Abstract
Candida parapsilosis is an important cause of bloodstream infections in the health care setting. We investigated a large C. parapsilosis outbreak occurring in a community hospital and conducted a case-control study to determine the risk factors for infection. We identified 22 cases of bloodstream infection with C. parapsilosis: 15 confirmed and 7 possible. The factors associated with an increased risk of infection included hospitalization in the intensive care unit (adjusted odds ratio, 16.4; 95% confidence interval, 1.8 to 148.1) and receipt of total parenteral nutrition (adjusted odds ratio, 9.2; 95% confidence interval, 0.9 to 98.1). Samples for surveillance cultures were obtained from health care worker hands, central venous catheter insertion sites, and medical devices. Twenty-six percent of the health care workers surveyed demonstrated hand colonization with C. parapsilosis, and one hand isolate was highly related to all case-patient isolates by tests with the DNA probe Cp3-13. Outbreak strain isolates also demonstrated reduced susceptibilities to fluconazole and voriconazole. This largest known reported outbreak of C. parapsilosis bloodstream infections in adults resulted from an interplay of host, environment, and pathogen factors. Recommendations for control measures focused on improving hand hygiene compliance.
In the United States, the incidence of bloodstream infections (BSIs) with Candida species is 6 to 14 per 100,000 persons per year (4, 11). The rate of mortality associated with Candida BSIs can approach 50% (10). Almost 80% of these infections, including those in the intensive care unit (ICU) (36%) and outpatient (25%) settings, occur in persons in whom a central venous catheter (CVC) has been placed (11). Candida parapsilosis is widely recognized as a cause of BSIs among hospitalized patients, particularly in neonates, and the association of C. parapsilosis fungemia with CVCs and parenteral nutrition is well appreciated (5, 11, 19, 26, 27). Outbreaks and clusters of cross-transmission, both in ICU patients and in outpatients, have been attributed to extrinsic contamination of CVCs, total parenteral nutrition (TPN) solutions, intravascular devices, and medications (3, 4, 13, 16, 22-25, 28).
In April 2001, infection control practitioners at a large community hospital (hospital A) noted an increase in the frequency of isolation of C. parapsilosis strains from cultures of blood and CVC tips from adult inpatients. We describe the results of the epidemiologic and laboratory investigations of this outbreak of C. parapsilosis BSIs.
(This study was presented at the 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy, September 2002, San Diego, Calif.)
MATERIALS AND METHODS
Definitions and ascertainment of cases.
To describe the extent of the outbreak and define a study period, we examined the annual catheter-associated BSI rates in the medical, surgical, and neurological ICUs of hospital A for 1999, 2000, and the first 6 months of 2001 using existing data from active surveillance for catheter-related BSIs. Hospital A used standard National Nosocomial Infection Surveillance System methods and case definitions for surveillance purposes (12).
We defined a confirmed case patient as a patient for whom C. parapsilosis was isolated from a blood culture at least 48 h after admission to hospital A during the outbreak period, defined as April 2001 (the first detectable increase in infection) through October 2001 (the date of the on-site investigation). We defined a possible case patient as a patient for whom C. parapsilosis was isolated from a CVC tip culture at least 48 h after admission to hospital A during the outbreak period, in the absence of a bloodstream isolate. Possible cases were believed to represent CVC colonization or, in some cases, a possible BSI (e.g., a CVC was removed because of a presumed BSI, but a culture of blood was sterile). Cases were identified by reviewing clinical microbiology laboratory records for all patients from whom Candida species was isolated in culture from any patient source during the study period.
Epidemiologic studies.
We conducted a case-control study to determine the potential risk factors for C. parapsilosis BSIs. Confirmed and possible cases were included in the study and the initial analysis. Two control patients were included for each case patient and were randomly selected from all inpatients of hospital A during the outbreak period. The case and the control patients were frequency matched by age group (18 to 44, 45 to 70, or >70 years) and the length of hospitalization prior to a positive culture for the case patient. Potential control patients were excluded if they had been hospitalized for less than 7 days, the minimum total length of stay for case patients.
Demographic and clinical data were abstracted from medical records by using a standardized form. Data collected included prior BSIs; procedures; and the use of CVCs, medications, and infusions. Data regarding the durations of exposure were recorded from the date of admission until the index day (the hospital day on which the first sample positive for C. parapsilosis by culture was collected) for case patients and the corresponding day for the matched controls.
Procedural investigation.
We examined patient care areas and other areas in the three ICUs and reviewed infection control and isolation policies and practices. We also conducted three surreptitious observational studies of health care worker (HCW) hand hygiene practices. These observations were 60 min each and were performed during separate nursing shifts. During the observational studies, we noted whether contact with patients occurred and whether hand hygiene was performed before and after each contact. In addition, we observed practices associated with TPN and lipid administration and the placement and care of CVCs.
Laboratory studies.
Eighteen patient isolates were sent to the Fungus Reference Unit, Centers for Disease Control and Prevention (CDC), for confirmation of the identification. These included five blood and two CVC tip isolates obtained from case patients during the outbreak period, three isolates obtained during the outbreak period from case-patient body sites other than blood or CVC tips, two isolates obtained during the outbreak period from patients in hospital A who did not meet the case definitions, and six blood or CVC tip isolates obtained from patients subsequent to the outbreak period. Isolates were identified at CDC by using ChromAgar Candida (DRG International, Mountainside, N.J.), the API 20C system (bioMerieux, St. Louis, Mo.), and cornmeal Dalmau plates.
Culture surveys and microbiologic methods.
To investigate the role of cross-transmission of C. parapsilosis in this outbreak, we determined the prevalence of Candida hand colonization in a sample of HCWs in the three ICUs by culturing samples from the hands by the handiwipe method (18). The HCWs surveyed included day- and night-shift nurses, physicians, phlebotomists, respiratory therapists, radiology technicians, and dialysis technicians. We surveyed HCWs regardless of the length of time on duty, the timing of hand hygiene, or their specific patient care activities.
To determine the prevalence of skin colonization with C. parapsilosis among ICU patients, a cross-sectional survey of medical-surgical and neurological ICU patients was done on 5 November 2001. Swabs for fungal culture were obtained from the CVC insertion sites and CVC hubs from all patients. We also cultured electrocardiograph leads and the blood pressure cuff tubes in each patient room in the medical-surgical ICU.
All environmental samples were sent to CDC for processing. Each handiwipe was returned in a sterile container. After the addition of 50 ml of sterile 0.02% Tween 80 in water, the container was agitated on a shaker for 30 min; the liquid was then drained into a 50-ml tube and centrifuged at 3,000 × g for 10 min. The pellet was resuspended in 200 μl of fresh Tween solution, and aliquots were plated on ChromAgar Candida (DRG International) and Sabouraud dextrose agar with chloramphenicol. The species of all yeast isolates were determined by using the API 20C system (bioMerieux) and cornmeal Dalmau plates.
Molecular typing of all C. parapsilosis isolates was performed by randomly amplified polymorphic DNA (RAPD) analysis, electrophoretic karyotyping, and Southern blotting with the complex Cp3-13 probe (6) by a nonisotopic development method. The Southern blot was analyzed with BioNumerics software (Applied Maths, Austin, Tex.) by using the unweighted pair-group method with arithmetic averages algorithm for cluster analysis (1, 6). Broth microdilution testing for susceptibilities to fluconazole, itraconazole, and voriconazole was performed by standard NCCLS methods (17).
Statistical analysis.
Univariate analysis was performed with SAS software (version 8.2, 1989-2000; SAS Institute, Cary, N.C.). Continuous data were compared by t tests, and categorical variables were compared by chi-square tests (α = 0.05). The Wilcoxon rank sum test was used for data that were not normally distributed. Odds ratios (ORs) were adjusted for age group and the duration of hospitalization prior to the index day by using the SAS logistic regression procedure to analyze the data for matched cases and controls. Variables were included in multivariable modeling when P was <0.1 by univariate analysis, and the results of multivariable modeling were considered significant when P was <0.1.
RESULTS
Outbreak confirmation.
The CVC-associated Candida BSI rate in the three adult ICUs of hospital A increased fivefold in the first 6 months of 2001 compared to the rates in 1999 and 2000, from 1.0 per 1,000 CVC-days in 1999 and 2000 to 5.3 per 1,000 CVC-days in the first 6 months of 2001. This increase was attributable to an almost sevenfold increase in the rate of C. parapsilosis BSIs, from 0 per 1,000 CVC-days in 1999 and 0.7 per 1,000 CVC-days in 2000 to 4.5 per 1,000 CVC-days in the first 6 months of 2001. During the same 6-month period in 2001, the rate of CVC-associated bacterial BSIs (3.8 per 1,000 CVC-days) remained unchanged from that in the previous year (3.6 per 1,000 CVC-days in 2000).
Descriptive characteristics.
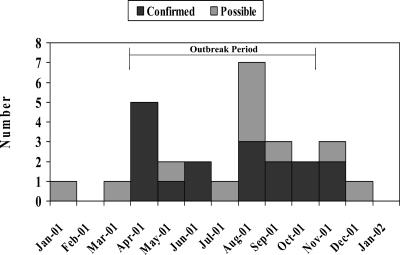
Figure 1 demonstrates the distributions of confirmed and possible C. parapsilosis cases by month of fungal isolation and includes incident isolates occurring before and after the outbreak period. We identified 15 confirmed cases and 7 possible cases. Of these 22 case patients, the median age was 55.5 years (range, 29 to 81 years), 12 (55%) were white, and 12 (55%) were male. All case patients had CVCs in place, and 18 (82%) had been in an ICU prior to the isolation of C. parapsilosis. The median time from CVC placement to the recovery of a C. parapsilosis isolate was 12 days (range, 3 to 29 days), and the median time from admission to isolate recovery was 20 days (range, 5 to 50 days). The median total length of hospital stay was 38 days. Nine (41%) case patients died, and six died within 7 days of recovery of an isolate.
FIG. 1.
Distribution of confirmed and probable C. parapsilosis infections by month of isolation, hospital A, Mississippi, 2001.
Assessment of risk factors.
Because complete medical records were unavailable for 2 of the 15 patients with confirmed cases, the case-control study included 20 case patients (13 with confirmed cases and 7 with possible cases) and 40 control patients. Case and control patients did not differ on matching characteristics; overall demographic and clinical characteristics were similar between the case and the control patients.
On univariate analysis case patients had significantly higher mean APACHE II scores than the control patients on the index day (19 and 13.7, respectively; P = 0.007) and a higher median number of catheters in place (2 and 1, respectively; P = 0.02). Case patients were significantly more likely than the controls to have received care in an ICU (OR, 8.9; 95% confidence interval [CI], 2.2 to 36.2) or on a surgical service (OR, 7.5; 95% CI, 1.4 to 38.4), receive mechanical ventilation (OR, 13.0; 95% CI, 2.7 to 61.7) or TPN (OR, 5.9; 95% CI, 1.6 to 21.4), or have had a previous case of bacteremia or candidemia (OR, 3.8; 95% CI, 1.2 to 11.6) (Table 1).
TABLE 1.
Univariate analysis of risk factors comparing all casesa and controls, hospital A, Mississippi, 2001
| Risk factor | All cases (n = 20) and controls
|
|
|---|---|---|
| ORb | 95% CI | |
| Any mechanical ventilation | 13 | 2.7-61.7 |
| TPN receipt | 5.9 | 1.6-21.4 |
| Mechanical ventilation on index day | 10 | 1.9-51.3 |
| ICU on index day | 8.9 | 2.2-36.2 |
| Surgical service | 7.5 | 1.4-38.4 |
| Previous bacteremia or candidemia | 3.8 | 1.2-11.6 |
Includes 13 confirmed cases and 7 possible cases, as 2 of the 15 confirmed cases were not included in the case-control study due to a lack of availability of medical records.
The ORs presented are significant at the P < 0.05 level.
The case patients were similar to the controls in terms of the type of indwelling vascular catheter (i.e., CVC, dialysis catheter, or peripherally inserted central catheter), the vessels in which the catheters were placed, the numbers of catheter lumens, the rates of dialysis, the numbers of major surgical procedures and nonsurgical procedures, and the numbers of medications received. Notably, the cases and the controls did not differ in the number or classes of antibiotics received or the receipt of fluconazole (55% of cases versus 35% of controls; P = 0.14). When we assessed the independent importance of each of these risk factors by multivariable analysis, concurrent hospitalization in an ICU (adjusted OR, 16.4; 95% CI, 1.8 to 148.1) and receipt of TPN (adjusted OR, 9.2; 95% CI, 0.9 to 98.1) were independently associated with an increased risk of being a case patient and were chosen as the most clinically relevant among the factors in predictive models.
Observational studies.
We observed 79 hand hygiene opportunities during the three observation periods. A hand hygiene opportunity was defined as the time from HCW entry into a patient room until departure when the episodes included direct contact with the patient or with objects in the environment. Overall, hand hygiene was performed before contact in 30 (38%) opportunities and after contact in 39 (49%) opportunities. Among the opportunities involving direct patient contact, nurses performed hand hygiene before contact in 42% of 43 opportunities and after contact in 60% of 43 opportunities, and physicians performed hand hygiene before contact in 18% of 11 opportunities and after contact in 18% of 11 opportunities. Of 64 observed hand hygiene events, 45 (70%) were with an alcohol foam waterless agent, 16 (25%) were with antibacterial soap and water, and 3 (5%) were with both.
We found no deviations from protocol or breaks in aseptic technique during the preparation of TPN solutions, microbiology specimen preparation and handling, or placement and maintenance of CVCs.
Laboratory studies.
We cultured swab specimens from the pairs of hands of 68 HCWs in the three ICUs, constituting 63% of the total ICU nursing staff (Table 2). Yeasts were recovered from 23 (34%) hand pairs; C. parapsilosis was recovered from 19 (28%) hand pairs, including those from 14 (26%) of 53 nurses, 3 (43%) of 7 physicians, and 2 (25%) of 8 other HCWs. C. parapsilosis was recovered from 1 (6%) of 16 cultures of specimens obtained from patient care devices (blood pressure cuff tubing), but no yeasts were recovered from cultures of any of eight CVC hubs or insertion sites sampled.
TABLE 2.
Description of environmental and hand carriage samples obtained and culture results by type of sample, hospital A, Mississippi, 2001
| Specimen cultured | No. (%) of samples:
|
|
|---|---|---|
| Positive for C. parapsilosis | Obtained | |
| Total HCW hand pairs | 19 (28) | 68 |
| Nurses | 14 (26) | 53 |
| Physicians | 3 (43) | 7 |
| Other | 2 (25) | 8 |
| CVC insertion sites | 0 | 8 |
| Medical device | 1 (6) | 16 |
| Blood pressure cuff tubing | 1 (13) | 8 |
| Electrocardiograph lead | 0 | 8 |
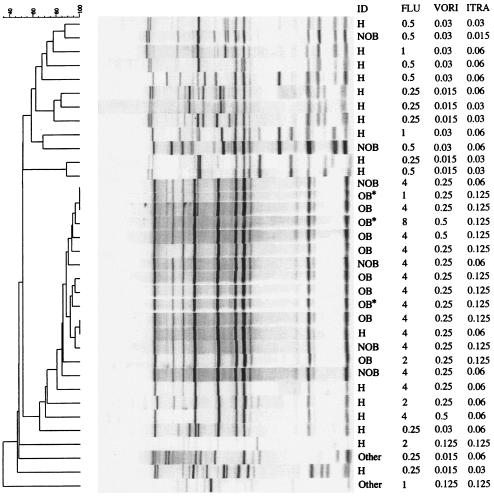
Molecular typing of these isolates with the Cp3-13 probe (Fig. 2) demonstrated 15 isolates with DNA banding patterns related to one another at a value of 85%. This cluster included DNA from all 10 case-patient isolates collected during the outbreak period, 4 (67%) of 6 blood or CVC tip isolates collected after the outbreak period, and 1 (6%) of 17 isolates from the hands of HCWs (Fig. 2). The banding patterns of three other isolates from the hands of HCWs displayed 70% relatedness to this cluster. However, DNA from other hand and patient isolates, including the two epidemiologically unrelated patient isolates, displayed banding patterns with less significant (40 to 60%) degrees of relatedness. Subtyping with RAPD primers and by electrophoretic karyotyping gave consistent results (data not shown), but with a lesser degree of discrimination.
FIG. 2.
Cluster analysis results by Southern blotting with the Cp3-13 probe and testing for susceptibilities to fluconazole (FLU), voriconazole (VORI), and itraconazole (ITRA), hospital A, Mississippi, 2001. ID, type of isolate; H, hand; NOB, bloodstream or CVC tip isolates from patients after the outbreak period; OB, bloodstream or CVC tip isolates from case patients during the outbreak; OB*, noninvasive isolates from case patients during the outbreak; Other, isolates from patients unrelated to the outbreak.
The antifungal susceptibility testing results for these isolates are shown in Fig. 2. The fluconazole MIC at which 50% of isolates are inhibited (MIC50) for the 10 outbreak-related patient isolates was 4 μg/ml (range, 1 to 8 μg/ml). The MIC50 for the hand isolates was 0.5 μg/ml (range, 0.25 to 4 μg/ml). The fluconazole MICs for all 15 clinical and hand isolates in the predominant DNA cluster as well as the 3 less closely related hand isolates were between 1 and 8 μg/ml, with a median of 4 μg/ml. Fluconazole MICs for patient and hand isolates outside of this cluster were from 0.25 to 2 μg/ml. The voriconazole MICs, but not those of itraconazole, were similarly elevated for the isolates within the predominant DNA cluster. The median voriconazole MIC for this cluster was 0.25 μg/ml (range, 0.25 to 0.5 μg/ml), and the mean itraconazole MIC was 0.125 μg/ml (range, 0.06 to 0.125 μg/ml).
DISCUSSION
This paper describes the largest outbreak, to our knowledge, of C. parapsilosis BSIs reported among adults. The cause of this outbreak was multifactorial, resulting from the interplay of host, environment, and pathogen contributors. Case patients had severe illnesses requiring ICU care, mechanical ventilation, CVCs, and TPN use. These factors require frequent contact with HCWs, magnifying the impact of lapses in appropriate hand hygiene and facilitating cross-transmission of yeasts from transiently colonized HCW hands to patients. Finally, the C. parapsilosis outbreak cluster demonstrated reduced susceptibilities to the azole antifungals fluconazole and voriconazole, a property that may have promoted persistence on CVCs and patient skin in this environment where fluconazole is administered for antifungal treatment.
Lapses in compliance with hand hygiene practices may allow the transmission of C. parapsilosis from HCW hands to CVCs. Published data indicate that C. parapsilosis can be recovered from 5 to 26% of cultures of swab specimens from the hands of HCWs in surgical and neonatal ICUs in nonoutbreak settings (21). Moreover, the transmission of C. parapsilosis via the hands of HCWs has been implicated in a number of outbreaks (4, 13, 16, 22, 27). The rate of compliance with hand hygiene practices among HCWs at hospital A was similar to that reported in prior studies, which typically observe rates of compliance of less than 50% (20).
Analysis of the Cp3-13 fingerprint patterns showed that this outbreak was caused by a cluster of related isolates. One isolate from the hand of an HCW can be placed firmly in this cluster, and three other hand isolates displayed lesser degrees of relatedness, although they still sorted within this cluster. The ability to discriminate among clinically relevant C. parapsilosis isolates has traditionally been limited due to similarities in electrophoretic karyotypes and RAPD profiles between strains and a lack of interlaboratory reproducibility among methods. The high degree of variability between strains demonstrated with the Cp3-13 probe has allowed us to discriminate among related isolates and to demonstrate a specific fingerprint profile among outbreak isolates.
Once the outbreak C. parapsilosis cluster was introduced onto catheters from HCW hands, several properties of the cluster may have promoted survival and introduction of the isolates into the bloodstreams of the case patients. Solutions that contain high concentrations of glucose and amino acids, such as TPN, confer a selective growth advantage to Candida species, including C. parapsilosis (8, 9, 23). Furthermore, several isolates from this outbreak cluster have been shown to form biofilms in vitro (14). C. parapsilosis is known to form extensive biofilms on bioprosthetic materials, and biofilm formation can confer relative resistance to antifungal drugs (2). Finally, reduced susceptibility to fluconazole and its derivative voriconazole, but not the structurally distinct antifungal itraconazole, may have promoted propagation and infection. While the MIC for this bloodstream cluster does not approach the cutoff value for resistance to fluconazole (MIC > 32 μg/ml), the median MIC of 4 μg/ml is unusual, as C. parapsilosis isolates typically exhibit extreme susceptibility to fluconazole (11). In this institutional setting of prior fluconazole use (55% of case patients and 35% of control patients had previously received fluconazole), such variance in susceptibility may provide an ecologic advantage over other skin and CVC hub flora and allow this yeast to remain viable in the local environment. The relative roles of these phenotypic factors in the epidemiology and pathogenesis of C. parapsilosis remain to be investigated, but the ability to correlate various molecular subtypes with unusual phenotypic properties will be useful.
As a result of this investigation, hospital A created a multidisciplinary program, with hospital administration support, designed to improve HCW adherence to recommended hand hygiene practices. The indications for hand hygiene were expanded, as outlined in the Hospital Infection Control Practices Advisory Committee hand hygiene guideline (7). In addition, fluconazole use practices in the ICUs were reviewed. Subsequent to the interventions, the number of cases of C. parapsilosis BSIs at hospital A diminished. Outbreak cluster isolates were still recovered from blood and CVC tips after the conclusion of the outbreak period; however, they were not of the single fingerprint type found to cause invasive disease among hospitalized patients.
Acknowledgments
We gratefully acknowledge David Soll for providing the Cp3-13 probe, Juanita Smith for assisting with the data collection, and hospital A and Mississippi State Health Department staff for facilitating the investigation.
The authors have no financial conflicts of interest to disclose.
REFERENCES
- 1.Blignaut, E., C. Pujol, S. Lockhart, S. Joly, and D. Soll. 2002. Ca3 fingerprinting of Candida albicans isolates from human immunodeficiency virus-positive and healthy individuals reveals a new clade in South Africa. J. Clin. Microbiol. 40:826-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branchini, M. L., M. A. Pfaller, J. Rhine-Chalberg, T. Frempong, and H. D. Isenberg. 1994. Genotypic variation and slime production among blood and catheter isolates of Candida parapsilosis. J. Clin. Microbiol. 32:452-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cano, M., J. Perz, M. Liu, G. Lyon, M. Brandt, B. Lasker, T. Lott, A. Craig, W. Schaffner, and R. Hajjeh. Candidemia among pediatric outpatients receiving home total parenteral nutrition. Med. Mycol., in press. [DOI] [PubMed]
- 4.Diekema, D. J., S. A. Messer, R. J. Hollis, R. P. Wenzel, and M. A. Pfaller. 1997. An outbreak of Candida parapsilosis prosthetic valve endocarditis. Diagn. Microbiol. Infect. Dis. 29:147-153. [DOI] [PubMed] [Google Scholar]
- 5.Edmond, M., S. Wallace, D. McClish, M. Pfaller, R. Jones, and R. Wenzel. 1999. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin. Infect. Dis. 29:239-244. [DOI] [PubMed] [Google Scholar]
- 6.Enger, L., S. Joly, C. Pujol, P. Simonson, M. Pfaller, and D. Soll. 2001. Cloning and characterization of a complex DNA fingerprinting probe for Candida parapsilosis. J. Clin. Microbiol. 39:658-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garner, J. S., et al. 1996. Guideline for isolation precautions in hospitals. Infect. Control Hosp. Epidemiol. 17:53-80. [DOI] [PubMed] [Google Scholar]
- 8.Gelbart, S. M., G. F. Reinhardt, and H. B. Greenlee. 1973. Multiplication of nosocomial pathogens in intravenous feeding solutions. Appl. Microbiol. 26:874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldmann, D. A., W. T. Martin, and J. W. Worthington. 1973. Growth of bacteria and fungi in total parenteral nutrition solutions. Am. J. Surg. 126:314-318. [DOI] [PubMed] [Google Scholar]
- 10.Gudlaugsson, O., S. Gillespie, K. Lee, J. Vande Berg, J. Hu, S. Messer, L. Herwaldt, M. Pfaller, and D. Diekema. 2003. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 37:1172-1177. [DOI] [PubMed] [Google Scholar]
- 11.Hajjeh, R., A. Sofair, L. Harrison, G. Lyon, B. Arthington-Skaggs, S. Mirza, M. Phelan, J. Morgan, W. Lee-Yang, M. Ciblak, L. Benjamin, L. Thomson Sanza, S. Huie, S. Yeo, M. Brandt, and D. Warnock. 2004. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J. Clin. Microbiol. 42:1519-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horan, T., and T. Emori. 1997. Definitions of key terms used in the NNIS system. Am. J. Infect. Control 25:112-116. [DOI] [PubMed] [Google Scholar]
- 13.Huang, Y. C., T. Y. Lin, H. S. Leu, H. L. Peng, J. H. Wu, and H. Y. Chang. 1999. Outbreak of Candida parapsilosis fungemia in neonatal intensive care units: clinical implications and genotyping analysis. Infection 27:97-102. [DOI] [PubMed] [Google Scholar]
- 14.Kuhn, D. M., P. K. Mukherjee, T. A. Clark, C. Pujol, J. Chandra, R. A. Hajjeh, D. W. Warnock, D. R. Soll, and M. A. Ghannoum. 2004. Characterization of Candida parapsilosis in an outbreak setting: comparison of genotypic and phenotypic markers, including biofilm production. Emerg. Infect. Dis. 10:1074-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasker, B. A., C. M. Elie, T. J. Lott, A. Espinel-Ingroff, L. Gallagher, R. J. Kuykendall, M. E. Kellum, W. R. Pruitt, D. W. Warnock, D. Rimland, M. M. McNeil, and E. Reiss. 2001. Molecular epidemiology of Candida albicans strains isolated from the oropharynx of HIV-positive patients at successive clinic visits. Med. Mycol. 39:341-352. [DOI] [PubMed] [Google Scholar]
- 16.Levin, A. S., S. F. Costa, N. S. Mussi, M. Basso, S. I. Sinto, C. Machado, D. C. Geiger, M. C. Villares, A. Z. Schreiber, A. A. Barone, and M. L. Branchini. 1998. Candida parapsilosis fungemia associated with implantable and semi-implantable central venous catheters and the hands of healthcare workers. Diagn. Microbiol. Infect. Dis. 30:243-249. [DOI] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth microdilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 18.Petersen, N. J., D. E. Collins, and J. H. Marshall. 1973. A microbiological assay technique for hands. Health Lab. Sci. 10:18-22. [PubMed] [Google Scholar]
- 19.Pfaller, M., and D. Diekema. 2002. Role of sentinel surveillance of candidemia: trends in species distribution and antifungal susceptibility. J. Clin. Microbiol. 40:3551-3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pittet, D., S. Dharan, S. Touveneau, V. Sauvan, and T. Perneger. 1999. Bacterial contamination of the hands of hospital staff during routine patient care. Arch. Intern. Med. 159:821-826. [DOI] [PubMed] [Google Scholar]
- 21.Rangel-Frausto, M., A. Houston, M. Bale, C. Fu, and R. Wenzel. 1994. An experimental model for study of Candida survival and transmission in human volunteers. Eur. J. Clin. Microbiol. Infect. Dis. 13:590-595. [DOI] [PubMed] [Google Scholar]
- 22.Sanchez, V., J. A. Vazquez, D. Barth-Jones, L. Dembry, J. D. Sobel, and M. J. Zervos. 1993. Nosocomial acquisition of Candida parapsilosis: an epidemiologic study. Am. J. Med. 94:577-582. [DOI] [PubMed] [Google Scholar]
- 23.Sherertz, R. J., K. S. Gledhill, K. D. Hampton, M. A. Pfaller, L. B. Givner, J. S. Abramson, and R. G. Dillard. 1992. Outbreak of Candida bloodstream infections associated with retrograde medication administration in a neonatal intensive care unit. J. Pediatr. 120:455-461. [DOI] [PubMed] [Google Scholar]
- 24.Solomon, S. L., H. Alexander, J. W. Eley, R. L. Anderson, H. C. Goodpasture, S. Smart, R. M. Furman, and W. J. Martone. 1986. Nosocomial fungemia in neonates associated with intravascular pressure-monitoring devices. Pediatr. Infect. Dis. J. 5:680-685. [DOI] [PubMed] [Google Scholar]
- 25.Solomon, S. L., R. F. Khabbaz, R. H. Parker, R. L. Anderson, M. A. Geraghty, R. M. Furman, and W. J. Martone. 1984. An outbreak of Candida parapsilosis bloodstream infections in patients receiving parenteral nutrition. J. Infect. Dis. 149:98-102. [DOI] [PubMed] [Google Scholar]
- 26.Trick, W., S. Fridkin, J. Edwards, R. Hajjeh, and R. Gaynes. 2002. Secular trends of hospital-acquired candidemia among intensive care unit patients in the United States during 1989-1999. Clin. Infect. Dis. 35:627-630. [DOI] [PubMed] [Google Scholar]
- 27.Weems, J. J., Jr. 1992. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin. Infect. Dis. 14:756-766. [DOI] [PubMed] [Google Scholar]
- 28.Weems, J. J., Jr., M. E. Chamberland, J. Ward, M. Willy, A. A. Padhye, and S. L. Solomon. 1987. Candida parapsilosis fungemia associated with parenteral nutrition and contaminated blood pressure transducers. J. Clin. Microbiol. 25:1029-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]