Abstract
Traditionally, researchers have believed that axons are highly dependent on their cell bodies for long-term survival. However, recent studies point to the existence of axon-autonomous mechanism(s) that regulate rapid axon degeneration after axotomy. Here, we review the cellular and molecular events that underlie this process, termed Wallerian degeneration. We describe the biphasic nature of axon degeneration after axotomy and our current understanding of how WldS—an extraordinary protein formed by fusing a Ube4b sequence to Nmnat1—acts to protect severed axons. Interestingly, the neuroprotective effects of WldS span all species tested, which suggests that there is an ancient, WldS-sensitive axon destruction program. Recent studies with WldS also reveal that Wallerian degeneration is genetically related to several dying back axonopathies, thus arguing that Wallerian degeneration can serve as a useful model to understand, and potentially treat, axon degeneration in diverse traumatic or disease contexts.
Keywords: axon, dying back disorder, axonal transport, Wallerian-like degeneration
INTRODUCTION
Axons are huge cellular structures. If a Volkswagen Beetle sprouted a tail proportional to the length of a human motor axon, it would be ~20 miles (or 30 km) long. Maintaining such an enormous cellular outgrowth is a major challenge for the nervous system, and it is accomplished through the combined support of neuronal cell bodies and axon-associated glial cells. Without the delivery of materials from cell bodies by axonal transport (De Vos et al. 2008), axons undergo Wallerian degeneration (Coleman 2005); and without glial support in vivo, axons also degenerate (Nave & Trapp 2008). Consequently, axon and synapse loss is increasingly recognized as a major contributor to neurodegenerative disease.
Despite the importance of extra-axonal support for long-term survival, axons are now thought to initiate their own degeneration when these systems fail. Moreover, the trigger is not a general lack of nutrients. Injured axons appear to self-destruct through a regulatable or active process (Buckmaster et al. 1995, Raff et al. 2002) that is distinct from apoptosis (Burne et al. 1996, Deckwerth & Johnson 1994, Finn et al. 2000). Related mechanisms are triggered in some dying back axonopathies that raise the prospect of intervention (Ferri et al. 2003, Mi et al. 2005, Samsam et al. 2003). All of this has come to light because of the fortuitous discovery of the slow Wallerian degeneration mutant mouse (WldS) (Lunn et al. 1989), in which axon stumps that are distal to an injury survive ten times longer than normal. Over the past decade, advances in this intriguing and sometimes controversial field have begun to shed light on a novel form of neuroprotection (Araki et al. 2004, Avery et al. 2009, Beirowski et al. 2009, Conforti et al. 2009, Mack et al. 2001, Sasaki et al. 2009b, Yahata et al. 2009).
In this review, we highlight the basic cell biology of Wallerian degeneration and our current understanding of the mechanism by which WldS delays injury-induced axon degeneration. Then, we review the relationship between Wallerian degeneration and central and peripheral neuropathies as defined by the use of WldS as a tool to block Wallerian-like degeneration. Next, we examine new opportunities created by transferring the WldS phenotype to other species. Finally, we discuss several outstanding questions, which include the identity of the molecular trigger for Wallerian degeneration, and we discuss future steps in understanding how WldS protects axons.
WALLERIAN DEGENERATION
Wallerian degeneration is classically defined as the degeneration of axons distal to an injury, following Augustus Waller’s original nerve transection experiments (Waller 1850). Here, we focus primarily on axonal events, which culminate in the granular disintegration of the axonal cytoskeleton and axon fragmentation that leaves characteristic myelin ovoids behind (Figure 1). However, the glial and macrophage clearance of degenerating axons, which we touch on briefly, is also an important part of Wallerian degeneration (Vargas & Barres 2006). Similar processes occur in unmyelinated axons in mammals and invertebrates (Avery et al. 2009, Ayaz et al. 2008, Macdonald et al. 2006) and in the mammalian central nervous system (CNS). CNS axons exhibit focal swellings that are many times wider than a normal axon many hours before axons fragment (Beirowski et al. 2010, Cajal 1928) and the slower clearance of axonal debris may contribute to the poor regenerative environment of the CNS (Vargas & Barres 2006).
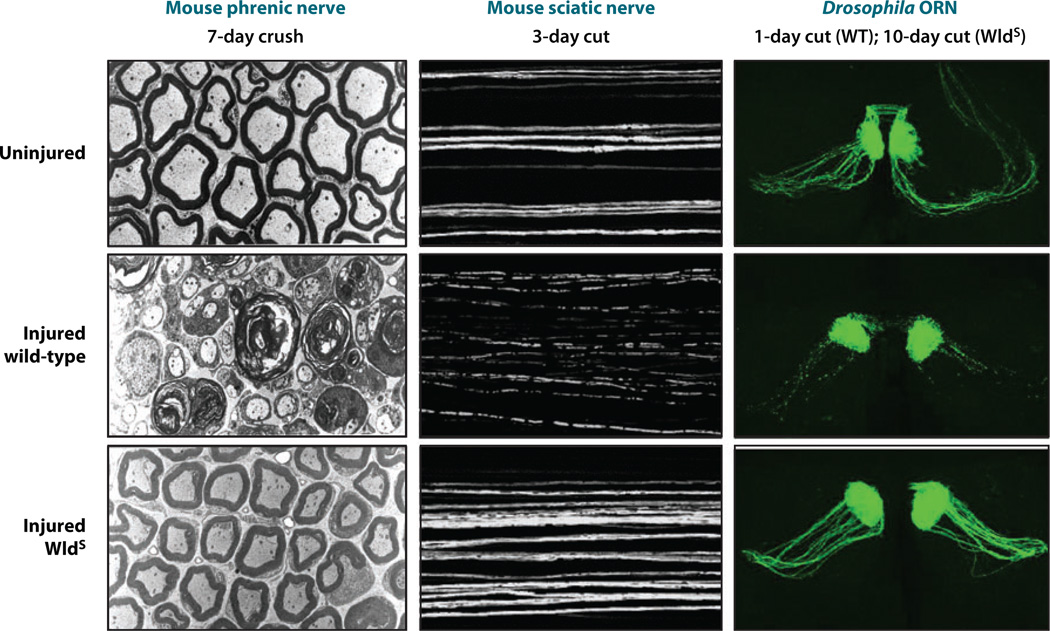
Figure 1.
Wallerian degeneration in wild-type axons and preservation in WldS. Injured wild-type axons (middle row) exhibit granular disintegration of the cytoskeleton seen in electron microscopy (left column) and fragmentation visualized by fluorescence microscopy (middle and right columns). Cytoskeletal integrity, unswollen mitochondria, and axon continuity are preserved by the WldS gene in each case (bottom row). Note the remarkable consistency of Wallerian degeneration and the neuroprotective WldS phenotype between mice and Drosophila. ORN, olfactory receptor neuron. Left column from Brown et al. (1994); reprinted with permission from Wiley-Blackwell. Middle column from Conforti et al. (2007b); reprinted by permission from Macmillan Publishers Ltd., Nature Publishing Group.
SLOW WALLERIAN DEGENERATION
The extended survival of WldS axons without their cell bodies has fundamentally changed our view of axon degeneration. WldS transected axons in the sciatic nerve survive for over two weeks, compared to approximately 1.5 days in wild-type mice, and conduct evoked action potentials when stimulated (Lunn et al. 1989) (Figure 1). Transected WldS axons in the CNS survive for similar extended periods (Perry et al. 1991). Surprisingly, WldS mice are viable and show normal motor function, although they exhibit a secondary delay in axon regeneration (Brown et al. 1994).
Transected WldS axons eventually degenerate in a process that is more atrophic and gradual than the sudden fragmentation that characterizes wild-type axons (Beirowski et al. 2005). This may reflect the gradual depletion of structural proteins from long-term anucleated axons. Thus, rapid Wallerian degeneration in wild-type nerves may be an active or at least regulated process, similar to apoptosis in principle.
WldS is a dose-dependent, semidominant phenotype that is inherited through a single locus (Mack et al. 2001, Perry et al. 1990b). It arose by spontaneous mutation at Harlan UK (then Harlan Olac, hence the original name C57BL/6/Ola) and was discovered by chance after it became homozygous (Lunn et al. 1989). The precise genetic background for WldS is uncertain (Lyon et al. 1993; V.H. Perry, personal communication), and there is further genomic divergence from C57BL/6 (A.L. Wilbrey, J.W. Tsao, and M.P. Coleman, manuscript in preparation).
The phenotype is intrinsic to nerves rather than macrophages (Perry et al. 1990a) and to axons rather than glia (Glass et al. 1993). In Schwann cell grafts between WldS and C57BL/6, host axons rather than donor Schwann cells determine the rate of degeneration (Glass et al. 1993); and primary neuronal cultures that lack glia show a remarkably similar delay in Wallerian degeneration after neurite transection, although neurites of both genotypes degenerate faster than in vivo (Buckmaster et al. 1995, Glass et al. 1993). Moreover, neuron-specific, but not glial, expression of the WldS gene confers the phenotype in Drosophila (Hoopfer et al. 2006, Macdonald et al. 2006). Such an axon-specific effect on Wallerian degeneration is quite unique. Other mutations have been reported to influence Wallerian degeneration but seem to act on Schwann cell or macrophage responses rather than on axons (Keilhoff et al. 2002, Levy et al. 2001, Lopez-Vales et al. 2008, Narciso et al. 2009, Ramaglia et al. 2007).
The use of WldS mice as a genetic tool to explore the basis of cellular destruction pathways shows that neurodegenerative mechanisms are highly compartmentalized. Despite its robust effect on axon degeneration, WldS has no effect on apoptotic death of the cell soma, either in NGF-deprived sympathetic neuronal cultures or in axotomized motor neurons (Adalbert et al. 2006, Deckwerth & Johnson 1994), and no phenotypic change in any other cell type has been reported. Conversely, neither Bcl-2 overexpression nor Bax and Bak deletion alters Wallerian degeneration (Burne et al. 1996, Whitmore et al. 2003), and caspase 3 activation is neither detected in nor required for rapid Wallerian degeneration (Finn et al. 2000). Similar experiments established that axons in several disease models also die by nonapoptotic mechanisms. Bcl-2 overexpression and Bax deletion, respectively, rescue cell bodies in pmn mice and the DBA/2J glaucoma model but have no effect on axon degeneration (Libby et al. 2005, Sagot et al. 1995). WldS rescues axons in both cases (Ferri et al. 2003, Howell et al. 2007).
Synaptic terminals are also protected by WldS but act as another, partially-distinct compartment with respect to the timing of degeneration after injury (Gillingwater et al. 2002). Transected WldS motor axons support evoked neurotransmitter release at intact neuromuscular junctions for approximately five days compared to the usual 12–20 h (Ribchester et al. 1995), and CNS synapses are also protected (Gillingwater et al. 2006a). However, NMJ denervation occurs far sooner in wild-type and WldS animals than degeneration of the axon trunk. Moreover, neuromuscular synapse preservation is lost in young adult WldS mice without any change in WldS expression, whereas WldS continues to preserve injured axon trunks (Gillingwater et al. 2002). Thus axonal and synaptic survival are both enhanced by WldS, but either the rate or the nature of the pathways involved differs.
New ENU mutant mice with enhanced synapse protection (Wong et al. 2009) and targeting of Nmnat1 to nerve terminals (E. Babetto, B. Beirowski, L. Janeckova, R. Brown, D. Thomson, R.R. Ribchester, M.P. Coleman, manuscript submitted) should shed more light on events at synapses. Interestingly, developmental synapse elimination is also unaltered in WldS mice (Parson et al. 1997), one of several findings that now distinguish developmental axon and synapse loss from injury-induced loss (Bishop et al. 2004, Hoopfer et al. 2006).
WALLERIAN-LIKE DEGENERATION
One central question is whether Wallerian degeneration is relevant to neurodegenerative disease. This occurred immediately to Waller, who stated: “It is particularly with reference to nervous diseases that it will be most desirable to extend these researches” (Waller 1850, p. 423). Decades later, dying-back-type axon degeneration in some peripheral nerve disorders was termed Wallerian-like degeneration based on morphological similarities (Cavanagh 1979, Griffin et al. 1996).
However, axon transection is rare in clinical neuroscience. Spinal injury and traumatic brain injury usually contuse and stretch axons respectively, which result in secondary axon interruption hours or days later. Peripheral nerves may be cut during surgery or wounding; but nerve damage by chronic pressure or metabolic, toxic, or inherited disorders is far more common. Axon transection has been observed directly in animal and cellular models of multiple sclerosis (Neumann et al. 2002; M. Kerschensteiner, personal communication), but whether this is the main mechanism in patients remains unclear. Axon endbulbs (Ferguson et al. 1997, Trapp et al. 1998) could alternatively begin as en passant swellings that precipitate distal axon degeneration (Coleman 2005).
Although axon transection is rare, the disruption of axonal transport is extremely common and also isolates distal axons from cell bodies. As a tool to block Wallerian degeneration, WldS mice made it possible to test the hypothesis that similar mechanisms are triggered in noninjury disorders. Dying back follows a Wallerian-like mechanism in some motor neuron disease and peripheral neuropathy models and in nitric oxide damage (Alvarez et al. 2008, Ferri et al. 2003, Samsam et al. 2003, Wang et al. 2002). CNS studies extended this mechanism to models of Parkinson’s disease, glaucoma, multiple sclerosis, and gracile axonal dystrophy (Beirowski et al. 2008, Hasbani & O’Malley 2006, Howell et al. 2007, Kaneko et al. 2006, Mi et al. 2005, Sajadi et al. 2004); and in primary culture, the neurotoxin vincristine and protein synthesis blockade also trigger Wallerian-like degeneration (Gilley & Coleman 2010, Wang et al. 2000). Thus, similar to using Bcl-2 to define apoptotic cell death, WldS sensitivity now provides a genetic definition for Wallerian-like degeneration.
WldS does not substantially block pathology in some models of amyotrophic lateral sclerosis or spinal muscular atrophy (Fischer et al. 2005, Kariya et al. 2009, Rose et al. 2008, Velde et al. 2004), whereas axonal swellings precede fragmentation by months in mouse models of familial Alzheimer’s disease (Adalbert et al. 2009, Spires et al. 2005). Thus, Wallerian-like degeneration may not be the only outcome when transport is impaired. This may reflect a loss of different transport cargoes in different disorders or the reversion of synapse degeneration to wild type in older animals (Gillingwater et al. 2002). New transgenic mice with stronger synapse protection could help distinguish these possibilities (Beirowski et al. 2009).
THE WLDS GENE AND PROTEIN
The WldS gene was mapped to mouse chromosome 4 (Lyon et al. 1993), in which an unusual genomic rearrangement brings two endogenous genes together. Their mRNAs splice to encode an in-frame fusion protein that is absent in wild-type mice (Figure 2) (Coleman et al. 1998, Conforti et al. 2000). The expression of this protein in transgenic mice replicates the WldS phenotype that identifies it as the WldS protein (Mack et al. 2001) (Figure 2), and the murine cDNA delays axon degeneration in rat, fly, and cell culture models (Adalbert et al. 2005, Araki et al. 2004, Hoopfer et al. 2006, Macdonald et al. 2006, Wang et al. 2001).
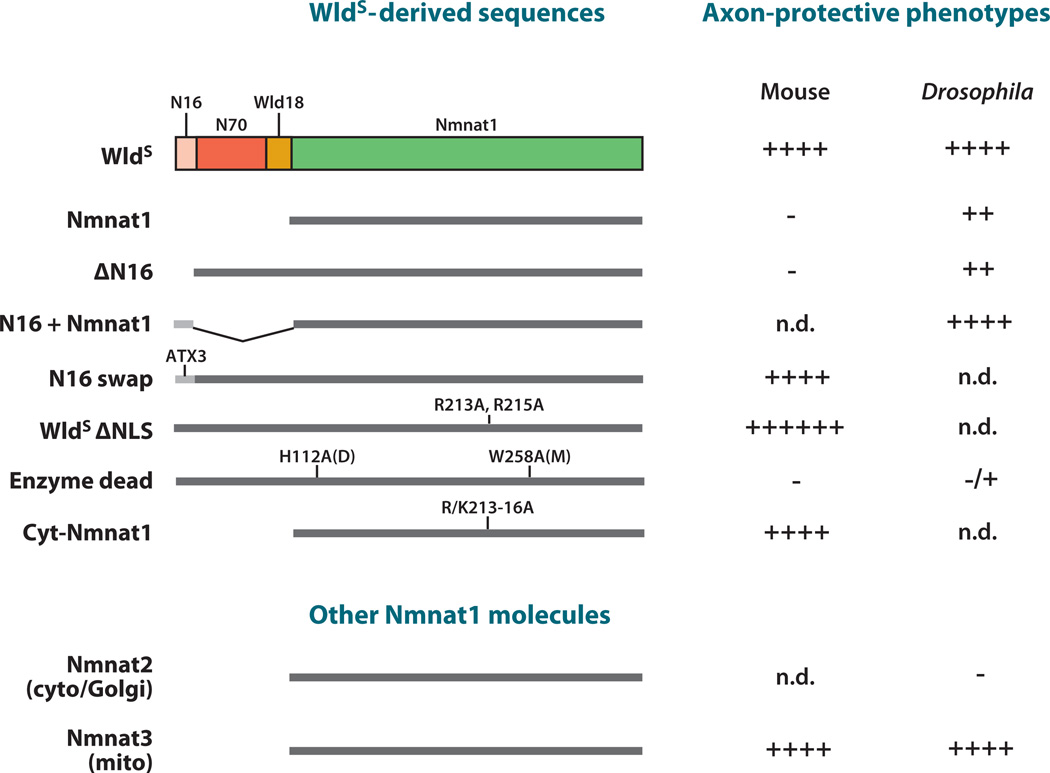
Figure 2.
Axon protection mediated by WldS, WldS domains, and Nmnat molecules. The in vivo protective effects have been studied extensively in mouse and Drosophila. (Top) WldS-derived sequences represent constructs where specific domains of WldS were deleted or mutated. (Bottom) Other Nmnat molecules are mouse Nmnat2 and Nmnat3. Protection in either mouse or Drosophila is shown to the right. Protection and its relative strength are indicated by (+), a lack of protection is indicated by (−), and those not determined in vivo are indicated by n.d. Point mutations or domain swaps are shown above the diagram of each molecule, with point mutation positions that refer to their relative position in WldS.
The C-terminal 285 amino acids comprise the complete protein sequence of nicotinamide mononucleotide adenylyltransferase 1 (Nmnat1), a key protein of the NAD+ salvage pathway in mammals. Nmnat1 normally resides predominantly or exclusively in nuclei (Magni et al. 2004), where WldS is also abundant (Mack et al. 2001), and new roles for NAD+ are emerging (Pollak et al. 2007). Other Nmnat isoforms synthesize NAD+ in mitochondria and the Golgi apparatus (Berger et al. 2005, Raffaelli et al. 2002). The WldS protein synthesizes NAD+ from nicotinamide mononucleotide and ATP but does not alter basal NAD+ levels (Araki et al. 2004, Mack et al. 2001), probably owing to rapid NAD+ catabolism (Pollak et al. 2007). The N-terminal 70 amino acids (N70) of WldS are derived from the N-terminus of Ube4b (or Ufd2a), an E4-type ubiquitin ligase that can add multiubiquitin chains to substrates of the ubiquitin fusion degradation (Ufd) pathway (Hatakeyama et al. 2001, Koegl et al. 1999). Only 6% of the Ube4b sequence is incorporated into WldS, which excludes the catalytic U box. Thus, WldS probably lacks ligase activity, but there is shared protein binding activity (Laser et al. 2006, Morreale et al. 2009). Finally, between the N70 and Nmnat1 sequences lies a unique 18 amino acid sequence generated by a read-through of Nmnat1 5′ UTR, the epitope of the specific Wld18 antibody (Samsam et al. 2003).
GAIN OF FUNCTION
Gain of function appears to be the most likely mechanism for WldS function. The genomic rearrangement retains the endogenous Nmnat1 and Ube4b genes, and the corresponding proteins are expressed at normal levels (Conforti et al. 2007b, Gillingwater et al. 2002). Thus, there is no obvious loss of function of either protein. Regarding a dominant negative mechanism, Nmnat activity is increased in WldS tissue (Mack et al. 2001), and deleting one allele of Nmnat1 does not alter Wallerian degeneration (L. Conforti, N. Smyth, and M.P. Coleman, manuscript in preparation), whereas haploin-sufficiency for Ube4b causes axon pathology rather than axon protection (Kaneko-Oshikawa et al. 2005). For a gain of function mechanism, the key question remains whether this is an entirely novel function or whether WldS strengthens or mimics the function of an endogenous protein. Recent data support the latter model, indicating that WldS substitutes for Nmnat2, an essential axonal protein that is rapidly lost after axon injury (Gilley & Coleman 2010).
DEFINING PROTECTIVE DOMAINS IN THE MOLECULE AND THE CELL
Which domains of WldS are essential for the protection of severed axons? This question has been a central focus of the field for the past five years, but attempts to answer it have raised several controversies. Studies in primary culture have generally produced less consistent results regarding whether, where, and how WldS and its constituent domains protect severed axons (Araki et al. 2004, Conforti et al. 2007b, Wang et al. 2005) than studies in vivo. Structure-function analyses in mice and Drosophila (Avery et al. 2009; Beirowski et al. 2009; Conforti et al. 2007b, 2009; Sasaki et al. 2009a; Yahata et al. 2009) are now converging on a model in which the combinatorial activity of two key domains of WldS acts somewhere outside the nucleus to confer maximal axon protection (Figure 2).
Theoretically, WldS could promote axon protection through N70, Wld18, Nmnat1, or some combination of these domains. In vitro data argued that Nmnat1 could protect severed neurites in primary culture, although to a significantly lower degree than WldS (Araki et al. 2004, Sasaki et al. 2009a, Wang et al. 2005). However, overexpressed Nmnat1 is not sufficient to protect severed axons in transgenic mice (Conforti et al. 2007b), whether they are driven by the same β actin promoter used to identify WldS previously (Mack et al. 2001) or they express up to threefold higher levels using the Prp promoter (Yahata et al. 2009).
Can Nmnat1 protect axons in vivo in any context? In Drosophila, the expression of mouse Nmnat1 in ORNs using the Gal4/UAS binary expression system resulted in the strong protection of severed axons (Macdonald et al. 2006), but, importantly, the protection it afforded was consistently weaker and lasted for a shorter period when compared to WldS (Avery et al. 2009). Thus, Nmnat1 is likely an important part of WldS neuroprotective function, but why is there a discrepancy in Nmnat1-mediated protection between flies and mice? The most plausible explanation is that Gal4/UAS expresses extremely high levels of Nmnat1 in Drosophila ORNs. The Gal4 driver line used (OR22a-Gal4) is quite strong, which results in high levels of Gal4, and the expression of Nmnat1 (i.e., UAS-Nmnat1) is further amplified because Gal4 is an efficient transcriptional activator of UAS-regulated target genes. Perhaps experiments aimed at dramatically increasing the levels of Nmnat1 in mouse axons might ultimately provide some level of axon protection in the mouse model. Alternatively, these discrepancies may reflect differences in axon length, diameter, or other characteristics among Drosophila and mice that affect the initiation and execution of Wallerian degeneration.
Although wild-type Nmnat1 is not sufficient for the robust protection of severed axons in mice, its activity is clearly an essential part of the protective action of WldS. Three groups recently generated animals that express WldS variants in which the enzymatic activity of Nmnat1 was disrupted by mutation (Conforti et al. 2009, Yahata et al. 2009). In each case, the neuroprotective effects of WldS were severely reduced (Avery et al. 2009) or abolished (Conforti et al. 2009, Yahata et al. 2009), this time consistent with primary culture results (Araki et al. 2004, Jia et al. 2007). These results are important because they implicate Nmnat1 enzymatic activity in WldS-dependent axon protection and because they begin to address a second possible role, that of a chaperone, for Nmnat1 in axon protection. An interesting recent study in Drosophila identified mutants in the sole fly Nmnat gene (dnmnat), whose neurons appear to develop normally, which extend axons to the appropriate targets but then exhibit age- and activity-dependent degeneration (Zhai et al. 2006). These data raised the intriguing possibility that basic axon integrity in the mature nervous system might be regulated by constitutive dNmnat-dependent suppression of neuronal degeneration.
Surprisingly, in rescue experiments, dNmnat enzymatic activity was found to be dispensable for the rescue of dnmnat mutant neurodegenerative phenotypes (Zhai et al. 2006). A subsequent study proposed a novel chaperone-like role for dNmnat and mammalian Nmnat3 (e.g., in the refolding of denatured proteins), independent of NAD+ biosynthetic activity; and the authors in turn proposed that this novel Nmnat chaperone activity might explain some aspects of the WldS-dependent protection of severed axons (Zhai et al. 2008). Such a chaperone-like role, which could stabilize axonal proteins, might fit nicely with the neuroprotective effects observed in WldS mice, which essentially express a modified Nmnat1.
However, as mentioned above, the mutation of either the ATP binding site of Nmnat1 (Avery et al. 2009) or the NMN+ binding site (Conforti et al. 2009, Yahata et al. 2009), which would be expected to leave chaperone function intact, potently blocked the ability of WldS to protect severed axons. Is Nmnat-dependent chaperone activity important for WldS-mediated axon protection? One possibility is that dNmnat and Nmnat3 chaperone-like functions have nothing to do with WldS-mediated axon protection and are more specific to the neurodegenerative phenotypes observed in dnmnat mutants. Alternatively, WldS may have two essential functions: to generate NAD+ or another biosynthetic product that would require enzymatic activity and to act as a chaperone. If both activities are essential, then the disruption of either should suppress the axon protective effects of WldS, as found in transgenic mice and flies. We can conclude that Nmnat-dependent chaperone activity cannot be the sole role for the Nmnat1 domain of WldS.
The weaker axon protective effect of Nmnat1 in flies and mice suggests that other portions of WldS are essential for WldS-like levels of axon protection, through affecting either its localization or activity. A protein interaction site within the N-terminal 16 amino acids (N16), which is derived from Ube4b, coimmunoprecipitates valosin containing protein (VCP/p97) from mouse brain homogenates (Laser et al. 2006). VCP is a AAA-ATPase with key roles in the UPS and membrane fusion (Wang et al. 2004) and is the only abundant, direct binding partner precipitated by this region.
Subsequent work has shown that N16 is necessary and sufficient to explain the differences in protective effects between WldS and Nmnat1 in vivo. The deletion of N16 from WldS completely suppresses the axon protection afforded by WldS in mice (Conforti et al. 2009) and greatly weakens the protection of axons in Drosophila to a level found by expressing Nmnat1 alone (Figure 2) (Avery et al. 2009). Moreover, fusing N16 directly to Nmnat1 results in levels of axon protection that are indistinguishable from WldS (Avery et al. 2009). Does N16 exert its effects on axon preservation through VCP? Two in vivo experiments support this notion. First, replacing N16 in WldS with a well-characterized VCP-binding motif from ataxin 3 (Morreale et al. 2009), which shares only five amino acids with N16, restores WldS-like axon protection in mice (Conforti et al. 2009). Second, RNAi knockdown of fly VCP is sufficient to suppress axon protection by WldS to levels indistinguishable from those afforded by Nmnat1 alone. Together, these data argue strongly that N16, likely working through VCP, and an enzymatically active Nmnat1, are the critical domains and essential activities for WldS-like levels of axon protection.
A second major question regarding WldS function relates to its site of action. Is it functioning in the nucleus or elsewhere in the cell? Clarifying this point is critical for understanding precisely how WldS can so potently suppress Wallerian degeneration. The striking nuclear localization of WldS has led to several studies of expression patterns in WldS versus wild-type neurons (Gillingwater et al. 2006b, Simonin et al. 2007), in the hope of identifying changes in the expression of key genes that modulate axon autodestruction (see below). However, more recent careful analysis of the localization of WldS and potential extranuclear sites of action proposes that WldS exerts its neuroprotective effects outside the nucleus.
Nmnat1 contains a strong nuclear localization sequence (NLS), which may account for the nuclear localization of WldS in vivo. If WldS is required in the nucleus, then deleting the NLS from Nmnat1 should weaken its protective effects. In striking contrast, the mutation of the Nmnat1 NLS from WldS doubled the latest timepoint when surviving axons could be identified and greatly enhanced synaptic protection especially in older mice (Beirowski et al. 2009). Moreover, although the expression of Nmnat1 alone in mice fails to suppress Wallerian degeneration (Conforti et al. 2007b, Yahata et al. 2009), the generation of mice that harbor a cytoplasmic mutant of Nmnat1 resulted in robust axon protection (Sasaki et al. 2009a), although the relative contributions of cytoplasmic targeting and high expression levels in these mice remain unclear. Thus, excluding WldS and Nmnat1 from the nucleus makes them more protective of severed axons. This observation hints at a possible role for N16 in relocalizing Nmnat1 activity outside the nucleus to another cellular location. Indeed, a careful analysis of WldS expression in peripheral nerves revealed low but detectable levels of expression (Beirowski et al. 2009, Yahata et al. 2009), consistent with a potential non-nuclear site of action for WldS in mice.
OUTSTANDING QUESTIONS ON THE WLDS AXON PROTECTIVE MECHANISM
What is the Cellular Site of WLDS Action?
The discussion above establishes a nonnuclear site of action for WldS; now, we need to identify the site. One approach is to distinguish between roles in the axon and soma, and another approach is to identify the appropriate organelle(s) or protein complex. This may be less straightforward than resolving nuclear or cytoplasmic actions. WldS localizes to multiple internal membranous organelles (Beirowski et al. 2009, Yahata et al. 2009). Mitochondria have attracted particular interest because injured axons are protected by overexpressed Nmnat3, the mitochondrial isoform (Avery et al. 2009, Yahata et al. 2009). However, overexpressed proteins may have ectopic locations, and the experience with nuclei reminds us that an observable location does not identify the site of action (Beirowski et al. 2009). Thus, other locations still need to be considered.
Other possible sites include the Golgi apparatus and the ER, particularly because these are sites where VCP is abundant. VCP binding redistributes WldS within nuclei, which suggests that cytoplasmic WldS is likely to migrate to sites where VCP is abundant (Wilbrey et al. 2008), although this also includes mitochondria (Braun et al. 2006). To complicate matters further, mitochondria are rapidly transported in axons (Misgeld et al. 2007), have close interactions with the ER that may influence axon survival (Merkwirth & Langer 2008), and exchange NAD+ with their surroundings (Todisco et al. 2006). Thus, any NAD+ produced within mitochondria could act on nearby structures to protect axons or vice versa. WldS becomes ineffective when targeted to the internal surface of the plasma membrane, so this may not be the site of action (Avery et al. 2009), but distinguishing between other sites could be far more complex.
Is NAD+ a Protective WLDS Product?
An alternative route forward is to identify functions downstream of WldS Nmnat activity. In addition to its long established role in bioenergetic metabolism, NAD+ is also a substrate for protein deacetylation by sirtuins, for synthesis of cyclic ADP ribose and ADP ribose, both regulators of internal calcium stores, and for mono- and poly-ADP ribosylation of proteins (Hassa et al. 2006, Pollak et al. 2007). It also potentiates the response of sodium-activated potassium channels to sodium (Tamsett et al. 2009), although an action of WldS at plasma membranes seems unlikely (see above). NAD+ is also used to synthesize NADP+, whose reduced form has roles in detoxification and oxidative defense (Pollak et al. 2007). Identifying one of these as an important downstream step could help resolve the site of action because rapid NAD+ catabolism limits its long-range diffusion (Pollak et al. 2007).
However, despite agreement that WldS needs Nmnat activity to protect axons (above), it is less clear whether NAD+ is the protective enzyme product. No increase in NAD+ is detectable with WldS (Araki et al. 2004, Mack et al. 2001) or when Nmnat activity is raised more than 15-fold (Sasaki et al. 2009a). Knockdown or inhibition of Nampt, the rate-limiting enzyme in the NAD+ salvage pathway, failed to revert WldS phenotype in one study (Sasaki et al. 2009b) and reverted it only partially in another (Conforti et al. 2009). Conversely, increasing cellular NAD+ by blocking NAD+ catabolyzing enzyme CD38 does not confer a WldS phenotype (Sasaki et al. 2009b; A.L. Wilbrey & M.P. Coleman, unpublished observations). One proposal is that Nmnat activity decreases reactive oxygen species in mitochondria because it protects axons from rotenone-induced damage without restoring normal ATP levels (Press & Milbrandt 2008). Another proposal is that Nmnat catalyzes the reverse reaction under stress conditions, which generates an emergency supply of ATP (Yahata et al. 2009), although this appears unlikely to preserve axons for several weeks. Many of these observations are also consistent with NAD+ acting at a highly localized site, but clearly, alternative substrates and products of Nmnat isoforms (Hassa et al. 2006, Sorci et al. 2007) need to be considered alongside NAD+ as candidates for the axon protective mechanism.
THE MOLECULAR TRIGGER FOR WALLERIAN DEGENERATION
To fully understand how WldS, or some Nmnats, delay Wallerian degeneration, we must understand the process they delay. Remarkably, we still do not know the molecular pathway for Wallerian degeneration, a question that far predates WldS mice (Lubinska 1977). Almost all recent studies have focused on overexpressed or modified proteins, including WldS itself, which is absent in wild-type organisms. The results are exciting but do not tell us directly how endogenous proteins behave when a wild-type axon is injured or sick. The “potential to throw light on the normal processes of nerve degeneration” was a major driving force for identifying WldS in the first place (Lyon et al. 1993, p. 9717), and realizing this goal remains a major gap in the field.
However, there are some clues from studying how WldS alters disease models. Two alternative models for how injury triggers Wallerian degeneration are a prodegeneration signal generated at the lesion site (e.g., calcium influx through the cut end) and the absence of a prosurvival signal derived from the cell body. The disease studies clearly show that Wallerian-like processes can be triggered in the complete absence of physical injury. In contrast, there is a strong correlation with disorders of axonal transport (Beirowski et al. 2008, Ferri et al. 2003, Howell et al. 2007, Wang et al. 2005). Non-lethal impairment of protein synthesis also triggers Wallerian-like degeneration (Gilley & Coleman 2010). These observations support the survival factor model and argue against the need for any signal derived from the injury site.
SURVIVAL AND CATASTROPHE
We have much to learn from the biphasic degeneration of wild-type axons. An initial latent phase lasts 36–44 hours after injury in a mouse sciatic nerve, followed by a sudden and catastrophic fragmentation phase (Beirowski et al. 2005, Kerschensteiner et al. 2005, Lubinska 1977). During the latent phase, nodal gaps widen and motor axons lose their terminals (Conforti et al. 2007a, Miledi & Slater 1970), but axon trunks remain continuous and can conduct evoked action potentials (Lunn et al. 1989, Moldovan et al. 2009). Fly axons, zebrafish axons (S. Martin & A. Sagasti, personal communication), and transected neurites in primary culture also show a latent phase, albeit shorter than in mice. Both between and within species, there is a correlation with axon stump length.
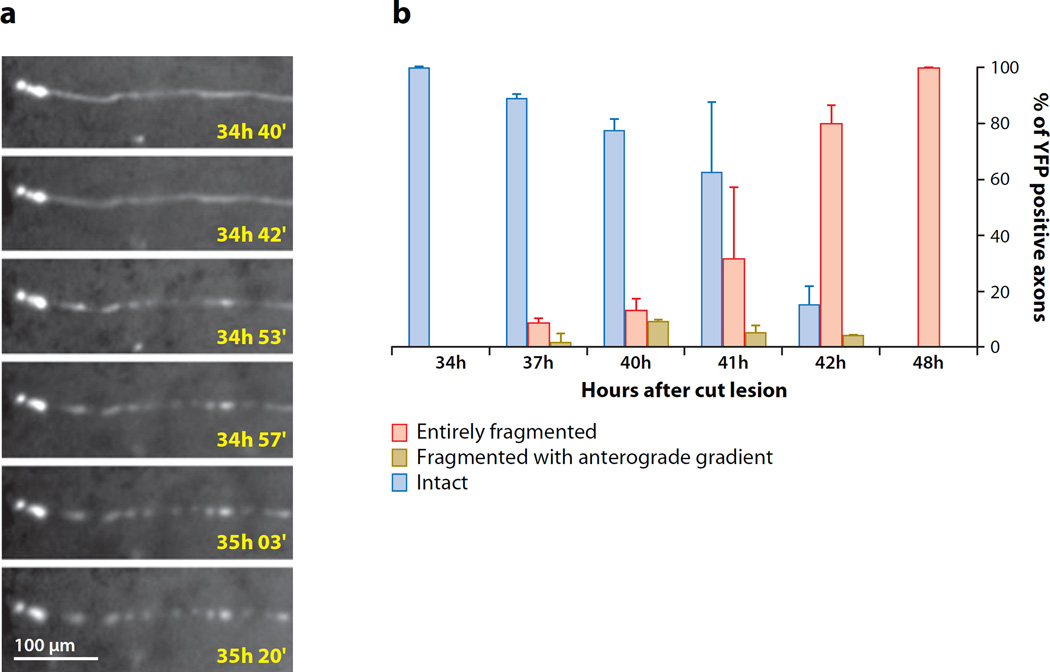
After surviving 1.5 days without a cell body, several centimeters of mouse wild-type axon undergoes catastrophic fragmentation, possibly in one hour (Figure 3) (Beirowski et al. 2005). An equally rapid process, captured in live in vivo imaging, occurs in CNS axons, where there is also more axon swelling (Kerschensteiner et al. 2005). The onset of this fragmentation phase is heterogeneous among axons in the same nerve and depends on intrinsic properties such as axon diameter and extrinsic properties such as temperature (Gamble & Jha 1958, Lubinska 1977).
Figure 3.
Wallerian degeneration in wild-type axons is a biphasic process. (a) Distal stumps of injured wild-type axons in the dorsal column of mouse spinal cord remain continuous for a latent phase of over 34 h before fragmenting over the course of a few minutes. From Kerschensteiner et al. (2005) reprinted by permission from Macmillan Publishers Ltd., Nature Publishing Group. (b) Quantification of fragmented, unfragmented, and partially fragmented wild-type axons in distal sciatic nerve after transection injury shows a similar latent phase followed by fragmentation of all axons in the nerve over the next few hours. The timing of fragmentation is heterogenous within the axon population, but once started, fragmentation is rapid, such that the percentage of partially fragmented axons never exceeds 10% at any one time. From Beirowski et al. (2005) reprinted with permission from BioMed Central.
The model posited to explain these data is strikingly similar to the survival signal discussed above. Lubinska suggested that distal axons require the constant delivery of a trophic factor, anterogradely transported from cell bodies (Lubinska 1977) but partially redistributed by retrograde transport (Lubinska 1982). Injured axons degenerate when this putative factor falls below a threshold level. This can explain why cold temperatures and proteasome inhibition extend the latent phase because these are likely to increase the trophic factor half-life (Gamble & Jha 1958, Macinnis & Campenot 2005). This also explains reports that fragmentation begins at the proximal end of a transected axon stump and progresses distally (Beirowski et al. 2005, Lubinska 1977) because steady degradation of the putative survival factor in distal axons will produce a net anterograde flux, which depletes the survival factor in regions closer to the injury sooner than in distal axons. However, the directionality remains controversial (Beirowski et al. 2005). The most consistent, and perhaps the most important, point is the sudden switch.
Lubinska’s reasons for suggesting a single trophic factor are unclear but probably relate to the simple biphasic kinetics. A sudden switch is best explained by a single event: the depletion of one axonal component. Multiple survival factors with slightly different half-lives and transport kinetics would be expected to produce a more gradual switch.
Any complete model for the molecular mechanism of Wallerian degeneration must explain what is happening during the latent phase and why it ends so suddenly. A complete model for the action of WldS should also explain whether and how the longer latent phase in WldS axons relates to the shorter latent phase in wild type.
WLDS AND SURVIVAL SIGNALING
The key to future progress on this question is to fit the WldS protective mechanism into the survival signal, or trophic factor model, of wild-type axon degeneration. The ability of axons to survive for 2–3 weeks originally cast doubt on the survival signal model and suggested that short-term axon survival is independent of cell bodies. However, these are mutant axons in which one or more axonal components must have been altered prior to any nerve lesion. WldS could not, and indeed does not (Wishart et al. 2007), cause major alterations to the axonal proteome, but if Lubinska’s proposal of a single, endogenous trophic factor is correct, only one or a few changes may be sufficient to circumvent its loss. This might be achieved by the following:
directly adding to the pool of a wild-type survival factor,
delivering an endogenous survival factor in greater quantities to axons,
depleting axons of a factor needed to execute degeneration,
stabilizing a survival factor in injured axons,
promoting local synthesis of an endogenous survival factor in axons, or
substituting for an endogenous survival factor.
Model (a) is clearly not the case. WldS cannot be identical to a wild-type survival factor because it is absent from wild-type cells.
GENE EXPRESSION
Models (b) and (c) rely on altering the supply of other axonal components. This could be achieved by altering their axonal transport or their expression level. WldS has no obvious similarity to any axonal transport motor or regulator, but it is abundant in nuclei (Mack et al. 2001), and several transcripts and proteins are expressed at different levels in WldS and C57BL/6 mice (Chitnis et al. 2007; Gillingwater et al. 2006b; Simonin et al. 2007; Wishart et al. 2007, 2008). Moreover, sirtuin-1, a nuclear NAD+ dependent gene silencing protein, was reported to be required for axon protection by exogenous NAD+ (Araki et al. 2004).
However, no causative link has been established yet between gene expression or proteomic changes and axon protection by WldS, and sirtuins are not required for WldS to protect axons (Avery et al. 2009, Wang et al. 2005). Many of these gene expression changes are also not conserved in WldS rats, and some changes reflect the genomic divergence of WldS and C57BL/6 mice directly (A.L. Wilbrey, J.W. Tsao, M.R. Cookson, and M.P. Coleman, manuscript in preparation). Moreover, the finding that WldS has a cytoplasmic site of action (above) argues against the existence of a mechanism that involves changes in gene expression.
UPS IMPAIRMENT
Model (d), stabilizing a survival factor in injured axons, most clearly fits with a possible impairment of the ubiquitin proteasome system (UPS) (Coleman & Ribchester 2004, Ehlers 2003). Impairing the UPS prolongs the survival of injured axons (Hoopfer et al. 2006, Macinnis & Campenot 2005, Zhai et al. 2003), but it is less clear whether this is how WldS prolongs axon survival. General impairment of the UPS is more likely to make axons sick than protect them. Proteasome inhibition causes neurite degeneration in culture and peripheral neuropathy in humans (Kane et al. 2003, Laser et al. 2003), whereas several mouse axonopathies are caused by UPS impairment (Kaneko-Oshikawa et al. 2005, Saigoh et al. 1999). An efficient UPS appears to be essential for axon health (Coleman & Ribchester 2004); so the robust health of WldS mice, rats, flies, and primary cultures argues against any general UPS impairment.
Interaction between WldS and VCP has the potential to impair specific UPS functions. In addition to Ube4b, several other ubiquitin ligases and the deubiquitinating enzyme ataxin 3 bind VCP at the same site, so WldS could compete for VCP binding with any or all of these proteins (Morreale et al. 2009). However, the VCP binding site is dispensable in the context of modified Nmnat1 or Nmnat3 (Avery et al. 2009, Sasaki et al. 2009a, Yahata et al. 2009); thus, these actions are not needed to protect axons.
LOCAL PROTEIN SYNTHESIS
Model (e) is based on the observation that injured axons elevate local protein synthesis on both sides of a lesion (Court et al. 2008, Perlson et al. 2005), so WldS might protect axons by stimulating the local synthesis of a survival factor. The local synthesis of WldS in axons has also been proposed (Fainzilber & Twiss 2006). However, the protein synthesis machinery appears at the same time in injured WldS and wild-type axons (Court et al. 2008), and the protective capacity of WldS is unabated when protein synthesis is suppressed (Gilley & Coleman 2010). Thus, local synthesis is not required for WldS to protect axons.
SUBSTITUTING FOR A SURVIVAL FACTOR
Model (f) would fit with WldS substituting for an endogenous Nmnat in injured axons. This concept seems implicit in the various Nmnat overexpression studies but has not been phrased this way, perhaps because the nucleus was previously seen as a likely site of action. The recent knowledge that WldS acts outside nuclei, and is present in axons, refocuses attention on what it may do there, consistent with an earlier report of a local site of action (Wang et al. 2005). A key requirement is that the respective Nmnat should also be present in axons. Interestingly, it now seems that Nmnat2, like some other Golgi proteins (Merianda et al. 2009), is present in axons and that its depletion is necessary for rapid Wallerian degeneration in vitro of injured axons and sufficient for Wallerian-like degeneration of uninjured axons (Gilley & Coleman 2010). This observation supports a model in which Nmnat2 is an endogenous axon survival factor, and WldS, a far more stable protein, can substitute for a prolonged period when it is present.
ACTIVE OR PASSIVE
A related discussion is whether Wallerian degeneration is an active process. Active could have several meanings. Unlike apoptosis, Wallerian degeneration does not require de novo protein synthesis (Gilley & Coleman 2010). Proteases are required to execute it because calpains are involved in the later stages (Schlaepfer 1974), but more exciting would be whether the molecular trigger involves a cascade of regulatory proteases. The involvement of caspase 6 in axonal pruning shows that such cascades can regulate axon degeneration (Nikolaev et al. 2009), although no evidence links this to Wallerian degeneration yet. Kinase cascades may also be involved. Deleting dual leucine kinase or applying a partially specific JNK inhibitor modestly delays Wallerian degeneration (Miller et al. 2009), and proteasome inhibition fails to delay Wallerian degeneration if MEK activity is blocked (Macinnis & Campenot 2005). In both cases, it will be interesting to know whether and how this relates to the axon protective effect of WldS. Finally, screens for loss-of-function mutations (below) could be informative. Even a single loss-of-function mutation in a neuronal gene that phenocopied WldS would demonstrate that Wallerian degeneration is indeed an active process, driven by an underlying and definable genetic program.
THE WLDS ZOO: FROM MICE TO FLIES AND BEYOND
For 16 years, WldS could only be studied in mice. In the past five years, this has changed dramatically with the generation of WldS rats and flies (Adalbert et al. 2005, Hoopfer et al. 2006, Macdonald et al. 2006) and recently also zebra fish (S. Martin & A. Sagasti, personal comunication). This replication in diverse species using mouse cDNA raises intriguing evolutionary questions and provides new tools for mechanism and disease studies.
WldS rats were generated for those surgical procedures and disease models in which rats have advantages over mice (Adalbert et al. 2005). For example, ventral root avulsion was used to show that WldS neuroprotection is compartment-specific in vivo (Adalbert et al. 2006), and laser photocoagulation of the trabecular meshwork was used to show axon protection in an induced glaucoma model (Beirowski et al. 2008). WldS rats have also made an unexpected contribution to mechanism studies. Changes that mediate WldS action in mice must also be present in rats, which enables us to eliminate CD200 as a candidate despite its elevation in WldS mice (Chitnis et al. 2007). WldS rats also provide an abundant source of tissue for biochemical and proteomic studies and establish a precedent for a replicating WldS phenotype in other mammals.
The serendipitous identification of WldS mice by Perry and colleagues was a fortunate event that revolutionized how we think about Wallerian degeneration, but the probability of further spontaneous mutants is low. Moreover, beyond the fact that WldS can block it, we know almost nothing about the molecular regulation of Wallerian degeneration. Is there a single or many genetic switches that must be thrown to activate axon autodestruction? What initiates and executes these events? And importantly, what is the mechanism by which WldS impinges upon these pathways?
One powerful approach to these and other outstanding questions that has been lacking in the field is unbiased forward genetic screening for mutants that modify Wallerian degeneration or axon protection by WldS. A recent screen for dominant ENU-induced mutants in mice has produced a new strain that strengthens protection of neuromuscular synapses (Wong et al. 2009), but screening for loss-of-function mutants in mice is labor intensive, expensive, and slow.
Two essential features to define an invertebrate system as tractable (and relevant) for the study of Wallerian degeneration are activation of an axon autodestruction program after axotomy that is morphologically similar to mammalian Wallerian degeneration and genetic regulation of this degenerative event by WldS. The recent development of a simple and reproducible approach for assaying axon degeneration in the adult Drosophila olfactory system allowed for the first detailed in vivo analysis of Wallerian degeneration and WldS function in invertebrate models (Macdonald et al. 2006) (Figure 1). Interestingly, this work revealed that the events that lead to axon destruction indeed appear morphologically similar to Wallerian degeneration in mammals: Severed axons remain intact for a defined latent phase of 6–8 h, subsequently show beading and cytoskeletal breakdown, and finally undergo wholesale fragmentation (Macdonald et al. 2006). Similarly, when PDF+ CNS axons were severed in primary cultures of the adult Drosophila brain, these axons also underwent degeneration within a day (Ayaz et al. 2008). Thus, when Drosophila axons are severed, the distal fragments degenerate after a latent phase and ultimately disappear from the CNS.
Can these injury-induced degenerative events in invertebrate axons be regulated by WldS? Impressively, in the case of Drosophila adult ORNs, mouse WldS suppresses Wallerian degeneration for >3 weeks after injury (Avery et al. 2009, Macdonald et al. 2006). Moreover, a recent structure-function study of WldS, Nmnat1, Nmnat2, and Nmnat3 in Drosophila (Avery et al. 2009) led to results strikingly similar to those found in mammalian systems with similar molecules (Conforti et al. 2009, Yahata et al. 2009) (Figure 2). Together, these observations argue strongly that the cellular and molecular mechanisms that mediate axon autodestruction are ancient features of neuronal cell types and are well conserved in mice and flies.
Laser axotomy, and the use of mutants with fragile axons that spontaneously break in response to worm movement, are emerging as useful techniques to sever axons in vivo in C. elegans. Although the focus of this work is exploring mechanisms of regeneration of the proximal stump (Guo et al. 2008; Hammarlund et al. 2007, 2009; Wu et al. 2007; Yanik et al. 2004), we can glean some information regarding degeneration of the distal fragment, which beads, degenerates, and disappears in a manner similar to Wallerian degeneration in mice and flies (Hammarlund et al. 2007, Wu et al. 2007). Whether WldS can suppress this degeneration remains to be determined.
Drosophila and C. elegans appear poised to contribute in major ways to understanding the basic cellular and molecular mechanisms that drive Wallerian degeneration and to further our understanding of how WldS protects severed axons. Foremost, these organisms are highly amenable to rapid forward genetic analysis, which allows for straightforward genetic screens for Wallerian degeneration mutants or modifiers of WldS-function. Exploiting this opportunity, along with other tools available in these systems such as genetically-encoded whole-genome RNAi collections, and genetic mosaic approaches to study cell autonomous roles of essential genes should help tremendously in rapidly defining the pathways that promote Wallerian degeneration or WldS function.
Finally, although WldS has been an extremely useful tool to explore the molecular relationship between Wallerian degeneration and mouse models of neurodegenerative disease, forward genetic screens in invertebrates are expected to lead to the identification of new WldS-independent tools that modulate other steps in the Wallerian degeneration pathway. These, in turn, will represent a new battery of genetic reagents with which to reassess this central question and could ultimately lead to the characterization of a core set of axon destruction genes used in diverse degenerative settings.
LESSONS FROM NATURE: DEGENERATION NOT REQUIRED
Do all severed axons have to degenerate? Some invertebrate axons exhibit very slow Wallerian degeneration, even in wild-type organisms (Benbassat & Spira 1994, Nordlander & Singer 1972). This is particularly well studied in crustaceans in which an evoked transmitter release can occur up to one year after axon transection (Parnas et al. 1991). Similarly, in two species of crickets, T. commodus and G. bimaculatus, severed PDF-positive axons survive for up to 90 days after axotomy, and behaviors subject to their control appear to remain intact (Stengl 1995). A few wild-type vertebrate axons show a similar phenotype, usually in extremely large-diameter axons (Zottoli et al. 1987). Unlike injured WldS axons, these axons are typically invaded by hypertrophic adaxonal glia, which are thought to transfer proteins to axons. Similar events may occur in mammalian axons, but this does not appear to contribute to the WldS phenotype (Court et al. 2008). Thus, the means of resisting Wallerian degeneration may be different, although the identities of any proteins supplied by glia would be very interesting for understanding axon survival mechanisms.
All this leads naturally to the question of whether a similar pathway operates in humans. A repeat of the tandem triplication that gave rise to WldS in mice seems unlikely. However, because minor changes to Nmnat1 or Nmnat3 can delay axon degeneration, it will be interesting to determine whether polymorphisms in these proteins, or in homologs of other proteins identified in invertebrate screens, alter susceptibility to neurodegenerative disorders.
ROLES FOR GLIA IN AXON SURVIVAL AND WALLERIAN DEGENERATION
The role of glia in Wallerian degeneration is normally thought to be limited to clearance of axonal debris and myelin ovoids (Vargas & Barres 2006), but could glia also play an instructive role in axon degeneration? To date, no clear genetic evidence links glial phagocytic activity to the destruction of target cell. For example, when glial engulfment activity is blocked by either mutations in the draper gene, which encodes an engulfment receptor required for clearance of axonal debris (Macdonald et al. 2006), or by suppressing endocytosis with a glial-expressed dominant temperature-sensitive dynamin (Doherty et al. 2009; J. Zeigenfuss and M.R. Freeman, unpublished observations), severed axons fragment on schedule. Likewise, although the glial clearance of fragmented axons and myelin debris is much slower in the CNS than in the PNS, CNS axons themselves fragment over a normal time frame (Vargas & Barres 2006). However, a potential role for Schwann cells has been suggested, based on the observation that Wallerian degeneration appears to be nucleated in the middle of each internode following the widening of nodes of Ranvier (Lubinska 1977). Future studies that directly address the precise sequence of these events and the neuron-glia signaling mechanisms involved in axon/myelin clearance should shed light on these important events.
What is the role of glia in supporting severed WldS-expressing axons during the many weeks they linger (without cell body support) in the CNS? It would be amazing if any axons survived on their own during this time without any contribution from glia. More likely, several key cellular components, including high energy metabolites, are passed from glia to surviving axon stumps (Court et al. 2008). Such a requirement might explain why WldS and wild-type axons degenerate more quickly in vitro than in vivo (Buckmaster et al. 1995). A major goal for the field should be to define precisely how axons are nourished by surrounding glia, and how these mechanisms impact axon survival or degeneration in Wallerian degeneration and neurodegenerative disease.
CONCLUSIONS
The past decade has seen a revolution in how we think about axon destruction after injury. We now understand that Wallerian degeneration is a highly regulated process, in which a poorly understood latent phase precedes the rapid and catastrophic destruction of the axon. Amazingly, Wallerian degeneration can be suppressed by a single protein, WldS, and this effect is robust even in diverse species. Two domains of WldS appear critical for its neuroprotective function and likely function at a highly-localized non-nuclear site, but precisely how and where they exert their effects remains unclear. There are several extremely interesting outstanding questions in the field, including the following:
What is the molecular trigger that activates Wallerian degeneration?
What other proteins regulate axon survival/destruction? Do these include executors of an active process as well as inhibitors?
What are the endogenous regulators of Wallerian degeneration in vivo, and what are their roles in axon degeneration disorders?
Where in the cytoplasm/axoplasm does WldS act?
Is NAD+ the Nmnat product responsible for axon survival, and if so, what does it do?
Why does WldS protect axons robustly in some axonopathies but not in others?
Does a WldS phenotype occur in the human population, and does this influence neurodegeneration?
It seems that Waller was right and that studies of Wallerian degeneration are informative about the molecular bases of axon degeneration in diverse injury and disease contexts. Answering the above questions is the next critical step in revealing the pathway and will advance our understanding of axon biology in fundamental ways.
Acknowledgments
This work was supported by funding from the Biotechnology and Biological Sciences Research Council (M.P.C.) and the U.S. National Institutes of Health (grant NS059991 to M.R.F.). M.R.F. is an Early Career Scientist with the Howard Hughes Medical Institute. We would like to thank members of the Coleman laboratory for critical reading of the text and Michelle Avery for Drosophila images in Figure 1.
Glossary
- Axonal transport
the bidirectional active transport of cargoes between axons and cell bodies and along axons
- Wallerian degeneration
the degeneration of an axon distal to a site of injury
- WldS
the slow Wallerian degeneration protein, an aberrant fusion protein that delays degeneration of injured axons by tenfold
- Wallerian-like degeneration
the degeneration of axons in the absence of transection or crush injury with Wallerian-like morphology and/or genetic regulation (WldS-sensitivity)
- Nmnat
nicotinamide mononucleotide adenylyltransferase; an enzyme that catalyzes NAD+ synthesis in the salvage pathway. There are three isoforms in mammals
- Dying back disorder
a neurodegenerative disorder in which axons die before cell bodies and/or in a retrograde pattern that begins with their distal ends
- N70
the N-terminal 70 amino acids of WldS and Ube4b
- Ube4b
ubiquitin ligase E4b, also known as Ufd2a. It carries out multiubiquitination of substrates in the ubiquitin fusion degradation pathway
Footnotes
DISCLOSURE STATEMENT
M.C. discloses that the patent for the Nmnat2 modulator is pending.
LITERATURE CITED
- Adalbert R, Gillingwater TH, Haley JE, Bridge K, Beirowski B, et al. A rat model of slow Wallerian degeneration (WldS) with improved preservation of neuromuscular synapses. Eur. J. Neurosci. 2005;21:271–277. doi: 10.1111/j.1460-9568.2004.03833.x. [DOI] [PubMed] [Google Scholar]
- Adalbert R, Nogradi A, Babetto E, Janeckova L, Walker SA, et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain. 2009;132:402–416. doi: 10.1093/brain/awn312. [DOI] [PubMed] [Google Scholar]
- Adalbert R, Nogradi A, Szabo A, Coleman MP. The slow Wallerian degeneration gene in vivo protects motor axons but not their cell bodies after avulsion and neonatal axotomy. Eur. J. Neurosci. 2006;24:2163–2168. doi: 10.1111/j.1460-9568.2006.05103.x. [DOI] [PubMed] [Google Scholar]
- Alvarez S, Moldovan M, Krarup C. Acute energy restriction triggers Wallerian degeneration in mouse. Exp. Neurol. 2008;212:166–178. doi: 10.1016/j.expneurol.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J. Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science. 2004;305:1010–1013. doi: 10.1126/science.1098014. [DOI] [PubMed] [Google Scholar]
- Avery MA, Sheehan A, Kerr KS, Wang J, Freeman MR. WldS requires Nmnat1 enzymatic activity and N16-VCP interactions to suppress Wallerian degeneration. J. Cell Biol. 2009;184:501–513. doi: 10.1083/jcb.200808042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz D, Leyssen M, Koch M, Yan J, Srahna M, et al. Axonal injury and regeneration in the adult brain of Drosophila. J. Neurosci. 2008;28:6010–6021. doi: 10.1523/JNEUROSCI.0101-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Adalbert R, Wagner D, Grumme D, Addicks K, et al. The progressive nature of Wallerian degeneration in wild-type and slow Wallerian degeneration (WldS) nerves. BMC Neurosci. 2005;6:6. doi: 10.1186/1471-2202-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Coleman MP, Martin KR. The WldS gene delays axonal but not somatic degeneration in a rat glaucoma model. Eur. J. Neurosci. 2008;28:1166–1179. doi: 10.1111/j.1460-9568.2008.06426.x. [DOI] [PubMed] [Google Scholar]
- Beirowski B, Babetto E, Gilley J, Mazzola F, Conforti L, et al. Non-nuclear Wld(S) determines its neuroprotective efficacy for axons and synapses in vivo. J. Neurosci. 2009;29:653–668. doi: 10.1523/JNEUROSCI.3814-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beirowski B, Nógrádi A, Babetto E, Garcia-Alias G, Coleman MP. Mechanisms of axonal spheroid formation in central nervous system Wallerian degeneration. J. Neuropath. Exp. Neurol. 2010 doi: 10.1097/NEN.0b013e3181da84db. In press. [DOI] [PubMed] [Google Scholar]
- Benbassat D, Spira ME. The survival of transected axonal segments of cultured aplysia neurons is prolonged by contact with intact nerve cells. Eur. J. Neurosci. 1994;6:1605–1614. doi: 10.1111/j.1460-9568.1994.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Berger F, Lau C, Dahlmann M, Ziegler M. Subcellular compartmentation and differential catalytic properties of the three human nicotinamide mononucleotide adenylyltransferase isoforms. J. Biol. Chem. 2005;280:36334–36341. doi: 10.1074/jbc.M508660200. [DOI] [PubMed] [Google Scholar]
- Bishop DL, Misgeld T, Walsh MK, Gan WB, Lichtman JW. Axon branch removal at developing synapses by axosome shedding. Neuron. 2004;44:651–661. doi: 10.1016/j.neuron.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Braun RJ, Zischka H, Madeo F, Eisenberg T, Wissing S, et al. Crucial mitochondrial impairment upon CDC48 mutation in apoptotic yeast. J. Biol. Chem. 2006;281:25757–25767. doi: 10.1074/jbc.M513699200. [DOI] [PubMed] [Google Scholar]
- Brown MC, Perry VH, Hunt SP, Lapper SR. Further studies on motor and sensory nerve regeneration in mice with delayed Wallerian degeneration. Eur. J. Neurosci. 1994;6:420–428. doi: 10.1111/j.1460-9568.1994.tb00285.x. [DOI] [PubMed] [Google Scholar]
- Buckmaster EA, Perry VH, Brown MC. The rate of Wallerian degeneration in cultured neurons from wild type and C57BL/WldS mice depends on time in culture and may be extended in the presence of elevated K+ levels. Eur. J. Neurosci. 1995;7:1596–1602. doi: 10.1111/j.1460-9568.1995.tb01155.x. [DOI] [PubMed] [Google Scholar]
- Burne JF, Staple JK, Raff MC. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J. Neurosci. 1996;16:2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajal SRy. Degeneration and Regeneration of the Nervous System. London: Oxford Univ. Press; 1928. [Google Scholar]
- Cavanagh JB. The ‘dying back’ process. A common denominator in many naturally occurring and toxic neuropathies. Arch. Pathol. Lab Med. 1979;103:659–664. [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, et al. Elevated neuronal expression of CD200. protects Wlds mice from inflammation-mediated neurodegeneration. Am. J. Pathol. 2007;170:1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman M. Axon degeneration mechanisms: commonality amid diversity. Nat. Rev. Neurosci. 2005;6:889–898. doi: 10.1038/nrn1788. [DOI] [PubMed] [Google Scholar]
- Coleman MP, Conforti L, Buckmaster EA, Tarlton A, Ewing RM, et al. An 85-kb tandem triplication in the slow Wallerian degeneration (Wlds) mouse. Proc. Natl. Acad. Sci. USA. 1998;95:9985–9990. doi: 10.1073/pnas.95.17.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MP, Ribchester RR. Programmed axon death, synaptic dysfunction and the ubiquitin proteasome system. Curr. Drug Targets CNS Neurol. Disord. 2004;3:227–238. doi: 10.2174/1568007043337436. [DOI] [PubMed] [Google Scholar]
- Conforti L, A W, Morreale G, Janeckova L, Beirowski B, et al. WldS protein requires Nmnat activity and a short N-terminal sequence to protect axons in mice. J. Cell Biol. 2009;184:491–500. doi: 10.1083/jcb.200807175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Adalbert R, Coleman MP. Neuronal death: Where does the end begin? Trends Neurosci. 2007a;30:159–166. doi: 10.1016/j.tins.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Conforti L, Fang G, Beirowski B, Wang MS, Sorci L, et al. NAD(+) and axon degeneration revisited: Nmnat1 cannot substitute for Wld(S) to delay Wallerian degeneration. Cell Death Differ. 2007b;14:116–127. doi: 10.1038/sj.cdd.4401944. [DOI] [PubMed] [Google Scholar]
- Conforti L, Tarlton A, Mack TG, Mi W, Buckmaster EA, et al. A Ufd2/D4Cole1e chimeric protein and overexpression of rbp7 in the slow Wallerian degeneration (WldS) mouse. Proc. Natl. Acad. Sci. USA. 2000;97:11377–11382. doi: 10.1073/pnas.97.21.11377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Hendriks WT, Macgillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J. Neurosci. 2008;28:11024–11029. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos KJ, Grierson AJ, Ackerley S, Miller CC. Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 2008;31:151–173. doi: 10.1146/annurev.neuro.31.061307.090711. [DOI] [PubMed] [Google Scholar]
- Deckwerth TL, Johnson EM., Jr Neurites can remain viable after destruction of the neuronal soma by programmed cell death (apoptosis) Dev. Biol. 1994;165:63–72. doi: 10.1006/dbio.1994.1234. [DOI] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J. Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Eppendorf 2003 prize-winning essay. Ubiquitin and the deconstruction of synapses. Science. 2003;302:800–801. doi: 10.1126/science.1092546. [DOI] [PubMed] [Google Scholar]
- Fainzilber M, Twiss JL. Tracking in the Wld(s)—the hunting of the SIRT and the luring of the draper. Neuron. 2006;50:819–821. doi: 10.1016/j.neuron.2006.05.023. [DOI] [PubMed] [Google Scholar]
- Ferguson B, Matyszak MK, Esiri MM, Perry VH. Axonal damage in acute multiple sclerosis lesions. Brain. 1997;120:393–399. doi: 10.1093/brain/120.3.393. [DOI] [PubMed] [Google Scholar]
- Ferri A, Sanes JR, Coleman MP, Cunningham JM, Kato AC. Inhibiting axon degeneration and synapse loss attenuates apoptosis and disease progression in a mouse model of motoneuron disease. Curr. Biol. 2003;13:669–673. doi: 10.1016/s0960-9822(03)00206-9. [DOI] [PubMed] [Google Scholar]
- Finn JT, Weil M, Archer F, Siman R, Srinivasan A, Raff MC. Evidence that wallerian degeneration and localized axon degeneration induced by local neurotrophin deprivation do not involve caspases. J. Neurosci. 2000;20:1333–1341. doi: 10.1523/JNEUROSCI.20-04-01333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Davis AA, Tennant P, Wang M, et al. The Wld(S) gene modestly prolongs survival in the SOD1(G93A) fALS mouse. Neurobiol. Dis. 2005;19:293–300. doi: 10.1016/j.nbd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Gamble HJ, Jha BD. Some effects of temperature upon the rate and progress of Wallerian degeneration in mammalian nerve fibers. J. Anat. 1958;92:171–177. [PMC free article] [PubMed] [Google Scholar]
- Gilley J, Coleman MP. Endogenous Nmnat2 is an essential survival factor for maintenance of health axons. PLoS Biol. 2010;8:e1000200. doi: 10.1371/journal.pbio.1000300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Ingham CA, Parry KE, Wright AK, Haley JE, et al. Delayed synaptic degeneration in the CNS of Wlds mice after cortical lesion. Brain. 2006a;129:1546–1556. doi: 10.1093/brain/awl101. [DOI] [PubMed] [Google Scholar]
- Gillingwater TH, Thomson D, Mack TG, Soffin EM, Mattison RJ, et al. Age-dependent synapse withdrawal at axotomised neuromuscular junctions in Wld(s) mutant and Ube4b/Nmnat transgenic mice. J. Physiol. 2002;543:739–755. doi: 10.1113/jphysiol.2002.022343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Wishart TM, Chen PE, Haley JE, Robertson K, et al. The neuroprotective WldS gene regulates expression of PTTG1 and erythroid differentiation regulator 1-like Gene in Mice and human cells. Hum. Mol. Genet. 2006b;15:625–635. doi: 10.1093/hmg/ddi478. [DOI] [PubMed] [Google Scholar]
- Glass JD, Brushart TM, George EB, Griffin JW. Prolonged survival of transected nerve fibres in C57BL/Ola mice is an intrinsic characteristic of the axon. J. Neurocytol. 1993;22:311–321. doi: 10.1007/BF01195555. [DOI] [PubMed] [Google Scholar]
- Griffin JW, George EB, Chaudhry V. Wallerian degeneration in peripheral nerve disease. Baillieres Clin. Neurol. 1996;5:65–75. [PubMed] [Google Scholar]
- Guo SX, Bourgeois F, Chokshi T, Durr NJ, Hilliard MA, et al. Femtosecond laser nanoaxotomy labon-a-chip for in vivo nerve regeneration studies. Nat. Methods. 2008;5:531–533. doi: 10.1038/nmeth.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Jorgensen EM, Bastiani MJ. Axons break in animals lacking beta-spectrin. J. Cell Biol. 2007;176:269–275. doi: 10.1083/jcb.200611117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbani DM, O’Malley KL. Wld(S) mice are protected against the Parkinsonian mimetic MPTP. Exp. Neurol. 2006;202:93–99. doi: 10.1016/j.expneurol.2006.05.017. [DOI] [PubMed] [Google Scholar]
- Hassa PO, Haenni SS, Elser M, Hottiger MO. Nuclear ADP-ribosylation reactions in mammalian cells: Where are we today and where are we going? Microbiol. Mol. Biol. Rev. 2006;70:789–829. doi: 10.1128/MMBR.00040-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama S, Yada M, Matsumoto M, Ishida N, Nakayama KI. U-Box proteins as a new family of ubiquitin-protein ligases. J. Biol. Chem. 2001;276:33111–33120. doi: 10.1074/jbc.M102755200. [DOI] [PubMed] [Google Scholar]
- Hoopfer ED, McLaughlin T, Watts RJ, Schuldiner O, O’Leary DD, Luo L. Wld(s) protection distinguishes axon degeneration following injury from naturally occurring developmental pruning. Neuron. 2006;50:883–895. doi: 10.1016/j.neuron.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Howell GR, Libby RT, Jakobs TC, Smith RS, Phalan FC, et al. Axons of retinal ganglion cells are insulted in the optic nerve early in DBA/2J glaucoma. J. Cell Biol. 2007;179:1523–1537. doi: 10.1083/jcb.200706181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, Yan T, Feng Y, Zeng C, Shi X, Zhai Q. Identification of a critical site in Wld(s): essential for Nmnat enzyme activity and axon-protective function. Neurosci. Lett. 2007;413:46–51. doi: 10.1016/j.neulet.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Wang J, Kaneko M, Yiu G, Hurrell JM, et al. Protecting axonal degeneration by increasing nicotinamide adenine dinucleotide levels in experimental autoimmune encephalomyelitis models. J. Neurosci. 2006;26:9794–9804. doi: 10.1523/JNEUROSCI.2116-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko-Oshikawa C, Nakagawa T, Yamada M, Yoshikawa H, Matsumoto M, et al. Mammalian E4 is required for cardiac development and maintenance of the nervous system. Mol. Cell Biol. 2005;25:10953–10964. doi: 10.1128/MCB.25.24.10953-10964.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya S, Mauricio R, Dai Y, Monani UR. The neuroprotective factor Wld(s) fails to mitigate distal axonal and neuromuscular junction (NMJ) defects in mouse models of spinal muscular atrophy. Neurosci. Lett. 2009;449:246–251. doi: 10.1016/j.neulet.2008.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilhoff G, Fansa H, Wolf G. Differences in peripheral nerve degeneration/regeneration between wild-type and neuronal nitric oxide synthase knockout mice. J. Neurosci. Res. 2002;68:432–441. doi: 10.1002/jnr.10229. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–644. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- Laser H, Conforti L, Morreale G, Mack TG, Heyer M, et al. The slow Wallerian degeneration protein, WldS, binds directly to VCP/p97 and partially redistributes it within the nucleus. Mol. Biol. Cell. 2006;17:1075–1084. doi: 10.1091/mbc.E05-04-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laser H, Mack TG, Wagner D, Coleman MP. Proteasome inhibition arrests neurite outgrowth and causes “dying-back” degeneration in primary culture. J. Neurosci. Res. 2003;74:906–916. doi: 10.1002/jnr.10806. [DOI] [PubMed] [Google Scholar]
- Levy D, Kubes P, Zochodne DW. Delayed peripheral nerve degeneration, regeneration, and pain in mice lacking inducible nitric oxide synthase. J. Neuropathol. Exp. Neurol. 2001;60:411–421. doi: 10.1093/jnen/60.5.411. [DOI] [PubMed] [Google Scholar]
- Libby RT, Li Y, Savinova OV, Barter J, Smith RS, et al. Susceptibility to neurodegeneration in glaucoma is modified by Bax gene dosage. PLoS Genet. 2005;1:e4. doi: 10.1371/journal.pgen.0010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vales R, Navarro X, Shimizu T, Baskakis C, Kokotos G, et al. Intracellular phospholipase A(2) group IVA and group VIA play important roles in Wallerian degeneration and axon regeneration after peripheral nerve injury. Brain. 2008;131:2620–2631. doi: 10.1093/brain/awn188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubinska L. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 1977;130:47–63. doi: 10.1016/0006-8993(77)90841-1. [DOI] [PubMed] [Google Scholar]
- Lubinska L. Patterns of Wallerian degeneration of myelinated fibres in short and long peripheral stumps and in isolated segments of rat phrenic nerve. Interpretation of the role of axoplasmic flow of the trophic factor. Brain Res. 1982;233:227–240. doi: 10.1016/0006-8993(82)91199-4. [DOI] [PubMed] [Google Scholar]
- Lunn ER, Perry VH, Brown MC, Rosen H, Gordon S. Absence of Wallerian degeneration does not hinder regeneration in peripheral nerve. Eur. J. Neurosci. 1989;1:27–33. doi: 10.1111/j.1460-9568.1989.tb00771.x. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Ogunkolade BW, Brown MC, Atherton DJ, Perry VH. A gene affecting Wallerian nerve degeneration maps distally on mouse chromosome 4. Proc. Natl. Acad. Sci. USA. 1993;90:9717–9720. doi: 10.1073/pnas.90.20.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR. The Drosophila cell corpse engulfment receptor draper mediates glial clearance of severed axons. Neuron. 2006;50:869–881. doi: 10.1016/j.neuron.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Macinnis BL, Campenot RB. Regulation of Wallerian degeneration and nerve growth factor withdrawal-induced pruning of axons of sympathetic neurons by the proteasome and the MEK/Erk pathway. Mol. Cell Neurosci. 2005;28:430–439. doi: 10.1016/j.mcn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Mack TG, Reiner M, Beirowski B, Mi W, Emanuelli M, et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 2001;4:1199–1206. doi: 10.1038/nn770. [DOI] [PubMed] [Google Scholar]
- Magni G, Amici A, Emanuelli M, Orsomando G, Raffaelli N, Ruggieri S. Structure and function of nicotinamide mononucleotide adenylyltransferase. Curr. Med. Chem. 2004;11:873–885. doi: 10.2174/0929867043455666. [DOI] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, et al. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol. Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C, Langer T. Mitofusin 2 builds a bridge between ER and mitochondria. Cell. 2008;135:1165–1167. doi: 10.1016/j.cell.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Mi W, Beirowski B, Gillingwater TH, Adalbert R, Wagner D, et al. The slow Wallerian degeneration gene, WldS, inhibits axonal spheroid pathology in gracile axonal dystrophy mice. Brain. 2005;128:405–416. doi: 10.1093/brain/awh368. [DOI] [PubMed] [Google Scholar]
- Miledi R, Slater CR. On the degeneration of rat neuromuscular junctions after nerve section. J. Physiol. 1970;207:507–528. doi: 10.1113/jphysiol.1970.sp009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat. Neurosci. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misgeld T, Kerschensteiner M, Bareyre FM, Burgess RW, Lichtman JW. Imaging axonal transport of mitochondria in vivo. Nat. Methods. 2007;4:559–561. doi: 10.1038/nmeth1055. [DOI] [PubMed] [Google Scholar]
- Moldovan M, Alvarez S, Krarup C. Motor axon excitability during Wallerian degeneration. Brain. 2009;132:511–523. doi: 10.1093/brain/awn332. [DOI] [PubMed] [Google Scholar]
- Morreale G, Conforti L, Coadwell J, Wilbrey AL, Coleman MP. Evolutionary divergence of valosin-containing protein/cell division cycle protein 48 binding interactions among endoplasmic reticulum-associated degradation proteins. FEBS J. 2009;276:1208–1220. doi: 10.1111/j.1742-4658.2008.06858.x. [DOI] [PubMed] [Google Scholar]
- Narciso MS, Mietto Bde S, Marques SA, Soares CP, Mermelstein Cdos S, et al. Sciatic nerve regeneration is accelerated in galectin-3 knockout mice. Exp. Neurol. 2009;217:7–15. doi: 10.1016/j.expneurol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Nave KA, Trapp BD. Axon-glial signaling and the glial support of axon function. Annu. Rev. Neurosci. 2008;31:535–561. doi: 10.1146/annurev.neuro.30.051606.094309. [DOI] [PubMed] [Google Scholar]
- Neumann H, Medana IM, Bauer J, Lassmann H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends Neurosci. 2002;25:313–319. doi: 10.1016/s0166-2236(02)02154-9. [DOI] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DD, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nordlander RH, Singer M. Electron microscopy of severed motor fibers in the crayfish. Z. Zellforsch. Mikrosk. Anat. 1972;126:157–181. doi: 10.1007/BF00307214. [DOI] [PubMed] [Google Scholar]
- Parnas I, Dudel J, Atwood HL. Synaptic transmission in decentralized axons of rock lobster. J. Neurosci. 1991;11:1309–1315. doi: 10.1523/JNEUROSCI.11-05-01309.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson SH, Mackintosh CL, Ribchester RR. Elimination of motor nerve terminals in neonatal mice expressing a gene for slow Wallerian degeneration (C57Bl/Wlds) Eur. J. Neurosci. 1997;9:1586–1592. doi: 10.1111/j.1460-9568.1997.tb01516.x. [DOI] [PubMed] [Google Scholar]
- Perlson E, Hanz S, Ben-Yaakov K, Segal-Ruder Y, Seger R, Fainzilber M. Vimentin-dependent spatial translocation of an activated MAP kinase in injured nerve. Neuron. 2005;45:715–726. doi: 10.1016/j.neuron.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Lunn ER. Very slow retrograde and Wallerian degeneration in the CNS of C57BL/Ola mice. Eur. J. Neurosci. 1991;3:102–105. doi: 10.1111/j.1460-9568.1991.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Brown MC, Lunn ER, Tree P, Gordon S. Evidence that very slow Wallerian degeneration in C57BL/Ola mice is an intrinsic property of the peripheral nerve. Eur. J. Neurosci. 1990a;2:802–808. doi: 10.1111/j.1460-9568.1990.tb00472.x. [DOI] [PubMed] [Google Scholar]
- Perry VH, Lunn ER, Brown MC, Cahusac S, Gordon S. Evidence that the rate of Wallerian degeneration is controlled by a single autosomal dominant gene. Eur. J. Neurosci. 1990b;2:408–413. doi: 10.1111/j.1460-9568.1990.tb00433.x. [DOI] [PubMed] [Google Scholar]
- Pollak N, Dolle C, Ziegler M. The power to reduce: pyridine nucleotides–small molecules with a multitude of functions. Biochem. J. 2007;402:205–218. doi: 10.1042/BJ20061638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J. Neurosci. 2008;28:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Raffaelli N, Sorci L, Amici A, Emanuelli M, Mazzola F, Magni G. Identification of a novel human nicotinamide mononucleotide adenylyltransferase. Biochem. Biophys. Res. Commun. 2002;297:835–840. doi: 10.1016/s0006-291x(02)02285-4. [DOI] [PubMed] [Google Scholar]
- Ramaglia V, King RH, Nourallah M, Wolterman R, de Jonge R, et al. The membrane attack complex of the complement system is essential for rapid Wallerian degeneration. J. Neurosci. 2007;27:7663–7672. doi: 10.1523/JNEUROSCI.5623-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribchester RR, Tsao JW, Barry JA, Asgari-Jirhandeh N, Perry VH, Brown MC. Persistence of neuromuscular junctions after axotomy in mice with slow Wallerian degeneration (C57BL/WldS) Eur. J. Neurosci. 1995;7:1641–1650. doi: 10.1111/j.1460-9568.1995.tb01159.x. [DOI] [PubMed] [Google Scholar]
- Rose FF, Jr, Meehan PW, Coady TH, Garcia VB, Garcia ML, Lorson CL. The Wallerian degeneration slow (Wld(s)) gene does not attenuate disease in a mouse model of spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2008;375:119–123. doi: 10.1016/j.bbrc.2008.07.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot Y, Dubois-Dauphin M, Tan SA, de Bilbao F, Aebischer P, et al. Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J. Neurosci. 1995;15:7727–7733. doi: 10.1523/JNEUROSCI.15-11-07727.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigoh K, Wang YL, Suh JG, Yamanishi T, Sakai Y, et al. Intragenic deletion in the gene encoding ubiquitin carboxy-terminal hydrolase in gad mice. Nat. Genet. 1999;23:47–51. doi: 10.1038/12647. [DOI] [PubMed] [Google Scholar]
- Sajadi A, Schneider BL, Aebischer P. Wld(s)-mediated protection of dopaminergic fibers in an animal model of parkinson disease. Curr. Biol. 2004;14:326–330. doi: 10.1016/j.cub.2004.01.053. [DOI] [PubMed] [Google Scholar]
- Samsam M, Mi W, Wessig C, Zielasek J, Toyka KV, et al. The Wlds mutation delays robust loss of motor and sensory axons in a genetic model for myelin-related axonopathy. J. Neurosci. 2003;23:2833–2839. doi: 10.1523/JNEUROSCI.23-07-02833.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing theNmnat1 protein manifest robust delay in axonal degeneration in vivo. J. Neurosci. 2009a;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Lund FE, Milbrandt J. Nicotinamide mononucleotide adenylyl transferase-mediated axonal protection requires enzymatic activity but not increased levels of neuronal nicotinamide adenine dinucleotide. J. Neurosci. 2009b;29:5525–5535. doi: 10.1523/JNEUROSCI.5469-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer WW. Effects of energy deprivation on Wallerian degeneration in isolated segments of rat peripheral nerve. Brain Res. 1974;78:71–81. doi: 10.1016/0006-8993(74)90354-0. [DOI] [PubMed] [Google Scholar]
- Simonin Y, Perrin FE, Kato AC. Axonal involvement in the Wlds neuroprotective effect: analysis of pure motoneurons in a mouse model protected from motor neuron disease at a presymptomatic age. J. Neurochem. 2007;101:530–542. doi: 10.1111/j.1471-4159.2006.04366.x. [DOI] [PubMed] [Google Scholar]
- Sorci L, Cimadamore F, Scotti S, Petrelli R, Cappellacci L, et al. Initial-rate kinetics of human NMN-adenylyltransferases: substrate and metal ion specificity, inhibition by products and multisubstrate analogues, and isozyme contributions to NAD+ biosynthesis. Biochemistry. 2007;46:4912–4922. doi: 10.1021/bi6023379. [DOI] [PubMed] [Google Scholar]
- Spires TL, Meyer-Luehmann M, Stern EA, McLean PJ, Skoch J, et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengl M. Pigment-dispersing hormone-immunoreactive fibers persist in crickets which remain rhythmic after bilateral transection of the optic stalks. J. Comp. Physiol. A. 1995;176:217–228. [Google Scholar]
- Tamsett TJ, Picchione KE, Bhattacharjee A. NAD+ activates KNa channels in dorsal root ganglion neurons. J. Neurosci. 2009;29:5127–5134. doi: 10.1523/JNEUROSCI.0859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todisco S, Agrimi G, Castegna A, Palmieri F. Identification of the mitochondrial NAD+ transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2006;281:1524–1531. doi: 10.1074/jbc.M510425200. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu. Rev. Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- Velde CV, Garcia ML, Yin X, Trapp BD, Cleveland DW. The neuroprotective factor Wlds does not attenuate mutant SOD1-mediated motor neuron disease. Neuromolecular Med. 2004;5:193–204. doi: 10.1385/NMM:5:3:193. [DOI] [PubMed] [Google Scholar]
- Waller A. Experiments on the section of glossopharyngeal and hypoglossal nerves of the frog and observations of the alternatives produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1850;140:423–429. [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, et al. A local mechanism mediates NAD-dependent protection of axon degeneration. J. Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MS, Davis AA, Culver DG, Glass JD. WldS mice are resistant to paclitaxel (taxol) neuropathy. Ann. Neurol. 2002;52:442–447. doi: 10.1002/ana.10300. [DOI] [PubMed] [Google Scholar]
- Wang MS, Fang G, Culver DG, Davis AA, Rich MM, Glass JD. The WldS protein protects against axonal degeneration: a model of gene therapy for peripheral neuropathy. Ann. Neurol. 2001;50:773–779. doi: 10.1002/ana.10039. [DOI] [PubMed] [Google Scholar]
- Wang MS, Wu Y, Culver DG, Glass JD. Pathogenesis of axonal degeneration: parallels between Wallerian degeneration and vincristine neuropathy. J. Neuropathol. Exp. Neurol. 2000;59:599–606. doi: 10.1093/jnen/59.7.599. [DOI] [PubMed] [Google Scholar]
- Wang Q, Song C, Li CC. Molecular perspectives on p97-VCP: progress in understanding its structure and diverse biological functions. J. Struct. Biol. 2004;146:44–57. doi: 10.1016/j.jsb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–261. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- Wilbrey AL, Haley JE, Wishart TM, Conforti L, Morreale G, et al. VCP binding influences intracellular distribution of the slow Wallerian degeneration protein, Wld(S) Mol. Cell Neurosci. 2008;38:325–340. doi: 10.1016/j.mcn.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wishart TM, Paterson JM, Short DM, Meredith S, Robertson KA, et al. Differential proteomic analysis of synaptic proteins identifies potential cellular targets and protein mediators of synaptic neuroprotection conferred by the slow Wallerian degeneration (Wlds) gene. Mol. Cell Proteomics. 2007;6(8):1318–1330. doi: 10.1074/mcp.M600457-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart TM, Pemberton HN, James SR, McCabe CJ, Gillingwater TH. Modified cell cycle status in a mouse model of altered neuronal vulnerability (slow Wallerian degeneration; Wlds) Genome Biol. 2008;9:R101. doi: 10.1186/gb-2008-9-6-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F, Fan L, Wells S, Hartley R, Mackenzie FE, et al. Axonal and neuromuscular synaptic phenotypes in WldS, SOD1(G93A) and ostes mutant mice identified by fiber-optic confocal microendoscopy. Mol. Cell Neurosci. 2009;42:296–307. doi: 10.1016/j.mcn.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Wu Z, Ghosh-Roy A, Yanik MF, Zhang JZ, Jin Y, Chisholm AD. Caenorhabditis elegans neuronal regeneration is influenced by life stage, ephrin signaling, and synaptic branching. Proc. Natl. Acad. Sci. USA. 2007;104:15132–15137. doi: 10.1073/pnas.0707001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Yuasa S, Araki T. Nicotinamide mononucleotide adenylyltransferase expression in mitochondrial matrix delays Wallerian degeneration. J. Neurosci. 2009;29:6276–6284. doi: 10.1523/JNEUROSCI.4304-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanik MF, Cinar H, Cinar HN, Chisholm AD, Jin Y, Ben-Yakar A. Neurosurgery: functional regeneration after laser axotomy. Nature. 2004;432:822. doi: 10.1038/432822a. [DOI] [PubMed] [Google Scholar]
- Zhai Q, Wang J, Kim A, Liu Q, Watts R, et al. Involvement of the ubiquitin-proteasome system in the early stages of wallerian degeneration. Neuron. 2003;39:217–225. doi: 10.1016/s0896-6273(03)00429-x. [DOI] [PubMed] [Google Scholar]
- Zhai RG, Cao Y, Hiesinger PR, Zhou Y, Mehta SQ, et al. Drosophila NMNAT maintains neural integrity independent of its NAD synthesis activity. PLoS Biol. 2006;4:e416. doi: 10.1371/journal.pbio.0040416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai RG, Zhang F, Hiesinger PR, Cao Y, Haueter CM, Bellen HJ. NAD synthase NMNAT acts as a chaperone to protect against neurodegeneration. Nature. 2008;452:887–891. doi: 10.1038/nature06721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli SJ, Marek LE, Agostini MA, Strittmatter SL. Morphological and physiological survival of goldfish Mauthner axons isolated from their somata by spinal cord crush. J. Comp. Neurol. 1987;255:272–282. doi: 10.1002/cne.902550210. [DOI] [PubMed] [Google Scholar]