Abstract
Background
Body mass index (BMI) has a U-shaped association with lung cancer risk. However, the effect of BMI on prognosis is controversial. This retrospective study aimed to investigate the effect of BMI on the survival of patients with stage I non-small cell lung cancer (NSCLC) after surgical resection.
Methods
In total, 624 consecutive stage I NSCLC patients who underwent radical resection were classified into four groups according to their BMI: underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5–22.4 kg/m2), overweight (BMI = 22.5–28.0 kg/m2), and obese (BMI > 28.0 kg/m2). The effect of BMI on progression-free survival (PFS) and overall survival (OS) was estimated using the Kaplan–Meier method and Cox proportional hazards model. Postoperative complications in each group were analyzed using the Chi square test or Fisher’s exact test.
Results
A univariate analysis showed that PFS and OS were longer in the overweight group than in other groups (both P < 0.05). A multivariate analysis showed that OS was longer in the overweight group than in other groups (compared with the other three groups in combination: hazard ratio [HR] = 1.87, 95% confidence interval [CI] 1.30–2.68, P = 0.003; compared with the underweight group: HR = 2.24, 95% CI 1.18–4.25, P = 0.013; compared with the normal weight group: HR = 1.58, 95% CI 1.07–2.33, P = 0.022; compared with the obese group: HR = 2.87, 95% CI 1.48–5.59, P = 0.002), but PFS was similar among the groups (HR = 1.28, 95% CI 0.97–1.68, P = 0.080). A subgroup analysis showed an association between being overweight and prolonged OS in patients at stage T1a (P = 0.024), T1b (P = 0.051), and T2a (P = 0.02), as well as in patients with a non-smoking history (P = 0.001). Overweight patients had lower rates of postoperative complications, such as respiratory failure (compared with the underweight and obese groups: P = 0.014), myocardial infarction (compared with the obese group: P = 0.033), and perioperative death (compared with the other three groups: P = 0.016).
Conclusions
Preoperative BMI is an independent prognostic factor for stage I NSCLC patients after resection, with overweight patients having a favorable prognosis.
Keywords: Non-small cell lung cancer, Early stage, Body mass index, Survival, Surgery
Background
The global incidence and mortality of lung cancer are the highest among all cancers. Furthermore, lung cancer is responsible for numerous public health problems [1–3]. Despite advances in surgical techniques and the incorporation of new therapeutic approaches, the 5-year survival rate for lung cancer patients has remained low [1, 4].
Strong evidence indicates that nutritional status, including weight and diet, might affect long-term survival of patients with certain types of cancer [5, 6]. The association between the body mass index (BMI) and the risk of lung cancer has been well established [7–12]. Some studies have shown that lung cancer mortality is lower in obese patients [13–16], but these studies have lacked details, such as histological type, disease stage, and treatment modality. Other studies, which only focused on advanced non-small cell lung cancer (NSCLC), showed that a lower BMI was related to a higher mortality [17]. An association between BMI and the survival of patients with operable early-stage NSCLC has not yet been definitively demonstrated. This association could help surgeons identify patients at high risk of recurrence or death.
Directing the treatment and accurately predicting the prognosis of early-stage NSCLC patients are important, as is increasing public awareness of the role lifestyle plays in cancer survivorship. The World Health Organization has recommended BMI cutoff points for underweight, normal weight, overweight, and obese, which are calculated to predict health risks, including the risks for all types of cancer and non-cancer disease [18, 19]. However, whether these cutoff points are suitable for the Asian population has remained a matter of debate [20]. In two large cohort studies that examined the association between BMI and lung cancer risk in the Chinese population, Yang et al. [13] used the BMI cutoff points of 18.5, 20.0, 22.4, and 25.0 kg/m2 for underweight, normal weight, overweight, and obese, respectively, whereas Koh et al. [21] proposed the cutoff points of 20.0, 24.0, and 28.0 kg/m2 for normal weight, overweight, and obese, respectively.
Adapting the BMI cutoff points proposed in Asian studies [13, 21], we analyzed a large cohort of Chinese patients with stage I NSCLC who underwent complete surgical resection to investigate the association between BMI and the survival of patients with stage I NSCLC, as well as complications after resection.
Patients and methods
Patient selection
This study was officially approved by the ethics committee of Sun Yat-sen University Cancer Center. We identified consecutive patients with stage I NSCLC (according to the 7th edition TNM classification) [22] who underwent complete surgical resection at Sun Yat-sen University Cancer Center between December 2005 and December 2010. Patients were excluded if they had received neoadjuvant chemotherapy, had an unknown BMI, had a history of other types of cancer, or had residual tumor tissue after surgery. Patient characteristics and postoperative complications were analyzed. All of the patients underwent a lobectomy, bilobectomy, or pneumonectomy with complete lymph node dissection through the surgical approaches of open thoracotomy or video-assisted thoracoscopy.
BMI
BMI was calculated based on direct height and weight measurements before treatment. Specific BMI cutoff points for the Asian population have yet to be defined [18–20]. Therefore, we modified the BMI cutoff points proposed by Yang et al. [13] (18.5, 20.0, 22.4, and 25.0 kg/m2) and Koh et al. [21] (20, 24, and 28 kg/m2) and divided the patients into four groups: underweight (BMI < 18.5 kg/m2), normal weight (BMI = 18.5–22.4 kg/m2), overweight (BMI = 22.5–28.0 kg/m2), and obese (BMI > 28.0 kg/m2).
Definitions of postoperative complications
All complications between the time of surgery and hospital discharge were documented. Respiratory failure was defined as the requirement for postoperative mechanical ventilation longer than 24 h. Postoperative pyrexia was defined as a body temperature ≥38°C for a period >24 h following surgery. Chylothorax was defined as elevated triglyceride levels in milky drained fluid. Postoperative hemorrhage required reoperation. Myocardial infarction and arrhythmia were detected by electrocardiogram. Pneumothorax was detected by clinical observation and chest X-ray radiography. Atelectasis was detected by clinical observation, chest X-ray radiography, and fiberoptic bronchoscopy. Perioperative death was defined as death within 1 month after surgery.
Follow-up
Generally, the patients were followed every 3 months for the first year and twice a year thereafter. However, follow-up interval was shortened for patients with specific symptoms. Regular follow-up examinations included a physical examination, blood chemistry analysis, tumor marker measurement, and computed tomography scan or chest X-ray radiography. The follow-up duration was calculated from the date of surgery to the date of the event or the last contact. Follow-up was continued until May 2015.
Statistical analysis
Statistical analyses were performed using SPSS 22.0 for Windows software (SPSS Inc., Chicago, IL, USA). Differences among the four BMI groups were tested using the Kruskal–Wallis test. The associations between BMI and clinicopathologic parameters or postoperative complications were analyzed using the Chi square test or Fisher’s exact test. Progression-free survival (PFS) was calculated from the date of surgery to the date of tumor recurrence, metastasis, or death from any cause. Overall survival (OS) was calculated from the date of surgery to the date of death from any cause. Survival curves were plotted using the Kaplan–Meier method and analyzed using the log-rank test. A multivariate analysis was performed using the Cox proportional hazards regression model with the backward stepwise procedure (the entry and removal probabilities were 0.05 and 0.10). A significant difference was declared if the P value from a two-tailed test was <0.05.
Results
Patient characteristics by BMI
Seven hundred consecutive patients with stage I NSCLC underwent complete surgical resection between December 2005 and December 2010. Among them, 624 patients were included in the study and were divided into four groups according to BMI. The other 76 patients were excluded because they had received neoadjuvant chemotherapy (17 patients), had an unknown BMI (3 patients), had a history of other types of cancer (50 patients), had residual tumor tissue after surgery (4 patients), or a combination of the above (2 patients).
The patient characteristics are shown in Table 1. As compared with a lower BMI, a higher BMI was associated with higher rates of hypertension (P < 0.001), smoking (P = 0.001), and high preoperative forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) ratios (P = 0.011). Normal weight patients were more likely to have visceral pleural invasion (P = 0.005) and to have a higher T stage (P < 0.001) and TNM stage (P < 0.001) than underweight, overweight, and obese patients.
Table 1.
The baseline clinicopathologic characteristics of underweight, normal weight, overweight, and obese patients with stage I NSCLC, stratified by BMI
| Characteristic | Overall (cases) | Underweight [cases (%)] | Normal weight [cases (%)] | Overweight [cases (%)] | Obese [cases (%)] | P value |
|---|---|---|---|---|---|---|
| Total | 624 | 44 | 245 | 306 | 29 | |
| Gender | 0.668 | |||||
| Male | 408 (65.4) | 26 (13.6) | 166 (67.8) | 198 (64.7) | 18 (62.1) | |
| Female | 216 (34.6) | 18 (86.4) | 79 (32.2) | 108 (35.3) | 11 (37.9) | |
| Age (years) | 0.991 | |||||
| <60 | 285 (45.7) | 20 (2.3) | 114 (46.5) | 138 (45.1) | 13 (44.8) | |
| ≥60 | 339 (54.3) | 24 (97.7) | 131 (53.5) | 168 (54.9) | 16 (55.2) | |
| Smoking | 0.001 | |||||
| Never | 318 (51.0) | 23 (4.5) | 101 (41.2) | 178 (58.2) | 16 (55.2) | |
| Current or ever | 306 (49.0) | 21 (95.5) | 144 (58.8) | 128 (41.8) | 13 (44.8) | |
| Hypertension | <0.001 | |||||
| With | 150 (24.0) | 6 (13.6) | 38 (15.5) | 93 (30.4) | 13 (44.8) | |
| Without | 474 (76.0) | 38 (86.4) | 207 (84.5) | 213 (69.6) | 16 (55.2) | |
| Diabetes | 0.318 | |||||
| With | 46 (7.4) | 1 (2.3) | 18 (7.3) | 23 (7.5) | 4 (13.8) | |
| Without | 578 (92.6) | 43 (97.7) | 227 (92.7) | 283 (92.5) | 25 (86.2) | |
| Heart disease | 0.482 | |||||
| With | 18 (2.9) | 2 (4.5) | 7 (2.9) | 7 (2.3) | 2 (6.9) | |
| Without | 606 (97.1) | 42 (95.5) | 238 (97.1) | 299 (97.7) | 27 (93.1) | |
| FEV1/FVC (%) | 0.011 | |||||
| <70 | 83 (13.3) | 11 (25.0) | 40 (16.3) | 30 (9.8) | 2 (6.9) | |
| ≥70 | 541 (86.7) | 33 (75.0) | 205 (83.7) | 276 (90.2) | 27 (93.1) | |
| Type of surgery | 0.979 | |||||
| Open thoracotomy | 537 (86.1) | 37 (84.1) | 210 (85.7) | 264 (86.3) | 25 (86.2) | |
| VATS | 87 (13.9) | 7 (15.9) | 35 (14.3) | 42(13.7) | 4 (13.8) | |
| Extent of resection | 0.681 | |||||
| Lobectomy or bilobectomy | 611 (97.9) | 44 (100.0) | 239 (97.6) | 299 (97.7) | 29 (100.0) | |
| Pneumonectomy | 13 (2.1) | 0 (0.0) | 6 (2.4) | 7 (2.3) | 0 (0.0) | |
| Adjuvant chemotherapy | 0.967 | |||||
| Absent | 486 (77.9) | 34 (77.3) | 193 (78.8) | 236 (77.1) | 23 (79.3) | |
| Present | 138 (22.1) | 10 (22.7) | 52 (21.2) | 70 (22.9) | 6 (20.7) | |
| Histological type | 0.432 | |||||
| Adenocarcinoma | 406 (65.1) | 29 (65.9) | 152 (62.0) | 203 (66.3) | 22 (75.9) | |
| Squamous cell carcinoma | 140 (22.4) | 12 (27.3) | 62 (25.3) | 63 (20.6) | 3 (10.3) | |
| Other NSCLC | 78 (12.5) | 3 (6.8) | 31 (12.7) | 40 (13.1) | 4 (13.8) | |
| Histologic differentiation | 0.131 | |||||
| Poor | 212 (34.0) | 17 (38.6) | 94 (38.4) | 92 (30.1) | 9 (31.0) | |
| Moderate | 300 (48.1) | 17 (38.6) | 113 (46.1) | 159 (52.0) | 11 (37.9) | |
| Well | 112 (17.9) | 10 (22.7) | 38 (15.5) | 55 (18.0) | 9 (31.0) | |
| Visceral pleural invasion | 0.005 | |||||
| Absent | 408 (65.4) | 32 (72.7) | 140 (57.1) | 212 (69.3) | 23 (79.3) | |
| Present | 216 (34.6) | 12 (27.3) | 105 (42.9) | 94 (30.7) | 6 (20.7) | |
| T stage | <0.001 | |||||
| T1a | 132 (21.2) | 10 (22.7) | 36 (14.7) | 78 (25.5) | 8 (27.6) | |
| T1b | 113 (18.1) | 13 (29.5) | 31 (12.7) | 59 (19.3) | 9 (31.0) | |
| T2a | 379 (60.7) | 21 (47.7) | 178 (72.7) | 169 (55.2) | 12 (41.4) | |
| Clinical TNM stage | <0.001 | |||||
| IA | 245 (39.3) | 23 (52.3) | 67 (27.3) | 137 (44.8) | 17 (58.6) | |
| IB | 379 (60.7) | 21 (47.7) | 178 (72.7) | 169 (55.2) | 12 (41.4) |
Underweight BMI < 18.5 kg/m2; normal weight BMI 18.5–22.4 kg/m2; overweight BMI = 22.5–28.0 kg/m2; obese BMI > 28.0 kg/m2
NSCLC non-small cell lung cancer, BMI body mass index, FEV1/FVC forced expiratory volume in 1 s/forced vital capacity, VATS video-assisted thoracic surgery
Overweight and normal weight patients were less likely to experience postoperative respiratory failure (P = 0.014) than underweight and obese patients, whereas obese patients were more likely to have myocardial infarction after surgery (P = 0.033) than underweight, normal weight, and overweight patients. Furthermore, perioperative death was significantly less common in the overweight group than in other groups (P = 0.016, Table 2).
Table 2.
Postoperative complications of underweight, normal weight, overweight, and obese patients with stage I NSCLC
| Characteristic | Overall (cases) | Underweight [cases (%)] | Normal weight [cases (%)] | Overweight [cases (%)] | Obese [cases (%)] | P value |
|---|---|---|---|---|---|---|
| Total | 624 | 44 | 245 | 306 | 29 | |
| Respiratory failure | 0.014 | |||||
| Absent | 605 (97.0) | 40 (90.9) | 240 (98.0) | 299 (97.7) | 26 (89.7) | |
| Present | 19 (3.0) | 4 (9.1) | 5 (2.0) | 7 (2.3) | 3 (10.3) | |
| Pyrexia | 0.965 | |||||
| Absent | 502 (80.4) | 36 (81.8) | 195 (79.6) | 247 (80.7) | 24 (82.8) | |
| Present | 122 (19.6) | 8 (18.2) | 50 (20.4) | 59 (19.3) | 5 (17.2) | |
| Chylothorax | 0.339 | |||||
| Absent | 616 (98.7) | 43 (97.7) | 241 (98.4) | 304 (99.3) | 28 (96.6) | |
| Present | 8 (1.3) | 1 (2.3) | 4 (1.6) | 2 (0.7) | 1 (3.4) | |
| Hemorrhage | 0.074 | |||||
| Absent | 612 (98.1) | 44 (100.0) | 236 (96.3) | 303 (99.0) | 29 (100.0) | |
| Present | 12 (1.9) | 0 (0.0) | 9 (3.7) | 3 (1.0) | 0 (0.0) | |
| Myocardial infarction | 0.033 | |||||
| Absent | 618 (99.0) | 44 (100.0) | 243 (99.2) | 304 (99.3) | 27 (93.1) | |
| Present | 6 (1.0) | 0 (0.0) | 2 (0.8) | 2 (0.7) | 2 (6.9) | |
| Arrhythmia | 0.484 | |||||
| Absent | 559 (89.6) | 38 (86.4) | 225 (91.8) | 270 (88.2) | 26 (89.7) | |
| Present | 65 (10.4) | 6 (13.6) | 20 (8.2) | 36 (11.8) | 3 (10.3) | |
| Pneumothorax | 0.100 | |||||
| Absent | 473 (75.8) | 29 (65.9) | 179 (73.1) | 240 (78.4) | 25 (86.2) | |
| Present | 151 (24.2) | 15 (34.1) | 66 (26.9) | 66 (21.6) | 4 (13.8) | |
| Atelectasis | 0.818 | |||||
| Absent | 579 (92.8) | 40 (90.9) | 228 (93.1) | 283 (92.5) | 28 (96.6) | |
| Present | 45 (7.2) | 4 (9.1) | 17 (6.9) | 23 (7.5) | 1 (3.4) | |
| Death | 0.016 | |||||
| Absent | 614 (98.4) | 42 (95.5) | 240 (98.0) | 305 (99.7) | 27 (93.1) | |
| Present | 10 (1.6) | 2 (4.5) | 5 (2.0) | 1 (0.3) | 2 (6.9) |
Univariate and multivariate analyses
The median follow-up duration of the entire cohort was 63.2 months (range 47.8–78.1 months). The univariate survival analysis showed significant differences in PFS and OS between the overweight group and other groups. The OS of overweight patients was significantly longer than those of underweight, normal weight, and obese patients (P = 0.001; Table 3; Fig. 1). The PFS of overweight patients was significantly longer than those of patients in other groups (P = 0.034, Table 4; Fig. 2). The multivariate survival analysis showed that BMI was an independent factor associated with OS, and that overweight patients had a significantly lower risk of death from stage I NSCLC after surgery than did patients in other groups (compared with the underweight group: hazard ratio [HR] = 2.24, 95% confidence interval [CI] 1.18–4.25, P = 0.013; compared with the normal weight group: HR = 1.58, 95% CI 1.07–2.33, P = 0.022; compared with the obese group: HR = 2.87, 95% CI 1.48–5.59, P = 0.002). Other independent factors that might affect OS included age, smoking history, extent of resection, histological differentiation, and T stage. Independent factors that were associated with PFS included age, smoking, extent of resection, adjuvant chemotherapy, BMI, and FEV1/FVC ratio.
Table 3.
Univariate and multivariate overall survival (OS) analyses for patients with stage I NSCLC
| Variate | OS rate (%) | Univariate analysis P value |
Multivariate analysis | ||
|---|---|---|---|---|---|
| 3-year | 5-year | HR (95% CI) | P value | ||
| Age (years) | 0.002 | 1.83 (1.26–2.64) | 0.001 | ||
| <60 | 92.6 ± 1.6 | 85.6 ± 2.2 | |||
| ≥60 | 85.8 ± 1.9 | 77.1 ± 2.4 | |||
| Smoking | <0.001 | 1.45 (1.05–2.10) | 0.047 | ||
| Never | 94.1 ± 1.3 | 86.4 ± 2.0 | |||
| Current or ever | 83.4 ± 2.2 | 75.1 ± 2.6 | |||
| BMI | 0.001 | 0.003 | |||
| Underweight | 84.0 ± 5.5 | 75.8 ± 6.7 | 0.012 | 2.24 (1.18–4.25) | 0.013 |
| Normal weight | 84.2 ± 2.4 | 75.5 ± 2.9 | <0.001 | 1.58 (1.07–2.33) | 0.022 |
| Overweight | 94.8 ± 1.3 | 87.7 ± 2.0 | Reference | Reference | Reference |
| Obese | 75.9 ± 7.9 | 65.3 ± 8.9 | 0.002 | 2.87 (1.48–5.59) | 0.002 |
| Extent of resection | <0.001 | 5.01 (2.26–11.07) | <0.001 | ||
| Lobectomy or bilobectomy | 89.6 ± 1.3 | 81.6 ± 1.6 | |||
| Pneumonectomy | 51.3 ± 15.8 | 41.0 ± 15.6 | |||
| Histological differentiation | 0.001 | 0.76 (0.58–0.99) | 0.039 | ||
| Poor | 83.0 ± 2.7 | 73.5 ± 3.2 | |||
| Moderate | 91.5 ± 1.6 | 84.0 ± 2.2 | |||
| Well | 93.2 ± 2.5 | 86.7 ± 3.5 | |||
| T stage | 0.005 | 1.40 (1.08–1.80) | 0.011 | ||
| T1a | 93.8 ± 2.1 | 90.4 ± 2.7 | |||
| T1b | 89.0 ± 3.0 | 82.0 ± 3.8 | |||
| T2a | 87.3 ± 1.8 | 77.3 ± 2.3 | |||
The data of survival rates are expressed as mean ± standard error
HR hazard ratio, CI confidence interval
Fig. 1.
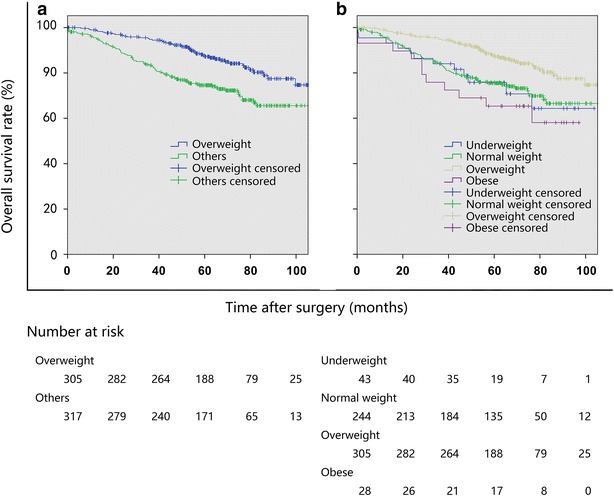
Kaplan–Meier overall survival (OS) curves of patients with stage I non-small cell lung cancer (NSCLC) stratified by body mass index (BMI). Underweight BMI < 18.5 kg/m2; normal weight BMI 18.5–22.4 kg/m2; overweight BMI 22.5–28.0 kg/m2; and obese: BMI > 28.0 kg/m2. a The OS was longer in the overweight group than in other groups in combination (P = 0.001). b The OS was longer in the overweight group than in the underweight group (P = 0.012), the normal weight group (P = 0.001), and the obese group (P = 0.002)
Table 4.
Univariate and multivariate progression-free survival (PFS) analyses for patients with stage I NSCLC
| Variate | PFS rate (%) | Univariate analysis P value |
Multivariate analysis | ||
|---|---|---|---|---|---|
| 3-year | 5-year | HR (95% CI) | P value | ||
| Age (years) | 0.068 | 1.45 (1.09–1.93) | 0.011 | ||
| <60 | 79.4 ± 2.4 | 69.9 ± 2.8 | |||
| ≥60 | 73.9 ± 2.4 | 65.0 ± 2.7 | |||
| Smoking | 0.003 | 1.43 (1.08–1.90) | 0.014 | ||
| Never | 80.8 ± 2.2 | 72.2 ± 2.6 | |||
| Current or ever | 71.9 ± 2.6 | 62.0 ± 2.9 | |||
| BMI | 0.034 | 1.28 (0.97–1.68) | 0.080 | ||
| Overweight | 80.2 ± 2.3 | 71.1 ± 2.7 | |||
| Others | 72.9 ± 2.5 | 63.6 ± 2.8 | |||
| Extent of resection | <0.001 | 4.71 (2.23–9.97) | <0.001 | ||
| Lobectomy or bilobectomy | 77.2 ± 1.7 | 68.0 ± 2.0 | |||
| Pneumonectomy | 38.4 ± 14.7 | 28.8 ± 13.8 | |||
| FEV1/FVC (%) | 0.910 | 0.70 (0.46–1.07) | 0.096 | ||
| <70 | 76.3 ± 1.9 | 67.3 ± 2.1 | |||
| ≥70 | 77.6 ± 4.7 | 66.8 ± 5.4 | |||
| Adjuvant chemotherapy | 0.128 | 1.39 (1.01–1.91) | 0.043 | ||
| Absent | 77.6 ± 1.9 | 68.7 ± 2.2 | |||
| Present | 72.3 ± 3.9 | 61.8 ± 4.4 | |||
Fig. 2.
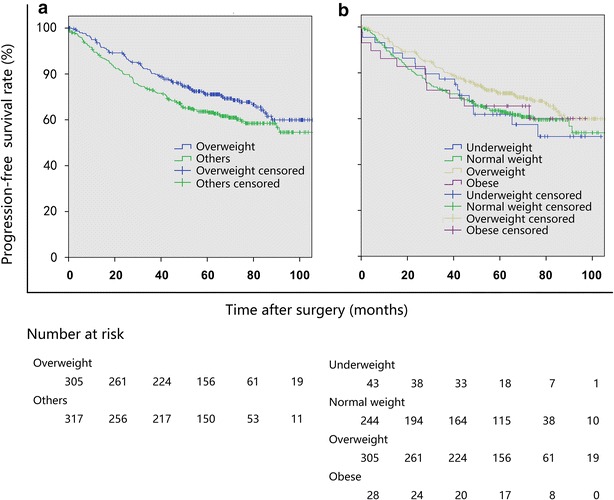
Kaplan–Meier progression-free survival (PFS) curves of patients with stage I NSCLC stratified by BMI. a The PFS was longer in the overweight group than in other groups in combination (P = 0.034). b The PFS was longer in the overweight group than in the normal weight group (P = 0.045), but differences were not significant between the overweight group and the underweight group (P = 0.172) and the obese group (P = 0.514)
Subgroup analysis
The univariate survival analyses showed that when stratified by smoking status and T stage, the association between being overweight and prolonged OS still existed (Table 5; Fig. 3).
Table 5.
Subgroup OS analysis for stage I NSCLC patients in the overweight group and other groups, stratified by smoking status and T stage
| Prognostic factor | OS rate (%) | Univariate analysis | ||
|---|---|---|---|---|
| 3-year | 5-year | P value (log-rank) | P value (Breslow) | |
| Non-smoking | 0.001 | <0.001 | ||
| Overweight | 98.2 ± 1.0 | 92.1 ± 2.2 | ||
| Others | 89.1 ± 2.7 | 79.4 ± 3.6 | ||
| Smoking | 0.083 | 0.024 | ||
| Overweight | 89.9 ± 2.8 | 81.2 ± 3.7 | ||
| Others | 78.8 ± 3.1 | 70.7 ± 3.5 | ||
| T1a stage | 0.024 | 0.004 | ||
| Overweight | 100.0 ± 0 | 97.0 ± 2.1 | ||
| Others | 85.0 ± 4.9 | 81.1 ± 5.4 | ||
| T1b stage | 0.051 | 0.040 | ||
| Overweight | 94.8 ± 2.9 | 87.0 ± 4.6 | ||
| Others | 82.4 ± 5.3 | 76.3 ± 6.0 | ||
| T2a stage | 0.020 | 0.005 | ||
| Overweight | 92.4 ± 2.1 | 83.5 ± 3.1 | ||
| Others | 83.2 ± 2.6 | 72.5 ± 3.2 | ||
The data of survival rates are expressed as mean ± standard error
Fig. 3.
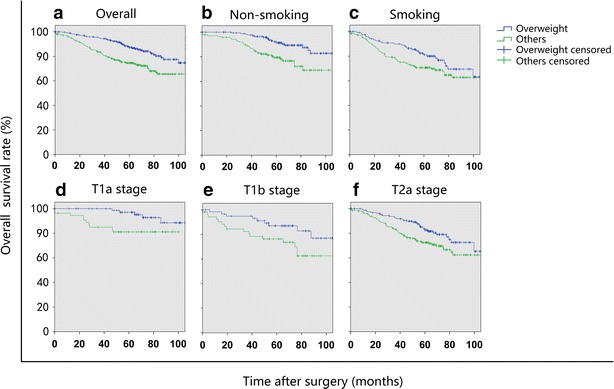
Kaplan–Meier OS curves of stage I NSCLC patients in the overweight group and other groups in combination stratified by smoking status and T stage. a The OS was longer in the overweight group than in other groups in combination (P = 0.001); b in non-smoking patients, the OS was longer in the overweight group than in other groups in combination (P = 0.001); c in smoking patients, the difference in OS was insignificant between the overweight group and other groups in combination (P = 0.083); d in patients with T1a stage NSCLC, the OS was longer in the overweight group than in other groups in combination (P = 0.024); e in patients with T1b stage NSCLC, the difference in OS was insignificant between the overweight group and other groups in combination (P = 0.051); and f in patients with T2a stage NSCLC, the OS of the overweight group was longer than that of other groups in combination (P = 0.020)
Discussion
In our study, overweight patients showed a significantly longer OS than patients in other groups, as determined with both the univariate and multivariate survival analyses. Overweight patients also had a longer PFS than patients in other groups, according to the univariate survival analysis. Furthermore, considering the potential effect of different T stages on the outcomes, we stratified the patients by T stage and re-tested the results. Using either the log-rank test or the Breslow test, we found that overweight patients had longer OS than did patients in other groups for each T stage, indicating the prognostic value of BMI in early-stage NSCLC.
The relationship between BMI and the risk of lung cancer has been studied widely in different patient populations [7, 8, 11–13, 21]. An inverse association between BMI and lung cancer mortality was reported in current smokers [13] and non-smokers [14, 15]. However, all of these studies focused primarily on epidemiology, including all histological types or stages of lung cancer, but the treatment details were not provided. In the present study, we focused on operable stage I NSCLC and found that being overweight had a significant positive effect on the survival of stage I NSCLC patients after surgery. A low BMI (<18.5 kg/m2) has been shown to increase the risk of early postoperative death following en bloc chest wall and lung resection for NSCLC patients (P = 0.009) [23]. In addition, in a study of 640 patients after lobectomy for NSCLC, Tewari et al. [24] reported that a BMI < 18.5 kg/m2 (as a surrogate for impaired nutrition) was a negative predictor of long-term survival independent of tumor extension and stage. In a large cohort study examining the effect of sex on the long-term outcomes of stage I NSCLC patients after curative resection, Warwick et al. [25] also found that BMI was an independent prognostic factor (HR = 0.98, 95% CI 0.96–1.00, P = 0.02). Although patients were not grouped by BMI, these studies demonstrated that a low BMI was a negative prognostic factor for NSCLC after resection. This finding may partly support our finding that survival was longer in overweight patients than in underweight and normal weight patients.
The mechanism by which being overweight might prolong patient survival is still not well understood. Some reports have explained that the association between BMI and lung cancer mortality is caused by smoking history [13]. A high smoking rate was found in patients with a low BMI, and smoking has been reported to have an negative effect on survival [26, 27]. In accordance with other studies [13, 16], we found that fewer overweight and obese patients were smokers as compared with underweight and normal weight patients. However, in the subgroup analysis, we found that the significant prognosis-protective effect of being overweight persisted in non-smokers. In smokers, the overweight group had significantly longer survival than did other groups, as determined using the Breslow test, and while not significant, this group also tended to show longer survival as measured using the log-rank test. Yang et al. [14] and Parr et al. [15] demonstrated that the inverse association between BMI and mortality persisted not only in smokers but also in non-smokers. This finding indicates that BMI is an independent risk factor for lung cancer patient survival. After excluding patients who died within 3 years after diagnosis, Leung et al. [16] also found that BMI was independently and negatively associated with death from lung cancer in smokers and never-smokers. This previous evidence and the findings of our study indicate that BMI is an independent factor for survival. The mechanism of the effect of BMI on the survival of NSCLC patients requires further investigation. Brennan et al. [28] found that the fat mass- and obesity-associated (FTO) gene, which is linked with increased BMI [29], was associated with a decreased risk of lung cancer. This finding provides us another perspective from which to consider how BMI affects the survival of lung cancer patients, although a direct association between lung cancer patient survival and the FTO gene has not yet been demonstrated.
In the current study, although being overweight appeared to prolong survival in stage I NSCLC patients, the obese group had a similar outcome as the normal and underweight groups. This finding contradicts those of other studies [13, 14], in which the obese group of lung cancer patients usually had the lowest mortality. However, these studies were based on all types of pulmonary malignancies, including small cell lung cancer and advanced-stage NSCLC, which have a poor prognosis. Stage I NSCLC in particular is biased toward non-cancer-related death because patients are more likely to be cured and to have a long life expectancy. The obese patients in our study actually had higher rates of preoperative diseases, such as hypertension, as well as postoperative complications, such as respiratory failure and myocardial infarction, compared with patients in other groups. These complications might cause poor health after surgery and may lead to death early after treatment. Using data from the Asia-Pacific Cohort Studies Collaboration, Parr et al. [14] examined the association between BMI and mortality from over 20 cancer sites in adults and found a result similar to ours. They compared normal weight and obese groups and found that significantly lower lung cancer mortality persisted in the overweight group in both smokers and non-smokers. Therefore, there was a U-shaped relation between BMI and lung cancer mortality. There have been few studies on the association between BMI and operable stage I NSCLC. The number of obese patients in our study was limited. Therefore, our results must be interpreted with caution.
We acknowledge some limitations in our study. Because BMI cutoff points have yet to be defined for the Asian population, patient grouping may lead to a selection bias. Additionally, we failed to explain the mechanism of how BMI affects lung cancer patient survival. Biomarkers and genetic markers should be used in further research on this topic.
Conclusions
Being overweight appears to be more favorable than being obese, normal weight, and underweight for the survival of patients with stage I NSCLC after complete surgical resection. Among either non-smokers or smokers, overweight patients had significantly longer survival than underweight, normal weight, and obese patients. The mechanism of how BMI affects lung cancer patient survival remains to be elucidated.
Authors’ contributions
XHJ, SXD, and ZX contributed to literature research, study design, interpretation of findings, and writing of the manuscript. WZQ contributed to data collection and data analysis. LH and RTH contributed to critical review of data analyses, interpretation of findings, and critical revising of the manuscript. All authors revised the final manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by Science and Technology Planning Projects of Guangdong Province (No. 01578040171810021).
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Hao-Jun Xie, Email: 348030874@qq.com.
Xu Zhang, Email: zhangx@sysucc.org.cn.
Zhen-Qiang Wei, Email: weizhenq@sysucc.org.cn.
Hao Long, Email: longhao@sysucc.org.cn.
Tie-Hua Rong, Email: rongth@sysucc.org.cn.
Xiao-Dong Su, Phone: +86 20 87343317, Email: suxd@sysucc.org.cn.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Li YG, Gao X. Epidemiologic studies of particulate matter and lung cancer. Chin J Cancer. 2014;33(8):376–380. doi: 10.5732/cjc.014.10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zeng H, Zhang S. The updated incidences and mortalities of major cancers in China 2011. Chin J Cancer. 2015;34:53. doi: 10.1186/s40880-015-0042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman MP, Forman D, Bryant H, Butler J, Rachet B, Maringe C, et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): an analysis of population-based cancer registry data. Lancet. 2011;377(9760):127–138. doi: 10.1016/S0140-6736(10)62231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies NJ, Batehup L, Thomas R. The role of diet and physical activity in breast, colorectal, and prostate cancer survivorship: a review of the literature. Br J Cancer. 2011;105(Suppl 1):S52–S73. doi: 10.1038/bjc.2011.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23(7):1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Dong J, Sun K, Zhao L, Zhao F, Wang L, et al. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132(5):1162–1169. doi: 10.1002/ijc.27719. [DOI] [PubMed] [Google Scholar]
- 8.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 9.Knekt P, Heliovaara M, Rissanen A, Aromaa A, Seppanen R, Teppo L, et al. Leanness and lung-cancer risk. Int J Cancer. 1991;49(2):208–213. doi: 10.1002/ijc.2910490211. [DOI] [PubMed] [Google Scholar]
- 10.Smith L, Brinton LA, Spitz MR, Lam TK, Park Y, Hollenbeck AR, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104(10):778–789. doi: 10.1093/jnci/djs179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jee SH, Yun JE, Park EJ, Cho ER, Park IS, Sull JW, et al. Body mass index and cancer risk in Korean men and women. Int J Cancer. 2008;123(8):1892–1896. doi: 10.1002/ijc.23719. [DOI] [PubMed] [Google Scholar]
- 12.Kollarova H, Machova L, Horakova D, Cizek L, Janoutova G, Janout V. Is obesity a preventive factor for lung cancer? Neoplasma. 2008;55(1):71–73. [PubMed] [Google Scholar]
- 13.Yang L, Yang G, Zhou M, Smith M, Ge H, Boreham J, et al. Body mass index and mortality from lung cancer in smokers and nonsmokers: a nationally representative prospective study of 220,000 men in China. Int J Cancer. 2009;125(9):2136–2143. doi: 10.1002/ijc.24527. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Cheung MC, Pedroso FE, Byrne MM, Koniaris LG, Zimmers TA. Obesity and weight loss at presentation of lung cancer are associated with opposite effects on survival. J Surg Res. 2011;170(1):e75–e83. doi: 10.1016/j.jss.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, et al. Body-mass index and cancer mortality in the Asia-Pacific cohort studies collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11(8):741–752. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leung CC, Lam TH, Yew WW, Chan WM, Law WS, Tam CM. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40(1):174–182. doi: 10.1093/ije/dyq134. [DOI] [PubMed] [Google Scholar]
- 17.Hsu CL, Chen KY, Shih JY, Ho CC, Yang CH, Yu CJ, et al. Advanced non-small cell lung cancer in patients aged 45 years or younger: outcomes and prognostic factors. BMC Cancer. 2012;12:241. doi: 10.1186/1471-2407-12-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 19.World Health Organization Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:1–253. [PubMed] [Google Scholar]
- 20.World Health Organization Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 21.Koh WP, Yuan JM, Wang R, Lee HP, Yu MC. Body mass index and smoking-related lung cancer risk in the Singapore Chinese health study. Br J Cancer. 2010;102(3):610–614. doi: 10.1038/sj.bjc.6605496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 23.Martin-Ucar AE, Nicum R, Oey I, Edwards JG, Waller DA. En-bloc chest wall and lung resection for non-small cell lung cancer. Predictors of 60-day non-cancer related mortality. Eur J Cardiothorac Surg. 2003;23(6):859–864. doi: 10.1016/S1010-7940(03)00120-9. [DOI] [PubMed] [Google Scholar]
- 24.Tewari N, Martin-Ucar AE, Black E, Beggs L, Beggs FD, Duffy JP, et al. Nutritional status affects long term survival after lobectomy for lung cancer. Lung Cancer. 2007;57(3):389–394. doi: 10.1016/j.lungcan.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Warwick R, Shackcloth M, Mediratta N, Page R, McShane J, Shaw M, et al. Female sex and long-term survival post curative resection for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2013;44(4):624–630. doi: 10.1093/ejcts/ezt139. [DOI] [PubMed] [Google Scholar]
- 26.Sidney S, Friedman GD, Siegelaub AB. Thinness and mortality. Am J Public Health. 1987;77(3):317–322. doi: 10.2105/AJPH.77.3.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrison RJ, Feinleib M, Castelli WP, McNamara PM. Cigarette smoking as a confounder of the relationship between relative weight and long-term mortality. The Framingham heart study. JAMA. 1983;249(16):2199–2203. doi: 10.1001/jama.1983.03330400045023. [DOI] [PubMed] [Google Scholar]
- 28.Brennan P, McKay J, Moore L, Zaridze D, Mukeria A, Szeszenia-Dabrowska N, et al. Obesity and cancer: Mendelian randomization approach utilizing the FTO genotype. Int J Epidemiol. 2009;38(4):971–975. doi: 10.1093/ije/dyp162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316(5826):889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]


