Abstract
BACKGROUND
Epilepsy with myoclonic-atonic seizures, also known as myoclonic-astatic epilepsy or Doose syndrome, has been recently linked to variants in the SLC6A1 gene. Epilepsy with myoclonic-atonic seizures is often refractory to antiepileptic drugs, and the ketogenic diet is known for treating medically intractable seizures, although the mechanism of action is largely unknown. We report a novel SLC6A1 variant in a patient with epilepsy with myoclonic-atonic seizures, analyze its effects, and suggest a mechanism of action for the ketogenic diet.
METHODS
We describe a ten-year-old girl with epilepsy with myoclonic-atonic seizures and a de novo SLC6A1 mutation who responded well to the ketogenic diet. She carried a c.491G>A mutation predicted to cause p.Cys164Tyr amino acid change, which was identified using whole exome sequencing and confirmed by Sanger sequencing. High-resolution structural modeling was used to analyze the likely effects of the mutation.
RESULTS
The SLC6A1 gene encodes a transporter that removes gamma-aminobutyric acid from the synaptic cleft. Mutations in SLC6A1 are known to disrupt the gamma-aminobutyric acid transporter protein 1, affecting gamma-aminobutyric acid levels and causing seizures. The p.Cys164Tyr variant found in our study has not been previously reported, expanding on the variants linked to epilepsy with myoclonic-atonic seizures.
CONCLUSION
A 10-year-old girl with a novel SLC6A1 mutation and epilepsy with myoclonic-atonic seizures had an excellent clinical response to the ketogenic diet. An effect of the diet on gamma-aminobutyric acid reuptake mediated by gamma-aminobutyric acid transporter protein 1 is suggested. A personalized approach to epilepsy with myoclonic-atonic seizures patients carrying SLC6A1 mutation and a relationship between epilepsy with myoclonic-atonic seizures due to SLC6A1 mutations, GABAergic drugs, and the ketogenic diet warrants further exploration.
Keywords: epilepsy with myoclonic-atonic seizures, myoclonic-astatic epilepsy, Doose syndrome, ketogenic diet, GABA, SLC6A1, ketosis
Epilepsy with myoclonic-atonic seizures (EMAS), also known as myoclonic-astatic epilepsy or Doose syndrome, consists of mixed generalized seizures of early childhood onset. The clinical presentation usually occurs in the first five years of life. It occurs more commonly in boys than in girls, with a ratio of 2:1. The types of seizures experienced include myoclonic, myoclonic absence, and atonic, but the defining symptomatology is the myoclonic-astatic seizure, characterized by abrupt cortical myoclonus or myoclonias followed by sudden loss of muscle control, often leading to a drop or fall. The abrupt drop, compared to cutting the strings of a marionette puppet, may lead to injury and is often followed by brief myoclonias. The disorder is usually difficult to control because of high resistance to medication and the occurrence of frequent daily seizures.1 A genetic etiology for EMAS has been proposed since its initial description by Doose and is supported by family studies.2 It is now included in the syndromes of genetic generalized epilepsies. Individuals with EMAS may or may not have a family history of seizures.3 Treatment is furthermore challenging because adverse effects and toxicity, including exacerbation of generalized seizures, may impact selection of antiseizure medication as much as efficacy.4 As such, the ketogenic diet has been suggested as an alternative or additional approach to treatment.
The use of a dietary approach to treat seizures dates to historic times when researchers found that a high-fat diet mimics aspects of fasting and results in antiseizure effects.5 The ketogenic diet has been used as a seizure treatment for years; however, its therapeutic mechanism remains largely unexplained. A study comparing various methods of EMAS treatment revealed the ketogenic diet to be the most successful.6
In a recent study, six of 160 probands with EMAS had mutations in SLC6A1, suggesting that mutations in this gene account for about 4% of individuals with this severe epilepsy syndrome and are more likely in individuals with preexisting developmental delay.2 The SLC6A1 gene encodes a gamma-aminobutyric acid (GABA) transporter, which removes GABA from the synaptic cleft. It was postulated that the mutations resulted in a loss of function and disrupted the transport of GABA from the extracellular space into the presynaptic terminal.2 The relationship between SLC6A1 mutations and the ketogenic diet was not described except for one patient who seemed to have improved on the diet.2 Here, we report a ten-year-old girl with Doose syndrome and a novel SLC6A1 mutation whose seizures were highly responsive to the ketogenic diet.
Patient Description
The proband is a ten-year-old female with onset of seizures at approximately 16 months of age. Her earliest events were characterized by initial cessation of activity and then progression to a fall sometimes followed by myoclonic movements. Those events each lasted for a few seconds. At age two years, she was diagnosed with epilepsy and was experiencing seizure clusters multiple times daily. Seizures were triggered by tiredness, hunger, illness, and overexcitation. Seizure types included myoclonic astatic and absence. Cranial magnetic resonance imaging at age two years was normal, but electroencephalogram was markedly abnormal because of paroxysms of bilateral independent and generalized 3- to 3.5-Hertz frontally dominant high-voltage rhythmic spike-and-wave activity. Valproate therapy was recommended and declined because of parental concern regarding potential adverse effects. There is no family history of seizures or other neurological disorders and no reported consanguinity. The patient was born at 8.5 months gestation after an uncomplicated pregnancy and delivery. She sat independently between six and seven months, spoke her first word at six months, and walked independently at 16 months. At age two years, her speech slowed, and she stopped acquiring new vocabulary.
At age three years, she was started on the modified Atkins diet (MAD), which reduced her absence seizures by 85% to 90%. The MAD was followed for more than a year, and she showed improvement in seizure frequency. She was taken off the MAD when she had a gastrointestinal illness causing vomiting, diarrhea, and increased ketosis. The diet was discontinued, although carbohydrates were restricted to 80 to 120 calories/day. On this diet, she had an average of 30 seizures/day. Most of her seizures were absence seizures which lasted two to four seconds each. Her speech development also regressed during this time, although she was receiving speech therapy. While off the MAD diet, the myoclonic jerks returned, and she had on average six to eight episodes per week. It was noted that the frequency of the myoclonias varied by week, depending on her diet. Given the apparent therapeutic nature of the MAD, a ketogenic diet was initiated at age four years.
By age six years, she was on a 2.25:1 ratio (fat-to-nonfat calories) diet, and her myoclonic seizures were controlled, with some breakthrough of absence seizures coinciding with intermittent viral infections. Her speech and development made significant progress. Electroencephalography showed diffuse background slowing with rare bursts of notched delta waves of shifting lateralization, but no definite epileptiform activity.
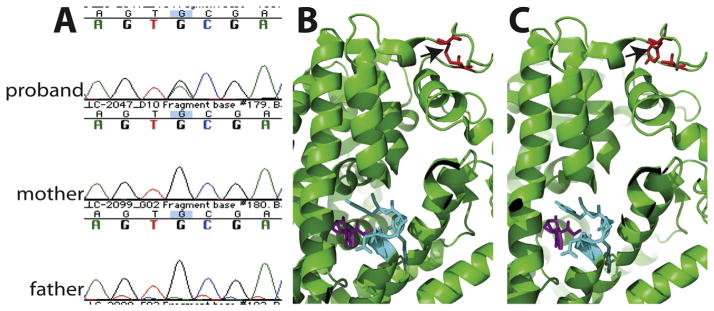
The family was enrolled in an institutional review board–approved study with full informed consent, and medical records and peripheral blood were collected from the proband and her parents. DNA was extracted from peripheral blood lymphocytes using standard methods and sent for whole exome sequencing at Claritas Genomics (Cambridge, MA). Exome libraries were constructed from genomic DNA using AmpliSeq Library 2.0 kits (Thermo Fisher Scientific, Waltham, MA), loaded into an Ion Torrent Proton PI v3 semiconductor sequencing chip (Thermo Fisher Scientific) and sequenced on an Ion Torrent Proton instrument with HiQ chemistry (Thermo Fisher Scientific). Data were analyzed with Ion Torrent Suite Software v4.4. Sequencing runs with at least 80% of targeted bases covered at 20X, and 90% of targeted bases at 10X, were used for analysis (AmpliSeqExome). An apparent de novo variant at chromosome 3:11061918 (hg19) within the SLC6A1 gene was identified in the proband and not seen in her parents. This finding was confirmed by Sanger sequencing of DNAs from all three family members. The SLC6A1 change, c.491G>A, is predicted to cause the missense amino acid change p.Cys164Tyr (NM_003042; Figure A). This variant was deemed pathogenic by various in silico methods including PolyPhen-2, Sorting Intolerant From Tolerant, and MutationTaster. The altered amino acid is highly conserved, present across both vertebrates and invertebrates including Drosophila melanogaster and Caenorhabditis elegans. It is not present in either the Exome Aggregation Consortium or 1000 genome databases.
FIGURE.
Genetic findings in the child with SLC6A1 mutation and molecular modeling. (A) Chromatograms from Sanger sequencing of genomic polymerase chain reaction products in the proband and her parents show the de novo c.491G>A change in the proband. (B, C) The high-resolution structure of the Drosophila dopamine transporter complexed with cocaine (PDB: 4XP4) is used to model the structure of SCL6A1. The Cys164 residue (red) is not close to cocaine (purple) in the structure (B), but they all connect to cocaine through transmembrane helices. Residue 164 (red) is in a helix holding residues 120 to 123 (cyan), which directly interact with cocaine (purple) (B). This residue Cys164 forms a disulfide bond with an adjust residue Cys173 (red) stabilizing the helix. The mutation p.Cys164Tyr (red) disrupts the disulfide bond and destabilizes the helix (C). (The color version of this figure is available in the online edition.)
SLC6A1 is similar to the Drosophila dopamine transporter with 66% homology at the protein level. For structural modeling, a high-resolution structure of the Drosophila dopamine transporter complexed with cocaine (PDB: 4XP4) is used.7 The C164 residue forms a disulfide bond (colored in red) with an adjacent residue C173 stabilizing the local structure, which includes a transmembrane helix towards the cocaine (colored in purple) binding residues 120–123 (colored in cyan). The mutation p.Cys164Tyr disrupts the disulfide bond (Figure B,C) which likely affects the conformation and dynamics of the transmembrane helix next to residues 120 to 123, and subsequently is predicted to alter the function of SLC6A1.
Discussion
The genetic cause of EMAS in some families has been recently linked to variants in the SLC6A1 gene that encodes for a GABA transporter protein 1 (GAT-1) responsible for the synaptic reuptake of GABA.2 GABA is an inhibitory neurotransmitter in the brain that counterbalances neuronal excitation that when disrupted may cause seizures. Carvill et al.2 reported six heterozygous SLC6A1 mutations, two truncations, and four missense mutations, associated with EMAS. They hypothesized that these alterations likely lead to a loss of function of the transporter leading to reduction in GABA reuptake from the synapse. The p.Cys164Tyr variant described by us has not been reported before,2,8 and it expands upon the SLC6A1 variants linked to EMAS.
The benefits of the ketogenic diet in epilepsy have been well described.9,10 Proposed mechanisms include direct effects of ketone bodies, glycolytic reduction, activation of adenosine triphosphate–sensitive potassium channels by mitochondrial metabolism, inhibition of the mammalian target of rapamycin pathway, and inhibition of glutamatergic excitatory synaptic transmission.5,11 However, the exact mechanism has not been proven.
Similarly, the beneficial effects of the ketogenic diet on GABA levels and activity remain inconclusive.12 It has been suggested that ketosis from the ketogenic diet causes a change in the equilibrium between the rate of glutamate transamination to aspartate and decarboxylation to GABA resulting in increased levels of GABA.13
The ketogenic diet has been noted as effective for some patients with epilepsy with myoclonic atonic seizures.14 We hypothesize that this involves an interaction with GABA reuptake into the presynaptic terminal mediated by GAT-1 and that the diet restores the requisite GABA concentration impaired by transporter dysfunction. Our patient had virtual cessation of seizures on the diet, although an incomplete response was previously reported.2 Enhanced understanding of genetic etiologies that may respond well to specific antiseizure therapies, such as the ketogenic diet, allows for a precision medicine approach to the treatment of epilepsy. SLC6A1 mutations affecting the GAT-1 appear to cause epilepsy with myoclonic atonic seizures that responds to the ketogenic diet.
Acknowledgments
The authors would like to thank the patient and her family for their participation in this report. We also thank the Doose Syndrome Epilepsy Alliance and Heather Jackson for assistance with this project. The authors gratefully acknowledge support from The Manton Center for Orphan Disease Research Gene Discovery Core. This work was funded by the Tommy Fuss fund, GETTYLAB, and the Research Connection at Boston Children’s Hospital. Sanger sequencing was performed by the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center Molecular Genetics Core, funded by NIH P30 HD18655.
References
- 1.Kelley SA, Kossoff EH. Doose syndrome (myoclonic-astatic epilepsy): 40 years of progress. Dev Med Child Neurol. 2010;52:988–993. doi: 10.1111/j.1469-8749.2010.03744.x. [DOI] [PubMed] [Google Scholar]
- 2.Carvill GL, McMahon JM, Schneider A, et al. Mutations in the GABA transporter SLC6A1 cause epilepsy with myoclonic-atonic seizures. Am J Hum Genet. 2015;96:808–815. doi: 10.1016/j.ajhg.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabbout R, Kozlovski A, Gennaro E, et al. Absence of mutations in major GEFS+ genes in myoclonic astatic epilepsy. Epilepsy Res. 2003;56:127–133. doi: 10.1016/j.eplepsyres.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Bromfield EB, Cavazos JE, Sirven JI, editors. An Introduction to Epilepsy. American Epilepsy Society; West Hartford (CT): 2006. [PubMed] [Google Scholar]
- 5.Rho JM. How does the ketogenic diet induce anti-seizure effects? Neurosci Lett. 2015;15:30054–30059. doi: 10.1016/j.neulet.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 6.Oguni H, Tanaka T, Hayashi K, et al. Treatment and long-term prognosis of myoclonic-astatic epilepsy of early childhood. Neuropediatrics. 2002;33:122–132. doi: 10.1055/s-2002-33675. [DOI] [PubMed] [Google Scholar]
- 7.Penmatsa A, Wang KH, Gouaux E. X-ray structures of Drosophila dopamine transporter in complex with nisoxetine and reboxetine. Nat Struct Mol Biol. 2015;22:506–508. doi: 10.1038/nsmb.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halvorsen M, Petrovski S, Shellhaas R, et al. Mosaic mutations in early-onset genetic diseases. Genet Med. 2016;18:746–749. doi: 10.1038/gim.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baranano KW, Hartman AL. The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol. 2008;10:410–419. doi: 10.1007/s11940-008-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2012;3:CD001903. doi: 10.1002/14651858.CD001903.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Danial NN, Hartman AL, Stafstrom CE, Thio LL. How does the ketogenic diet work? Four potential mechanisms. J Child Neurol. 2013;28:1027–1033. doi: 10.1177/0883073813487598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yudkoff M, Daikhin Y, Horyn O, Nissim I, Nissim I. Ketosis and brain handling of glutamate, glutamine, and GABA. Epilepsia. 2008;49:73–75. doi: 10.1111/j.1528-1167.2008.01841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caraballo RH, Cersosimo RO, Sakr D, et al. Ketogenic diet in patients with myoclonic-astatic epilepsy. Epileptic Disord. 2006;8:151–155. [PubMed] [Google Scholar]