Abstract
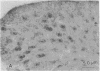
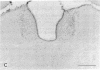
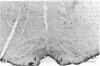
The distribution of the P-type calcium channel in the mammalian central nervous system has been demonstrated immunohistochemically by using a polyclonal specific antibody. This antibody was generated after P-channel isolation via a fraction from funnel-web spider toxin (FTX) that blocks the voltage-gated P channels in cerebellar Purkinje cells. In the cerebellar cortex, immunolabeling to the antibody appeared throughout the molecular layer, while all the other regions were negative. Intensely labeled patches of reactivity were seen on Purkinje cell dendrites, especially at bifurcations; much weaker reactivity was present in the soma and stem segment. Electron microscopic localization revealed labeled patches of plasma membrane on the soma, main dendrites, spiny branchlets, and spines; portions of the smooth endoplasmic reticulum were also labeled. Strong labeling was present in the periglomerular cells of the olfactory bulb and scattered neurons in the deep layer of the entorhinal and pyriform cortices. Neurons in the brainstem, habenula, nucleus of the trapezoid body and inferior olive and along the floor of the fourth ventricle were also labeled intensely. Medium-intensity reactions were observed in layer II pyramidal cells of the frontal cortex, the CA1 cells of the hippocampus, the lateral nucleus of the substantia nigra, lateral reticular nucleus, and spinal fifth nucleus. Light labeling was seen in the neocortex, striatum, and in some brainstem neurons.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Koyano K., Saisu H., Nishiuchi Y., Sakakibara S. Binding of omega-conotoxin to receptor sites associated with the voltage-sensitive calcium channel. Neurosci Lett. 1986 Nov 11;71(2):203–208. doi: 10.1016/0304-3940(86)90559-8. [DOI] [PubMed] [Google Scholar]
- Ahlijanian M. K., Westenbroek R. E., Catterall W. A. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990 Jun;4(6):819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Cortés R., Supavilai P., Karobath M., Palacios J. M. Calcium antagonist binding sites in the rat brain: quantitative autoradiographic mapping using the 1,4-dihydropyridines [3H]PN 200-110 and [3H]PY 108-068. J Neural Transm. 1984;60(3-4):169–197. doi: 10.1007/BF01249092. [DOI] [PubMed] [Google Scholar]
- Fortier L. P., Tremblay J. P., Rafrafi J., Hawkes R. A monoclonal antibody to conotoxin reveals the distribution of a subset of calcium channels in the rat cerebellar cortex. Brain Res Mol Brain Res. 1991 Feb;9(3):209–215. doi: 10.1016/0169-328x(91)90004-h. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Activity of dendrites of single Purkinje cells and its relationship to so-called inactivation response in rabbit cerebellum. J Neurophysiol. 1968 Mar;31(2):131–141. doi: 10.1152/jn.1968.31.2.131. [DOI] [PubMed] [Google Scholar]
- Hillman D. E., Chen S. Reciprocal relationship between size of postsynaptic densities and their number: constancy in contact area. Brain Res. 1984 Mar 19;295(2):325–343. doi: 10.1016/0006-8993(84)90981-8. [DOI] [PubMed] [Google Scholar]
- Hockberger P. E., Tseng H. Y., Connor J. A. Fura-2 measurements of cultured rat Purkinje neurons show dendritic localization of Ca2+ influx. J Neurosci. 1989 Jul;9(7):2272–2284. doi: 10.1523/JNEUROSCI.09-07-02272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr L. M., Filloux F., Olivera B. M., Jackson H., Wamsley J. K. Autoradiographic localization of calcium channels with [125I]omega-conotoxin in rat brain. Eur J Pharmacol. 1988 Jan 27;146(1):181–183. doi: 10.1016/0014-2999(88)90501-8. [DOI] [PubMed] [Google Scholar]
- Leonard J. P., Nargeot J., Snutch T. P., Davidson N., Lester H. A. Ca channels induced in Xenopus oocytes by rat brain mRNA. J Neurosci. 1987 Mar;7(3):875–881. doi: 10.1523/JNEUROSCI.07-03-00875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. W., Rudy B., Llinás R. Funnel-web spider venom and a toxin fraction block calcium current expressed from rat brain mRNA in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4538–4542. doi: 10.1073/pnas.87.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R., Nicholson C. Electrophysiological properties of dendrites and somata in alligator Purkinje cells. J Neurophysiol. 1971 Jul;34(4):532–551. doi: 10.1152/jn.1971.34.4.532. [DOI] [PubMed] [Google Scholar]
- Llinás R. R. The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science. 1988 Dec 23;242(4886):1654–1664. doi: 10.1126/science.3059497. [DOI] [PubMed] [Google Scholar]
- Llinás R., Hess R. Tetrodotoxin-resistant dendritic spikes in avian Purkinje cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2520–2523. doi: 10.1073/pnas.73.7.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Nicholson C., Freeman J. A., Hillman D. E. Dendritic spikes and their inhibition in alligator Purkinje cells. Science. 1968 Jun 7;160(3832):1132–1135. doi: 10.1126/science.160.3832.1132. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980 Aug;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda N., Wada K., Yuzaki M., Mikoshiba K. Autoradiographic visualization of a calcium channel antagonist, [125I]omega-conotoxin GVIA, binding site in the brains of normal and cerebellar mutant mice (pcd and weaver). Brain Res. 1989 Jun 5;489(1):21–30. doi: 10.1016/0006-8993(89)90004-8. [DOI] [PubMed] [Google Scholar]
- Mori Y., Friedrich T., Kim M. S., Mikami A., Nakai J., Ruth P., Bosse E., Hofmann F., Flockerzi V., Furuichi T. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991 Apr 4;350(6317):398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Nowycky M. C., Fox A. P., Tsien R. W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985 Aug 1;316(6027):440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- Olivera B. M., McIntosh J. M., Cruz L. J., Luque F. A., Gray W. R. Purification and sequence of a presynaptic peptide toxin from Conus geographus venom. Biochemistry. 1984 Oct 23;23(22):5087–5090. doi: 10.1021/bi00317a001. [DOI] [PubMed] [Google Scholar]
- Regan L. J., Sah D. W., Bean B. P. Ca2+ channels in rat central and peripheral neurons: high-threshold current resistant to dihydropyridine blockers and omega-conotoxin. Neuron. 1991 Feb;6(2):269–280. doi: 10.1016/0896-6273(91)90362-4. [DOI] [PubMed] [Google Scholar]
- Ross W. N., Werman R. Mapping calcium transients in the dendrites of Purkinje cells from the guinea-pig cerebellum in vitro. J Physiol. 1987 Aug;389:319–336. doi: 10.1113/jphysiol.1987.sp016659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura M., Fukui H., Wada H. Different localization of receptors for omega-conotoxin and nitrendipine in rat brain. Biochem Biophys Res Commun. 1987 Dec 31;149(3):982–988. doi: 10.1016/0006-291x(87)90505-5. [DOI] [PubMed] [Google Scholar]
- Takemura M., Kiyama H., Fukui H., Tohyama M., Wada H. Autoradiographic visualization in rat brain of receptors for omega-conotoxin GVIA, a newly discovered calcium antagonist. Brain Res. 1988 Jun 7;451(1-2):386–389. doi: 10.1016/0006-8993(88)90790-1. [DOI] [PubMed] [Google Scholar]
- Tank D. W., Sugimori M., Connor J. A., Llinás R. R. Spatially resolved calcium dynamics of mammalian Purkinje cells in cerebellar slice. Science. 1988 Nov 4;242(4879):773–777. doi: 10.1126/science.2847315. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Lipscombe D., Madison D. V., Bley K. R., Fox A. P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988 Oct;11(10):431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- Turner T. J., Goldin S. M. Do dihydropyridine-sensitive calcium channels play a role in neurosecretion in the central nervous system? Ann N Y Acad Sci. 1988;522:278–283. doi: 10.1111/j.1749-6632.1988.tb33365.x. [DOI] [PubMed] [Google Scholar]
- Wenthold R. J., Altschuler R. A., Hampson D. R. Immunocytochemistry of neurotransmitter receptors. J Electron Microsc Tech. 1990 May;15(1):81–96. doi: 10.1002/jemt.1060150108. [DOI] [PubMed] [Google Scholar]