Abstract
Background
The ratio of C-reactive protein to albumin, as a novel inflammation-based prognostic score, is associated with outcomes in cancer and septic patients. The diagnostic accuracy of the CRP/albumin ratio has not been assessed in colorectal surgery for postoperative complications.
Methods
A total of 359 patients undergoing major colorectal surgery between 2012 and 2015 were eligible for this study. Uni- and multivariate analyses were performed to identify risk factors for postoperative complications. Receiver operating characteristic curves were developed to examine the cutoff values and diagnostic accuracy of the CRP/albumin ratio and postoperative CRP levels.
Results
Among all the patients, 139 (38.7%) were reported to have postoperative complications. The CRP/albumin ratio was an independent risk factor for complications (OR 4.413; 95% CI 2.463–7.906; P < 0.001), and the cutoff value was 2.2, which had a higher area under the curve compared to CRP on postoperative day 3 (AUC 0.779 vs 0.756). The CRP/albumin ratio also had a higher positive predictive value than CRP levels on postoperative day 3. Patients with CRP/albumin ≥2.2 suffered more postoperative complications (60.8% vs 18.6%, P < 0.001), longer postoperative stays (10 (4–71) vs 7 (3–78) days, P < 0.001), and increased surgical site infections (SSIs) (21.1% vs 4.8%, P < 0.001) than those with CRP/albumin <2.2.
Conclusions
The ratio of C-reactive protein to albumin could help to identify patients who have a high probability of postoperative complications, and the ratio has higher diagnostic accuracy than C-reactive protein alone for postoperative complications in colorectal surgery.
Keywords: C-reactive protein to albumin ratio, Colorectal surgery, Diagnoses
Background
There are various postoperative complications occurring after surgery for both benign and malignant colorectal disease [1, 2], and they cause increased treatment costs, infections, longer hospital stays, a slower return to premorbid status and worse long-term survival [3–5]. Therefore, successful early detection and treatment of any complications are paramount to improve outcomes, especially under the enhanced recovery after surgery (ERAS) program.
As surgical interventions lead to well-understood metabolic, neuroendocrine, and immune responses, the stress responses to surgery contribute to increased postoperative complications [6]. Pro-inflammatory cytokines will increase due to surgical injury, which leads to changes of circulating acute phase proteins, such as albumin and C-reactive protein (CRP) [6]. It is widely known that CRP is used to detect postoperative complications resulting from surgical injury. Additionally, several studies found CRP to be a useful tool to ensure a safe early discharge following colorectal surgery [7]. Warschkow et al. [1] reported that CRP on postoperative day 3 or 4 (POD 4) had the best performance in predicting postoperative complications. On the other hand, some studies have proven that low albumin levels are related to postoperative complications. Increased capillary leakage of albumin is one of the features of systemic inflammatory response syndrome (SIRS), which leads to low plasma albumin levels in patients undergoing major abdominal surgery [8]. A pilot study has reported that an early postoperative albumin drop was associated with adverse clinical outcomes and was due to surgical trauma [9]. Lee et al. [10] reported that patients with postoperative hypoalbuminemia were more likely to experience poorer outcomes undergoing off-pump coronary artery bypass graft surgery.
A recent study has proven that a novel inflammation-based prognostic score, the CRP/albumin ratio (CAR), is a useful predictive index for outcome in patients with sepsis or colorectal cancer [11, 12]. However, almost no study has investigated the diagnostic accuracy of CAR for postoperative complications in patients undergoing colorectal surgery. Therefore, we hypothesized that CAR could be used as a reliable and accurate predictor of postoperative outcome after colorectal surgery.
In the present study, we investigated the association between CAR and postoperative complications after colorectal surgery and compared the diagnostic accuracy between CAR and postoperative CRP in these patients.
Methods
Patients
This study was approved by the ethics committee of Jinling Hospital. The information on patients undergoing elective colectomy or rectal resection for malignant or non-malignant disease was retrospectively analyzed at the Department of General Surgery in Jinling Hospital between October 2012 and December 2015. Patients with reoperation prior to postoperative day 3, albumin infusion either preoperatively or within 2 postoperative days, closing of ileostomy or colostomy, multivisceral resection, inflammatory bowel disease, or incomplete laboratory data were excluded. Patients who underwent primary intestinal resection were generally included.
Data collection
The data collected included baseline characteristics, intraoperative data and laboratory data from the database. Baseline characteristics included age, sex, body mass index, American Society of Anesthesiologists (ASA) grade, comorbidities, and surgical indication. Laboratory data included hemoglobin, preoperative CRP and albumin, CRP on POD 3 [7], and albumin on POD 3 [13]. Intraoperative data included operation time, type of surgery, surgical approach (open vs laparoscopy), stoma creation, intraoperative blood transfusion, and estimated blood loss during surgery.
Definition of outcomes
Postoperative outcomes were evaluated, including days of postoperative hospital stay, surgical site complications (surgical site infections (SSIs)), and postoperative complications (Clavien-Dindo classification) [14]. The primary outcome was overall postoperative complications graded according to the Clavien-Dindo system. Grades I to II were defined as mild complications, and grades III to IV were defined as major complications. Postoperative complications were defined as those that occurred before hospital discharge or <30 days after surgery. Surgical site complications included superficial incisional, deep incisional, or organ/space SSIs. For evaluation, CAR was defined as (CRP level on POD 3)/(ALB level on POD 3) [1, 13, 15]. The CAR cutoff values were determined using receiver operating characteristic (ROC) curve analysis [11].
Statistical analysis
All of the data were analyzed using SPSS version 19.0 (SPSS, Inc., Chicago, IL). Continuous data are presented as the mean ± SD or median (range), while categorical data are presented as n (%). Continuous variables were analyzed using Student’s t test, categorical variables were analyzed using Pearson’s chi-square test or the Fisher exact test as appropriate. Significant associations (P < 0.05) on univariate analysis were submitted to multivariate logistic regression analysis to verify independent predictors of postoperative complications. ROC curve analysis was used to assess the predictive accuracy. P values <0.05 were considered statistically significant.
Results
Study population and baseline characteristics
A total of 359 patients were enrolled for the final analysis (male to female = 179:180), of which 83.3% had malignant disease, as shown in Table 1. Among those with benign disease, 60 (16.7%) were chronic radiation enteropathy, diverticular disease or others. There were 139 (38.7%) patients who had postoperative complications. In addition, 96 (26.7%) patients had mild complications, and 55 (15.3%) patients had major complications (Clavien-Dindo grade III−IV), and 12.5% patients had SSIs. The length of the postoperative hospital stay was 11.2 ± 10.2 days. The clinical background characteristics of the two groups of studied patients with or without postoperative complications are shown in Table 1.
Table 1.
Univariate analysis of risk factors associated with postoperative complications
| Characteristics | All | Postoperative complications | P value | |
|---|---|---|---|---|
| (n = 359) | Yes (n = 139) | No (n = 220) | ||
| Age (year)a | 53.4 ± 10.4 | 53.1 ± 9.6 | 53.5 ± 10.8 | 0.717 |
| Sex, male, n (%)c | 179 (49.9) | 52 (37.4) | 127 (57.7) | <0.001 |
| BMI (kg/m2)a | 22.3 ± 3.7 | 21.6 ± 3.5 | 22.7 ± 3.7 | 0.004 |
| Comorbiditiesc | ||||
| Diabetes mellitus | 49 (13.6) | 16 (11.5) | 38 (17.3) | 0.137 |
| Hypertension | 113 (31.5) | 40 (28.8) | 73 (33.2) | 0.381 |
| Preoperative hemoglobin (g/L)a | 120.7 ± 58.6 | 109.4 ± 26.9 | 127.8 ± 70.9 | 0.004 |
| Preoperative ALB (g/L)a | 40.6 ± 3.8 | 40.2 ± 4.0 | 40.8 ± 3.7 | 0.185 |
| Preoperative CRP (mg/L)a | 9.5 ± 23.8 | 12.2 ± 27.1 | 7.9 ± 21.3 | 0.097 |
| Operation time (min)b | 160 (70–383) | 160 (70–365) | 165 (70–383) | 0.483 |
| Type of surgery, n (%)c | 0.117 | |||
| Right colectomy | 126 (35.1) | 60 (43.2) | 66 (30.0) | |
| Transverse colectomy | 14 (3.9) | 5 (3.6) | 9 (4.1) | |
| Left colectomy | 44 (12.3) | 19 (13.7) | 25 (11.4) | |
| Sigmoid resection | 41 (11.4) | 12 (8.6) | 29 (13.2) | |
| Rectal resection | 116 (32.3) | 38 (27.3) | 78 (35.5) | |
| Total colectomy | 18 (5.0) | 5 (3.6) | 13 (5.9) | |
| Laparoscopic surgery, n (%)c | 142 (39.6) | 49 (35.3) | 93 (42.3) | 0.185 |
| Conversion, n (%)c | 25 (7.0) | 11 (7.9) | 14 (6.4) | |
| Estimated blood loss (ml)a | 226 ± 137 | 245 ± 139 | 214 ± 134 | 0.035 |
| Stoma creation, n (%)c | 58 (16.2) | 30 (21.6) | 28 (12.7) | 0.026 |
| CRP on POD 3 (mg/L)a | 89.1 ± 64.4 | 123.1 ± 66.9 | 67.7 ± 52.5 | <0.001 |
| Postoperative CARa | 2.8 ± 2.3 | 4.2 ± 2.6 | 2.0 ± 1.6 | <0.001 |
| Surgical indication, n (%)c | <0.001 | |||
| Malignant | 299 (83.3) | 97 (69.8) | 202 (91.8) | |
| Non-malignant | 60 (16.7) | 42 (30.2) | 18 (8.2) | |
| ASA ≥3, n (%)c | 27 (7.5) | 16 (11.5) | 11 (5.0) | 0.023 |
| Intraoperative blood transfusion, n (%)c | 19 (5.3) | 11 (7.9) | 8 (3.6) | 0.078 |
CRP C-reactive protein, ALB albumin, POD postoperative day, CAR CRP to albumin (CRP/ALB) ratio
aValues are expressed as the mean ± SD
bValues are expressed as the median (range)
cValues are expressed as n (%)
Analysis of possible risk factors for postoperative complications
As shown in Table 1, univariate analysis revealed factors that were significantly associated with postoperative complications, including sex, BMI, preoperative hemoglobin, estimated blood loss, stoma creation, CRP on POD 3, postoperative CAR, surgical indication, and ASA. Then, a multivariate analysis model was used to determine the risk factors from the univariate analysis that were independently associated with postoperative complications. In the multivariate analysis, postoperative CAR (OR 4.413; 95% CI 2.463–7.906; P < 0.001) was found to be the only significant independent risk factor for postoperative complications (Table 2). However, we cannot draw conclusions solely from this result that postoperative CAR can act as an accurate predictor for postoperative complications.
Table 2.
Multivariate analysis of factors associated with postoperative complications
| Risk factor | OR | 95% CI | P value |
|---|---|---|---|
| Male sex | 1.307 | 0.738–2.313 | 0.359 |
| BMI (<18.5 kg/m2) | 0.928 | 0.389–2.216 | 0.867 |
| Preoperative hemoglobin (<120 g/L) | 1.805 | 1.053–3.093 | 0.032 |
| Estimated blood loss | 1.002 | 1.000–1.004 | 0.021 |
| Stoma creation | 1.095 | 0.542–2.209 | 0.801 |
| CRP on POD 3 (>135 mg/L) | 1.826 | 0.938–3.556 | 0.077 |
| Postoperative CAR (>2.2) | 4.413 | 2.463–7.906 | <0.001 |
| Surgical indication | 0.466 | 0.194–1.116 | 0.087 |
| ASA ≥3 | 1.380 | 0.525–3.624 | 0.514 |
CRP C-reactive protein, ALB albumin, POD postoperative day, CAR CRP to albumin (CRP/ALB) ratio
Predictive accuracy of postoperative CAR and CRP on POD 3 for postoperative complications
Many studies have already suggested that CRP on POD 3 or 4 could be a practical predictive index for postoperative complications in colorectal surgery [1]. ROC curve analysis was used to examine the predictive accuracy of postoperative CAR vs CRP on POD 3. The ROC curve parameters are shown in Table 3 and Fig. 1. For CRP on POD 3, the area under the curve (AUC) was 0.756, sensitivity was 0.698, specificity was 0.723, positive predictive value was 79.1%, negative predictive value was 61.4%, Youden’s index was 0.421, and the cutoff point was 85.1. On the other hand, the AUC of CAR was 0.779, sensitivity was 0.748, specificity was 0.695, positive predictive value was 81.4%, negative predictive value was 60.8%, Youden’s index was 0.444, and the cutoff point was 2.2. Clinically, the positive predictive value (PPV) is used as a practical predictive parameter. For example, the PPV of CRP was 79.1%, and the PPV of CAR was 81.4%, which means the probability of correctly predicting complications in a patient with CRP ≥ 85.1 was 79.1%; however, the probability was 81.4% in a patient with CAR ≥2.2. Therefore, CAR was a better predictor than CRP on POD 3 in patients undergoing colorectal surgery.
Table 3.
ROC curve showing Postoperative CAR levels and CRP on POD 3 levels predictive of postoperative overall complications
| Postoperative CAR | CRP on POD 3 | |
|---|---|---|
| Cutoff point | 2.2 | 85.1 |
| AUC | 0.779 | 0.756 |
| Sensitivity | 0.748 | 0.698 |
| Specificity | 0.695 | 0.723 |
| Positive predictive value | 81.4% | 79.1% |
| Negative predictive value | 60.8% | 61.4% |
| Youden’s index | 0.444 | 0.421 |
CAR CRP to albumin (CRP/ALB) ratio, ROC receiver operating characteristic, CRP C-reactive protein, ALB albumin, POD postoperative day
Fig. 1.
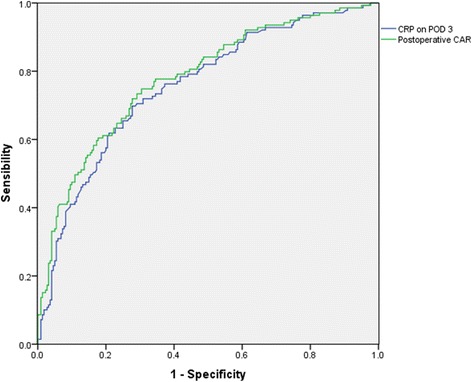
ROC curve showing postoperative CAR levels and CRP on POD 3 levels predictive of postoperative overall complications
Use of CAR to predict postoperative complications
Patients were separated into two groups based on the cutoff values. Patients with CAR ≥2.2 were found to have more postoperative complications than those with CAR <2.2 (60.8% vs 18.6%, P < 0.001). Patients with CAR ≥2.2 had more mild complications (grade I to II) and major complications than those with CAR <2.2 (39.8% vs 14.9%, P < 0.001; 25.1% vs 6.4%, P < 0.001, respectively). Moreover, patients with CAR ≥2.2 were more likely to have a longer postoperative stay and surgical site infection (P < 0.001) (Table 4).
Table 4.
Comparison of postoperative complications associated with postoperative CAR
| Characteristics | CAR <2.2 (n = 188) | CAR ≥2.2 (n = 171) | P value |
|---|---|---|---|
| Overall, n (%)a, c | 35 (18.6) | 104 (60.8) | <0.001 |
| Grade I, n (%)a, c | 12 (6.4) | 25 (14.6) | 0.010 |
| Ileus | 4 (2.1) | 5 (2.9) | |
| Fever >38.5 °C after surgery | 5 (2.7) | 10 (5.8) | |
| Delayed gastric emptying | 1 (0.5) | 3 (1.8) | |
| Wound infection | 2 (1.1) | 5 (2.9) | |
| Urinary retention | 0 | 1 (0.6) | |
| Transient confusion | 0 | 1 (0.6) | |
| Grade II, n (%)a, c | 16 (8.5) | 43 (25.1) | <0.001 |
| Postoperative blood transfusions >2U | 9 (4.8) | 25 (14.6) | |
| TPN >2 weeks | 3 (1.6) | 5 (2.9) | |
| Pneumonia | 2 (1.1) | 2 (1.2) | |
| Urinary tract infection | 0 | 1 (0.6) | |
| Wound infection | 0 | 7 (4.1) | |
| Early postoperative bowel obstruction | 2 (1.1) | 0 | |
| Line sepsis | 0 | 3 (1.8) | |
| Grade III, n (%)a, c | 11 (5.9) | 38 (22.2) | <0.001 |
| Fascial dehiscence | 2 (1.1) | 2 (1.2) | |
| Abdomino-pelvic collection | 2 (1.1) | 3 (1.8) | |
| Pleural effusion | 1 (0.5) | 7 (4.1) | |
| Gastrointestinal bleeding | 0 | 1 (0.6) | |
| Acalculous choleycystitis | 0 | 3 (1.8) | |
| Intraabdominal bleeding | 0 | 1 (0.6) | |
| Anastomotic leakage | 3 (1.6) | 15 (8.8) | |
| Lymphatic leakage | 1 (0.5) | 0 | |
| Stoma complications | 0 | 1 (0.6) | |
| Early postoperative bowel obstruction | 0 | 1 (0.6) | |
| Intraabdominal abscess | 2 (1.1) | 4 (2.3) | |
| Grade IV, n (%)a, c | 1 (0.5) | 5 (2.9) | 0.176 |
| Septic shock | 0 | 1 (0.6) | |
| Sepsis | 0 | 1 (0.6) | |
| Respiratory failure | 0 | 1 (0.6) | |
| Kidney failure | 0 | 1 (0.6) | |
| MODS | 1 (0.5) | 1 (0.6) | |
| Grade V, n (%)a, c | 0 | 0 | |
| Grade III or greater, n (%)a, c | 12 (6.4) | 43 (25.1) | <0.001 |
| Postoperative stay (days)b | 7 (3–78) | 10 (4–71) | <0.001 |
| Surgical site infection (+), SSI (+), n (%)c | 9 (4.8) | 36 (21.1) | <0.001 |
CAR CRP to albumin (CRP/ALB) ratio, CRP C-reactive protein, ALB albumin
aClavien-Dindo’s classification of surgical complication
bValues are expressed as the median (range)
cValues are expressed as n (%)
Predictive value of CAR in patients with colorectal cancer
A subgroup analysis of the predictive value of CAR in patients with colorectal cancer was performed. Receiver operating characteristic curve analysis showed that the AUC of CAR in patients with colorectal cancer was 0.764, with a sensitivity of 0.691 and a specificity of 0.728. The cutoff value was 2.3. The AUC of CRP on POD 3 was 0.747. The sensitivity was 0.670, and the specificity was 0.743. The cutoff value was 85.1. Univariate and multivariate analyses showed that CAR ≥2.3 (OR 4.152, 95% CI 2.233–7.721, P < 0.001) was an independent risk factor for postoperative morbidity. Patients with colorectal cancer with CAR ≥2.3 were more likely to have longer postoperative hospital stays (P < 0.001), more SSIs (P < 0.001), and more postoperative complications (P < 0.001).
Discussion
The main findings of our study can be summarized as follows. First, CAR was an independent and significant risk factor for postoperative complications in patients undergoing colorectal surgery. Second, CAR appeared to show higher accuracy than CRP on POD 3 alone as a predictor of postoperative complications. Third, patients with CAR ≥2.2, which was more accurate than CRP level on POD 3, were at higher risk of postoperative complications, prolonged postoperative hospital stay and more SSIs after colorectal surgery.
Increasing evidence shows that a systemic inflammatory response following surgical trauma is associated with poor outcome in patients after surgery, and this was revealed by serum C-reactive protein and albumin [9, 16, 17]. There are some inflammation-based prognostic scores, such as the modified Glasgow Prognostic Score (mGPS), neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), postoperative Glasgow Prognostic Score (poGPS), and CRP to albumin ratio (CAR), that predict outcomes for surgical patients [15, 18–20]. Watt et al. [20] suggested that a postoperative systemic inflammation score including postoperative C-reactive protein and albumin levels could predict short- and long-term outcomes in patients undergoing colorectal surgery. Among these, CAR is novel. Haruki et al. [19] suggested that for pancreatic cancer patients after pancreatic resection, CAR could be an independent and significant indicator of poor long-term outcome. Shibutani et al. [21] found preoperative CAR to be a useful prognostic marker in patients with colorectal cancer undergoing potentially curative surgery. In septic patients, Ranzani et al. [15] also reported that residual inflammation at ICU discharge as assessed by CAR was an independent risk factor for outcome.
It is well known that postoperative CRP levels can be used as a practical marker to assess postoperative inflammation and to predict postoperative complications [1]. However, some studies still find certain drawbacks to CRP as a predictor of stress-related complications [9, 22], including less accuracy in some patients. However, CAR is based on circulating levels of two acute phase proteins, CRP and albumin, which are also associated with inflammation from surgical trauma [19]. In our study, CAR was an independent prognostic factor for postoperative complications in multivariate analysis (OR 4.413; 95% CI 2.463–7.906; P < 0.001). In contrast, CRP on POD 3 was not an independent risk factor for complications after colorectal surgery in multivariate analysis (OR 1.826; 95% CI 0.938–3.556; P = 0.077). ROC analysis also revealed that the AUC of CAR was higher than that of CRP, and CAR had a higher positive predictive value than CRP on POD 3. Combined with ROC analysis, these results suggest that the predictive value of CAR was superior to that of CRP on POD 3, and CAR appeared to show higher accuracy than CRP alone.
The reasons why CAR could be more accurate than CRP alone for predicting postoperative complications might be that CRP combined with albumin is more likely to reflect inflammation from surgical stress. Studies have already proven that the surgical stress response can be evidenced by CRP concentrations on POD 3 or 4 [1, 7, 23]. Moreover, serum albumin acting as a negative acute-phase protein also decreases in response to surgical stress [24]. Increased capillary leakage of albumin is one of the features of the systemic inflammatory response after colorectal surgery, which leads to changes in serum albumin levels [16]. In addition, collagen synthesis and granuloma formation, which impairs the innate immune response, will be influenced by hypoalbuminemia; therefore, wound healing is delayed, and the systemic immune status is predisposing to infection [25]. Although many studies have focused on serum albumin before surgery, Hubner et al. [9] showed an early postoperative albumin drop to be related to adverse clinical outcomes. Lee et al. [26] found that after oral cancer surgery, patients with postoperative hypoalbuminemia were at risk of surgical site infection. Hypoalbuminemia is thought to be associated with inflammation or previous malnutrition [27]. Some studies have already merged albumin and CRP into a single index to predict outcomes [12, 28]. In our study, we found that an increased CRP/albumin ratio could reflect higher diagnostic accuracy for postoperative complications than CRP alone. As a result, it is better to put albumin and CRP together to predict postoperative complications.
The current study had several limitations. First, it is a retrospective observational analysis, and the effects of residual confounding factors cannot be excluded completely. Second, it is a single-center study, and it is necessary to conduct multicenter studies for further confirmation, as perioperative management is dependent on our local experience. Third, other surgeries, such as gastrectomy, esophagectomy resection, and liver resection, are needed to verify these results.
Conclusions
The current study confirmed that the CRP/albumin ratio predicted postoperative outcomes in patients who underwent elective colorectal surgery. Merging CRP and albumin into a single index could be more accurate than CRP alone. Patients with CAR greater than 2.2 should be intensively monitored for early detection of postoperative outcome to reduce postoperative complications, shorten hospital stay, quickly return to premorbid functional activity, and reduce hospital cost.
Acknowledgements
The authors gratefully acknowledge all of the investigators for their contributions to the trial, as well as Huatong Liu from the Australian National University and Kaixuan Wu from University of Bath, UK, who provided medical writing assistance.
Funding
This study was funded in part by the National Natural Science Foundation of China (81270006) and National Gastroenterology Research Project (2015BAI13B07).
Availability of data and materials
Access to the data and the calculation method can be obtained from the authors by email (njumedgxl09@126.com).
Authors’ contributions
XG, YC, and NL contributed to study conception and design. XG, JG, and YC contributed to acquisition of data. CD, XZ, and HT contributed to analysis and interpretation of data. XG and HW contributed to drafting of manuscript. WZ and NL contributed to critical revision. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from all patients enrolled in the investigation. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and the guidelines of the regional ethical committees of Jinling Hospital, Medical School of Nanjing University, Nanjing, China.
Ethics approval and consent to participate
This study was approved by the ethics committee of Jinling Hospital. Written informed consent was obtained from all participants.
Abbreviations
- ALB
Albumin
- ASA
American Society of Anesthesiologists
- CRP
C-reactive protein
- POD
Postoperative day
- ROC
Receiver operating characteristic
- SIRS
Systemic inflammatory response syndrome
Contributor Information
Xiaolong Ge, Email: gxlnjumed09@126.com.
Yu Cao, Email: njumedcy@126.com.
Hongkan Wang, Email: xiaolongge9118@126.com.
Chao Ding, Email: jinlingh_dc@163.com.
Hongliang Tian, Email: 531539603@qq.com.
Xueying Zhang, Email: jinlingh_ljs@sina.com.
Jianfeng Gong, Email: jinlingh_gongjf@163.com.
Weiming Zhu, Email: jinlingh_zwm@163.com.
Ning Li, Email: jinlingh_lining@163.com.
References
- 1.Warschkow R, Beutner U, Steffen T, Muller SA, Schmied BM, Guller U, et al. Safe and early discharge after colorectal surgery due to C-reactive protein: a diagnostic meta-analysis of 1832 patients. Ann Surg. 2012;256:245–50. doi: 10.1097/SLA.0b013e31825b60f0. [DOI] [PubMed] [Google Scholar]
- 2.Zhang T, Cao L, Cao T, Yang J, Gong J, Zhu W, et al. Prevalence of sarcopenia and its impact on postoperative outcome in patients with Crohn’s disease undergoing bowel resection. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115612054. [DOI] [PubMed] [Google Scholar]
- 3.Frolkis AD, Dykeman J, Negron ME, Debruyn J, Jette N, Fiest KM, et al. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013;145:996–1006. doi: 10.1053/j.gastro.2013.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930–8. doi: 10.1097/SLA.0b013e3182a6f2fc. [DOI] [PubMed] [Google Scholar]
- 5.Ortega-Deballon P, Radais F, Facy O, d'Athis P, Masson D, Charles PE, et al. C-reactive protein is an early predictor of septic complications after elective colorectal surgery. World J Surg. 2010;34:808–14. doi: 10.1007/s00268-009-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt DG, McSorley ST, Horgan PG, McMillan DC. Enhanced recovery after surgery: which components, if any, impact on the systemic inflammatory response following colorectal surgery?: a systematic review. Medicine. 2015;94(36):e1286. doi: 10.1097/MD.0000000000001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Facy O, Paquette B, Orry D, Binquet C, Masson D, Bouvier A, et al. Diagnostic accuracy of inflammatory markers as early predictors of infection after elective colorectal surgery: results from the IMACORS study. Ann Surg. 2016;263(5):961–6. doi: 10.1097/SLA.0000000000001303. [DOI] [PubMed] [Google Scholar]
- 8.Norberg A, Rooyackers O, Segersvard R, Wernerman J. Albumin kinetics in patients undergoing major abdominal surgery. PLoS One. 2015;10:e0136371. doi: 10.1371/journal.pone.0136371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hubner M, Mantziari S, Demartines N, Pralong F, Coti-Bertrand P, Schafer M. Postoperative albumin drop is a marker for surgical stress and a predictor for clinical outcome: a pilot study. Gastroenterol Res Pract. 2016;2016:8743187. doi: 10.1155/2016/8743187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EH, Chin JH, Choi DK, Hwang BY, Choo SJ, Song JG, et al. Postoperative hypoalbuminemia is associated with outcome in patients undergoing off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2011;25:462–8. doi: 10.1053/j.jvca.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Ishizuka M, Nagata H, Takagi K, Iwasaki Y, Shibuya N, Kubota K. Clinical significance of the C-reactive protein to albumin ratio for survival after surgery for colorectal cancer. Ann Surg Oncol. 2016;23:900–7. doi: 10.1245/s10434-015-4948-7. [DOI] [PubMed] [Google Scholar]
- 12.Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a modified early warning system for acute medical admissions and comparison with C-reactive protein/albumin ratio as a predictor of patient outcome. Clin Med. 2009;9:30–3. doi: 10.7861/clinmedicine.9-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sang BH, Bang JY, Song JG, Hwang GS. Hypoalbuminemia within two postoperative days is an independent risk factor for acute kidney injury following living donor liver transplantation: a propensity score analysis of 998 consecutive patients. Crit Care Med. 2015;43:2552–61. doi: 10.1097/CCM.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranzani OT, Zampieri FG, Forte DN, Azevedo LC, Park M. C-reactive protein/albumin ratio predicts 90-day mortality of septic patients. PLoS One. 2013;8:e59321. doi: 10.1371/journal.pone.0059321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleck A, Raines G, Hawker F, Trotter J, Wallace PI, Ledingham IM, et al. Increased vascular permeability: a major cause of hypoalbuminaemia in disease and injury. Lancet. 1985;1:781–4. doi: 10.1016/S0140-6736(85)91447-3. [DOI] [PubMed] [Google Scholar]
- 17.Angiolini MR, Gavazzi F, Ridolfi C, Moro M, Morelli P, Montorsi M, et al. Role of C-reactive protein assessment as early predictor of surgical site infections development after pancreaticoduodenectomy. Dig Surg. 2016;33:267–75. doi: 10.1159/000445006. [DOI] [PubMed] [Google Scholar]
- 18.Proctor MJ, Morrison DS, Talwar D, Balmer SM, Fletcher CD, O'Reilly DS, et al. A comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow Inflammation Outcome Study. Eur J Cancer. 2011;47:2633–41. doi: 10.1016/j.ejca.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Haruki K, Shiba H, Shirai Y, Horiuchi T, Iwase R, Fujiwara Y, et al. The C-reactive protein to albumin ratio predicts long-term outcomes in patients with pancreatic cancer after pancreatic resection. World J Surg. 2016;40(9):2254–60. doi: 10.1007/s00268-016-3491-4. [DOI] [PubMed] [Google Scholar]
- 20.Watt DG, McSorley ST, Park JH, et al. A postoperative systemic inflammation score predicts short- and long-term outcomes in patients undergoing surgery for colorectal cancer. Ann Surg Oncol. 2016 doi: 10.1245/s10434-016-5659-4. [DOI] [PubMed] [Google Scholar]
- 21.Shibutani M, Maeda K, Nagahara H, Iseki Y, Ikeya T, Hirakawa K. Prognostic significance of the preoperative ratio of C-reactive protein to albumin in patients with colorectal cancer. Anticancer Res. 2016;36:995–1001. [PubMed] [Google Scholar]
- 22.Easton R, Balogh ZJ. Peri-operative changes in serum immune markers after trauma: a systematic review. Injury. 2014;45:934–41. doi: 10.1016/j.injury.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Korner H, Nielsen HJ, Soreide JA, Nedrebo BS, Soreide K, Knapp JC. Diagnostic accuracy of C-reactive protein for intraabdominal infections after colorectal resections. J Gastrointest Surg. 2009;13:1599–606. doi: 10.1007/s11605-009-0928-1. [DOI] [PubMed] [Google Scholar]
- 24.Sung J, Bochicchio GV, Joshi M, Bochicchio K, Costas A, Tracy K, et al. Admission serum albumin is predicitve of outcome in critically ill trauma patients. Am Surg. 2004;70:1099–102. [PubMed] [Google Scholar]
- 25.Otranto M, Souza-Netto I, Aguila MB, Monte-Alto-Costa A. Male and female rats with severe protein restriction present delayed wound healing. Appl Physiol Nutr Metab. 2009;34:1023–31. doi: 10.1139/H09-100. [DOI] [PubMed] [Google Scholar]
- 26.Lee JI, Kwon M, Roh JL, Choi JW, Choi SH, Nam SY, et al. Postoperative hypoalbuminemia as a risk factor for surgical site infection after oral cancer surgery. Oral Dis. 2015;21:178–84. doi: 10.1111/odi.12232. [DOI] [PubMed] [Google Scholar]
- 27.Vincent JL, Dubois MJ, Navickis RJ, Wilkes MM. Hypoalbuminemia in acute illness: is there a rationale for intervention? A meta-analysis of cohort studies and controlled trials. Ann Surg. 2003;237:319–34. doi: 10.1097/01.SLA.0000055547.93484.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinilla JC, Hayes P, Laverty W, Arnold C, Laxdal V. The C-reactive protein to prealbumin ratio correlates with the severity of multiple organ dysfunction. Surgery. 1998;124:799–805. doi: 10.1067/msy.1998.91365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Access to the data and the calculation method can be obtained from the authors by email (njumedgxl09@126.com).


