |
| VS 85 | SHV-12 | ≤4/2 | >64 | ≤16/4 | ≤2 | 128 | ≤0.12/4 | 128 | ≤0.12/4 | 32 | 32 | ≤0.5 | Tet Sul |
| VS 62 | CTX-M-14 | ≤4/2 | >64 | ≤16/4 | 4 | >128 | ≤0.12/4 | 2 | ≤0.12/4 | >32 | >64 | ≤0.5 | St Tet Sul Tp |
AMC, amoxicillin-clavulanate; PIP, piperacillin; P/T, piperacillin-tazobactam; CTX, cefotaxime; CTX-C, cefotaxime-clavulanate; CAZ, ceftazidime; CAZ-C, ceftazidime-clavulanate; CPM, cefepime; ATM, aztreonam; IMP, imipenem.
DISCUSSION
Since 1987, the Microbiology Department of the Hospital Universitario Ramón y Cajal has continuously screened all isolates of the family Enterobacteriaceae recovered from all routinely studied clinical samples for ESBL production. Like other institutions (16, 40), patients' fecal carriage of ESBL-producing isolates was investigated only when the emergence of an outbreak situation was suspected. Fecal colonization status was not routinely determined for all patients, even if they were admitted to an intensive care unit. In 1991, we investigated the prevalence of fecal carriage of ESBL-producing Enterobacteriaceae in our institution during a nonoutbreak situation. In that study, we also included fecal samples from community patients submitted to our laboratory for stool culture. The results revealed that the prevalence of fecal carriers among ambulatory patients was 0.7%, more than twofold that among hospitalized patients (0.3%). In 2003, we repeated the study, using identical methods and consecutive stool specimens submitted to our laboratory. A dramatic, significant increase in the frequency of fecal carriage of ESBL-producing isolates was demonstrated in 2003. In contrast to the results obtained in 1991, in 2003 the prevalence of fecal carriage of ESBL-producing isolates among outpatients (5.5%) was lower than that among hospitalized patients (11.8%). The rate of fecal carriage among outpatients was consistent with but slightly higher than that observed among healthy volunteers (3.7%) enrolled during the same period of time. It should be noted that this group of healthy volunteers had no history of antibiotic consumption or admission to a hospital during at least the 3 months before sampling.
Asymptomatic colonization of the intestinal compartment with ESBL-producing isolates has been described previously (12, 17, 38). Most of those studies were performed at the time of nosocomial outbreak situations and showed the dispersion and transmission of specific clones in specific wards or even in the same institution. In 2002, a study carried out in a hospital in Barcelona, Spain, revealed that the incidence of strains of the Enterobacteriaceae producing ESBLs in the stools of outpatients was up to 7.5% (29). This value is even higher than those reported in the present work for outpatients (5.4%) and healthy volunteers (3.7%). In 2001 and 2002, 2% of outpatients admitted to an intensive care unit in a hospital in Baltimore, Md., were colonized with ESBL-producing isolates (16). A higher proportion, 4.2%, was found in hospitalized neonates on admission to a neonatal intensive care unit in Washington, D.C. (40). Conversely, other investigators did not detect ESBL-producing isolates in stool specimens from 1993 to 1997 in Finland (35) or from 1997 to 1999 in Spain (9), but those studies did not include antibiotic-supplemented selective agar plates. The cefotaxime or ceftazidime concentration in our MacConkey agar plates was 1 μg/ml, which corresponds to that recommended by the NCCLS for initial screening of K. pneumoniae, Klebsiella oxytoca, and E. coli for ESBL production. In our experience, the use of MacConkey selective broth supplemented with 2 μg of ceftazidime per ml or 1 μg of cefotaxime per ml in order to detect carriers with low bacterial loads did not increase the rate of detection of ESBL-producing organisms (data not shown).
Colonization with multiresistant isolates, including ESBL-producing isolates, is considered a prerequisite for infection. The importance of the detection of carriers of antimicrobial-resistant bacteria has recently been highlighted not only in patient populations but also in healthy people (41). The increase in the proportion of carriers in the community increases the risk that other individuals will become carriers as a consequence of human-to-human transmission of resistant bacteria or through the environment (22), enriching the resistance gene pool and thus facilitating the acquisition of resistance mechanisms by susceptible bacteria (10). The reduction in the proportion of susceptible microbiota in the community reduces the possibility that the proportion of resistant bacteria in the nosocomial setting will decrease (23). Finally, the admission of carriers harboring resistant bacteria to hospitals increases the risk of infection for other hospitalized patients (6, 16).
Antibiotic selective pressure in hospitals may amplify the number of carriers harboring resistant bacteria (6) and enhance the opportunity for these bacteria to cause infections. This fact could also be responsible for the higher prevalence of fecal carriage of ESBL-producing Enterobacteriaceae in the nosocomial setting than in the community (this study) or the finding that the rate of ESBL colonization is higher among patients admitted to high-risk units with high levels of antibiotic consumption (15). A similar situation can be applicable to nursing homes and residents of health care or skilled care facilities, among whom the rates of colonization with multiresistant pathogens, including ESBL producers, is higher than that among true community patients or healthy volunteers (44, 45).
It is worth noting that in our study the majority of ESBL-producing isolates (and all isolates recovered in 2003) were E. coli and there was a high degree of genetic diversity among the different isolates, which is indicative of an allodemic situation rather than the dissemination of specific clones (3). This suggests that the huge increment in ESBL production is a consequence of horizontal gene transfer rather than the spread of specific clones, particularly for the CTX-M β-lactamases. In a recent Spanish study on the epidemiology and clinical features of infections caused by ESBL-producing E. coli isolates in nonhospitalized patients, Rodríguez-Baño et al. (39) also detected clonally unrelated isolates from each patient studied. Those investigators found the CTX-M-9-type enzymes to be the most prevalent ESBLs, with a prevalence of up to 64%. In our study, nearly 60% of the patients studied during 2003 were colonized with isolates harboring CTX-M-type enzymes, mainly CTX-M-9 and CTX-M-14 (Table 1). Although many CTX-M ESBL types are widely distributed geographically, both the CTX-M-9 and the CTX-M-14 enzymes are also widespread in other countries (4). In our study, nearly 70% of patients colonized with CTX-M ESBL types were not hospitalized, demonstrating that the community compartment is essential for the maintenance of these enzymes. Moreover, the community can be a reservoir of ESBLs not yet detected in clinical isolates. For example, a CTX-M-2 like enzyme was found only in healthy volunteers and not in patients. ESBLs from the CTX-M-2 group have mainly been found in South America and other European countries but have not yet been found in Spain (4).
The SHV-12 enzyme was also prevalent in our study both in the community and in healthy volunteers. This enzyme has risen to prominence in recent years and has been detected in different species of the Enterobacteriaceae in many parts of the world, including typical opportunistic environmental isolates and healthy animals (8, 26, 36). TEM-type ESBLs were found only in hospitalized patients in 2003. It should also be noted that a K. pneumoniae strain harboring TEM-4 was responsible for a clonal and plasmid outbreak in our hospital from 1997 to 1999 (2, 11).
Multidrug resistance profiles involving non-β-lactam antibiotics in ESBL-producing isolates may also contribute to the increase in colonization pressure. Fluoroquinolone resistance is becoming a common feature rather than an exception in ESBL-producing isolates (43, 37, 46, 39). In our study, the decrease in the rate of fluoroquinolone susceptibility was high both in the community (62.5%) and in the hospital (41.6%) isolates recovered from patients during 2003 (Table 3). Previous fluoroquinolone use has been demonstrated to be a risk factor for the acquisition of ESBL-producing isolates, particularly isolates producing the CTX-M-type enzymes in the community setting (39, 45). Other antimicrobial agents such as trimethoprim-co-trimoxazole or tetracyclines may also contribute to the acquisition of ESBL-producing isolates. In 2003, the prevalence of tetracycline, streptomycin, trimethoprim, and sulfonamide resistance among ESBL-producing E. coli fecal isolates was greater than 50%, similar to that observed among ESBL-producing E. coli clinical isolates (46, 47). In our case, this could be related to the locations of blaESBL genes in the integron structures encoding these resistances (E. Machado et al., 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. C2-53, 2003).
In summary, a dramatic increase in the prevalence of fecal carriage of ESBL-producing isolates has been observed over the last decade. This increase was associated with a higher diversity of ESBLs, although more than 50% of the ESBLs corresponded to CTX-M-type enzymes. Our results denote the importance of the intestinal tract as a reservoir for ESBL-producing isolates and also illustrate the possibility that colonized patients may act as a source of ESBLs for clinical strains. This work also points out the increasing trend toward the endemic nature of these isolates.
Acknowledgments
A. Valverde is the recipient of a grant (grant PI02043) from the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad of Spain. A. Rollan was supported by the “Red Española de Investigación en Patología Infecciosa” (REIPI-ISCIII-C03/14), Instituto Carlos III, Ministerio de Sanidad of Spain. This work was partially supported by research grants from the Fondo de Investigaciones Sanitarias, Ministerio de Sanidad of Spain (grant PI02043); Ministerio de Ciencia y Tecnología of Spain (grant SAF 2003-09285); and the European Commission (grant SLMM-CT-2003-503335).
REFERENCES
- 1.Arduino, S. M., M. Catalano, B. E. Orman, P. H. Roy, and D. Centron. 2003. Molecular epidemiology of orf513-bearing class 1 integrons in multiresistant clinical isolates from Argentinean hospitals. Antimicrob. Agents Chemother. 47:3945-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asensio, A., A. Oliver, P. Gonzalez-Diego, F. Baquero, J. C. Pérez-Díaz, P. Ros, J. Cobo, M. Palacios, D. Lasheras, and R. Canton. 2000. Outbreak of a multiresistant Klebsiella pneumoniae strain in an intensive care unit: antibiotic use as risk factor for colonization and infection. Clin. Infect. Dis. 30:55-60. [DOI] [PubMed] [Google Scholar]
- 3.Baquero, F., T. M. Coque, and R. Cantón. 2002. Allodemics. Lancet Infect. Dis. 2:591-592. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet, R. 2004. Growing group of extended-spectrum beta-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomo, R. A., C. J. Donskey, J. L. Blumer, A. M. Hujer, C. K. Hoyenm, M. R. Jacobs, C. C. Whalen, and R. A. Salata. 2003. Cefotaxime-resistant bacteria colonizing older people admitted to an acute care hospital. J. Am. Geriatr. Soc. 51:519-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonten, M. J., S. Slaughter, A. W. Ambergen, M. K. Hayden, J. van Voorhis, C. Nathan, and R. A. Weinstein. 1998. The role of “colonization pressure” in the spread of vancomycin-resistant enterococci: an important infection control variable. Arch. Intern. Med. 158:1127-1132. [DOI] [PubMed] [Google Scholar]
- 7.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Briñas, L., M. A. Moreno, M. Zarazaga, C. Porrero, Y. Saenz, M. García, L. Domínguez, and C. Torres. 2003. Detection of CMY-2, CTX-M-14, and SHV-12 β-lactamases in Escherichia coli fecal-sample isolates from healthy chickens. Antimicrob. Agents Chemother. 47:2056-2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Briñas, L., M. Zarazaga, Y. Saenz, F. Ruiz-Larrea, and C. Torres. 2002. β-Lactamases in ampicillin-resistant Escherichia coli isolates from foods, humans, and healthy animals. Antimicrob. Agents Chemother. 46:3156-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cantón, R., T. M. Coque, and F. Baquero. 2003. Multi-resistant gram-negative bacilli: from epidemics to endemics. Curr. Opin. Infect. Dis. 16:315-325. [DOI] [PubMed] [Google Scholar]
- 11.Coque, T. M., A. Oliver, J. C. Perez-Diaz, F. Baquero, and R. Cantón. 2002. Genes encoding TEM-4, SHV-2, and CTX-M-10 extended-spectrum β-lactamases are carried by multiple Klebsiella pneumoniae clones in a single hospital (Madrid, 1989 to 2000). Antimicrob. Agents Chemother. 46:500-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Champs, C., M. P. Sauvant, C. Chanal, D. Sirot, N. Gazuy, R. Malhuret, J. C. Baguet, and J. Sirot. 1989. Prospective survey of colonization and infection caused by expanded-spectrum-β-lactamase-producing members of the family Enterobacteriaceae in an intensive care unit. J. Clin. Microbiol. 27:2887-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Essack, S. Y., L. M. Hall, D. G. Pillay, M. L. McFadyen, and D. M. Livermore. 2001. Complexity and diversity of Klebsiella pneumoniae strains with extended-spectrum β-lactamases isolated in 1994 and 1996 at a teaching hospital in Durban, South Africa. Antimicrob. Agents Chemother. 45:88-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gniadkowski, M. 2001. Evolution and epidemiology of extended-spectrum β-lactamases (ESBLs) and ESBL-producing microorganisms. Clin. Microbiol. Infect. 7:597-608. [DOI] [PubMed] [Google Scholar]
- 15.Green, M., and K. Barbadora. 1998. Recovery of ceftazidime-resistant Klebsiella pneumoniae from pediatric liver and intestinal transplant recipients. Pediatr. Transplant. 2:224-230. [PubMed] [Google Scholar]
- 16.Harris, A. D., L. Nemoy, J. A. Johnson, A. Martin-Carnahan, D. L. Smith, H. Standiford, and E. N. Perencevich. 2004. Co-carriage rates of vancomycin-resistant Enterococcus and extended-spectrum beta-lactamase-producing bacteria among a cohort of intensive care unit patients: implications for an active surveillance program. Infect. Control Hosp. Epidemiol. 25:105-108. [DOI] [PubMed] [Google Scholar]
- 17.Hollander, R., M. Ebke, H. Barck, and E. von Pritzbuer. 2001. Asymptomatic carriage of Klebsiella pneumoniae producing extended-spectrum β-lactamase by patients in a neurological early rehabilitation unit: management of an outbreak. J. Hosp. Infect. 48:207-213. [DOI] [PubMed] [Google Scholar]
- 18.Huovinen, S. 1988. Rapid isoelectric focusing of plasmid-mediated β-lactamases with Pharmacia PhastSystem. Antimicrob. Agents Chemother. 32:1730-1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarlier, V., M. Nicolas, G. Fournier, and A. Philippon. 1988. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev. Infect. Dis. 10:867-878. [DOI] [PubMed] [Google Scholar]
- 20.Jutersek, B., A. Baraniak, T. Zohar-Cretnik, A. Storman, E. Sadowy, and M. Gniadkowski. 2003. Complex endemic situation regarding extended-spectrum β-lactamase-producing Klebsiella pneumoniae in a hospital in Slovenia. Microb. Drug Resist. 9(Suppl. 1):S25-S33. [DOI] [PubMed] [Google Scholar]
- 21.Kaufmann, M. E. 1998. Pulsed-field-electrophoresis, p. 33-50. In N. Woodford and A. P. Johnson (ed.), Molecular bacteriology. Protocols and clinical applications. Humana Press, Totowa, N.J.
- 22.Levin, B. R. 2001. Minimizing potential resistance: a population dynamics view. Clin. Infect. Dis. 33(Suppl. 3):S161-S169. [DOI] [PubMed] [Google Scholar]
- 23.Lipsitch, M., and M. H. Samore. 2002. Antimicrobial use and antimicrobial resistance: a population perspective. Emerg. Infect. Dis. 8:347-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livermore, D. M. 1995. β-Lactamases in laboratory and clinical resistance. Clin. Microbiol. Rev. 8:557-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mabilat, C., and S. Goussard. 1995. PCR detection and identification of genes for extended-spectrum β-lactamases, p. 553-557. In D. H. Persing, T. F. Smith, F. C. Tenover, and T. J. White (ed.), Diagnostic molecular microbiology: principles and applications. American Society for Microbiology, Washington, D.C.
- 26.Mazzariol, A., J. Zuliani, R. Fontana, and G. Cornaglia. 2003. Isolation from blood culture of a Leclercia adecarboxylata strain producing an SHV-12 extended-spectrum β-lactamase. J. Clin. Microbiol. 41:1738-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medeiros, A. A. 1997. Evolution and dissemination of β-lactamases accelerated by generations of β-lactam antibiotics. Clin. Infect. Dis. 24(Suppl. 1):S19-S45. [DOI] [PubMed] [Google Scholar]
- 28.Millar, M. R., T. R. Walsh, C. J. Linton, S. Zhang, J. P. Leeming, P. M. Bennett, et al. 2001. Avon Longitudinal Study of Pregnancy and Childhood. Carriage of antibiotic-resistant bacteria by healthy children. J. Antimicrob. Chemother. 47:605-610. [DOI] [PubMed] [Google Scholar]
- 29.Mirellis, B., F. Navarro, E. Miró, R. J. Mesa, P. Coll, and G. Prats. 2003. Community transmission of extended-spectrum β-lactamase. Emerg. Infect. Dis. 9:1024-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moland, E. S., J. A. Black, A. Hossain, N. D. Hanson, K. S. Thomson, and S. Pottumarthy. 2003. Discovery of CTX-M-like extended-spectrum beta-lactamases in Escherichia coli isolates from five U.S. states. Antimicrob. Agents Chemother. 47:2382-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial disk susceptibility tests. NCCLS document M2-A8. Approved standard, 8th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.National Committee for Clinical Laboratory Standards. 2003. MIC methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS document M7-A6. Approved standard, 6th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 33.Oliver, A., L. M. Weigel, J. K. Rasheed, J. E. McGowan Juniorperiod, P. Raney, and F. C. Tenover. 2002. Mechanisms of decreased susceptibility to cefpodoxime in Escherichia coli. Antimicrob. Agents Chemother. 46:3829-3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver, A., J. C. Pérez-Díaz, T. M. Coque, F. Baquero, and R. Cantón. 2001. Nucleotide sequence and characterization of a novel cefotaxime-hydrolyzing β-lactamase (CTX-M-10) isolated in Spain. Antimicrob. Agents Chemother. 45:616-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osterblad, M., A. Hakanen, R. Manninen, T. Leistevuo, R. Peltonen, O. Meurman, P. Huovinen, and P. Kotilainen. 2000. A between-species comparison of antimicrobial resistance in enterobacteria in fecal flora. Antimicrob. Agents Chemother. 44:1479-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paterson, D. L., K. M. Hujer, A. M. Hujer, B. Yeiser, M. D. Bonomo, L. B. Rice, R. A. Bonomo, and the International Klebsiella Study Group. 2003. Extended-spectrum β-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type β-lactamases. Antimicrob. Agents Chemother. 47:3554-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paterson, D. L., L. Mulazimoglu, J. M. Casellas, W. C. Ko, H. Goossens, A. Von Gottberg, S. Mohapatra, G. M. Trenholme, K. P. Klugman, J. G. McCormack, and V. L. Yu. 2000. Epidemiology of ciprofloxacin resistance and its relationship to extended-spectrum beta-lactamase production in Klebsiella pneumoniae isolates causing bacteremia. Clin. Infect. Dis. 30:473-478. [DOI] [PubMed] [Google Scholar]
- 38.Peña, C., M. Pujol, C. Ardanuy, A. Ricart, R. Pallares, J. Linares, J. Ariza, and F. Gudiol. 1998. Epidemiology and successful control of a large outbreak due to Klebsiella pneumoniae producing extended-spectrum β-lactamases. Antimicrob. Agents Chemother. 42:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodríguez-Baño, J., M. D. Navarro, L. Romero, L. Martínez-Martínez, M. A. Muniain, E. J. Perea, R. Pérez-Cano, and A. Pascual. 2004. Epidemiology and clinical features of infections caused by extended-spectrum β-lactamase-producing Escherichia coli in nonhospitalized patients. J. Clin. Microbiol. 42:1089-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh, N., K. M. Patel, M. M. Leger, B. Short, B. M. Sprague, N. Kalu, and J. M. Campos. 2002. Risk of resistant infections with Enterobacteriaceae in hospitalized neonates. Pediatr. Infect. Dis. J. 21:1029-1033. [DOI] [PubMed] [Google Scholar]
- 41.Smith, D. L., J. Dushoff, E. N. Perencevich, A. D. Harris, and S. A. Levin. 2004. Persistent colonization and the spread of antibiotic resistance in nosocomial pathogens: resistance is a regional problem. Proc. Natl. Acad. Sci. USA 101:3709-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tolun, V., O. Kucukbasmaci, D. Torumkuney-Akbulut, C. Catal, M. Ang-Kucuker, and O. Ang. 2004. Relationship between ciprofloxacin resistance and extended-spectrum β-lactamase production in Escherichia coli and Klebsiella pneumoniae strains. Clin. Microbiol. Infect. 10:72-75. [DOI] [PubMed] [Google Scholar]
- 44.Trick, W. E., R. A. Weinstein, P. L. DeMarais, M. J. Kuehnert, W. Tomaska, C. Nathan, T. W. Rice, S. K. McAllister, L. A. Carson, and W. R. Jarvis. 2001. Colonization of skilled-care facility residents with antimicrobial-resistant pathogens. J. Am. Geriatr. Soc. 49:270-276. [DOI] [PubMed] [Google Scholar]
- 45.Wiener, J., J. P. Quinn, P. A. Bradford, R. V. Goering, C. Nathan, K. Bush, and R. A. Weinstein. 1999. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA 281:517-523. [DOI] [PubMed] [Google Scholar]
- 46.Winokur, P. L., R. Cantón, J. M. Casellas, and N. Legakis. 2001. Variations in the prevalence of strains expressing an extended-spectrum β-lactamase phenotype and characterization of isolates from Europe, the Americas, and the Western Pacific region. Clin. Infect. Dis. 32(Suppl. 2):S94-S103. [DOI] [PubMed] [Google Scholar]
- 47.Xiong, Z., D. Zhu, F. Wang, Y. Zhang, R. Okamoto, and M. Inoue. 2002. Investigation of extended-spectrum beta-lactamase in Klebsiellae pneumoniae and Escherichia coli from China. Diagn. Microbiol. Infect. Dis. 44:195-200. [DOI] [PubMed] [Google Scholar]