In this perspective, Simian and Bissell discuss the evolution of the 3D culture and organoid research field up to now as well as its future directions.
Abstract
In the last ten years, there has been a dramatic surge in the number of publications where single or groups of cells are grown in substrata that have elements of basement membrane leading to the formation of tissue-like structures referred to as organoids. However, this field of research began many decades ago, when the pioneers of cell culture began to ask questions we still ask today: How does organogenesis occur? How do signals integrate to make such vastly different tissues and organs given that the sequence of the genome in our trillions of cells is identical? Here, we summarize how work over the past century generated the conceptual framework that has allowed us to make progress in the understanding of tissue-specific morphogenetic programs. The development of cell culture systems that provide accurate and physiologically relevant models are proving to be key in establishing appropriate platforms for the development of new therapeutic strategies.
How do we define organoids and 3D cultures?
That functional differentiation is dependent on 3D architecture has become accepted recently. Many papers over the last 50 years have shown that cells cultured in 2D are not representative of the in vivo situation. Structurally, 2D cultures do not provide the conditions for the organization and cellular relationships observed in vivo. Moreover, cell signaling networks are altered in 2D versus 3D, and this probably explains why drug screening outcomes many times do not reproduce the in vivo setting (Wang et al., 1998; Weaver et al., 2002). It is encouraging to see the recognition of the importance of 3D cultures to model signaling, differentiation, and drug development. Many of the studies use elegant images and sophisticated animations that are a delight to see and hear about and clearly show the similarity between organoids and the tissues and organs from which the cells were derived in vivo. We applaud the excitement and cheer the general enthusiasm that the work has deservedly generated. What is most exciting is that the combined effort is finally a critical mass and as a result has caught the attention of many new scientists who are emphasizing the importance of 3D culture by pointing out the relevance and the significance of this work to clinical research. However, the term “organoid” is being treated, or has come to imply, that this is a completely new field, separate from what several scientists from as early as the turn of the previous century have been engaged in for years, essentially in isolation, introducing the term 3D cultures, beginning the field of microenvironment, and pointing out the significance of tissue architecture.
The first use of the words “three-dimensional culture models,” we believe, started with the assays developed by Barcellos-Hoff et al. (1989) and Petersen et al. (1992), although floating collagen gels were described in the 1970s and were certainly 3D (see Fig. 2). Before 2005, the word organoid was an extension of 3D cultures. Typically, it referred to small tissue fragments taken from organs, mostly epithelial tissues, separated from stroma by mechanical and enzymatic digestion and grown in different types of 3D gels to produce an organ-like structure. As an example, see Simian et al. (2001), in which rodent mammary fragments were grown in collagen gels to produce a branching structure resembling branching in the mammary gland of virgin mice, or Fata et al. (2007), in which rodent mammary fragments were grown in laminin-rich gels giving rise to alveogenesis. However, in the last decade, the meaning of “organoid” has lost precision and has come to cover a series of cell culture techniques that are not necessarily a single technique. Below are examples of definitions of organoids taken from some recent papers in appropriate journals for the field. We come across the following definitions:
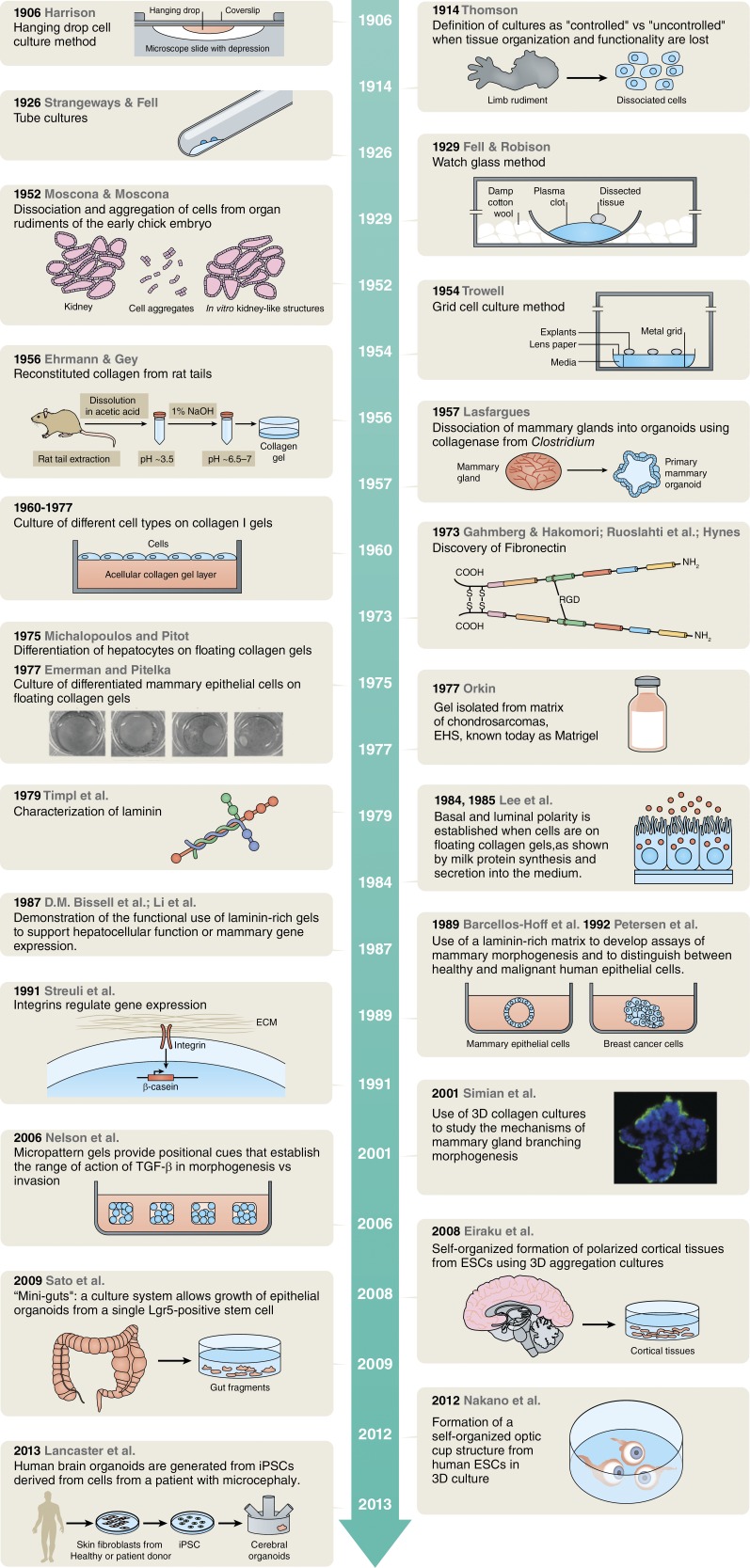
Figure 2.
Timeline of techniques and experiments leading to the current organoid field. The images shown in the 1975–1977 box are from Lyon et al. (2015), and are displayed under the terms of a Creative Commons License. Figure courtesy of Neil Smith.
(1) “Various subfields use these terms either interchangeably or distinctly; for example, in the field of mammary gland biology, the term organoids refers to primary explants of epithelial ducts into 3D extracellular matrix (ECM) gels. Conversely, in studies of intestinal biology, organoids can refer to clonal derivatives of primary epithelial stem cells that are grown without mesenchyme or can refer to epithelial–mesenchymal co-cultures that are derived from embryonic stem cells or induced pluripotent stem cells” (Shamir and Ewald, 2014).
(2) “Thus, we would like to define an organoid as containing several cell types that develop from stem cells or organ progenitors and self-organize through cell sorting and spatially restricted lineage commitment, similar to the process in vivo” (Lancaster and Knoblich, 2014).
(3) “An organoid is now defined as a 3D structure grown from stem cells and consisting of organ-specific cell types that self-organizes through cell sorting and spatially restricted lineage commitment…” (Clevers, 2016).
(4) “Here we define an organoid as an in vitro 3D cellular cluster derived exclusively from primary tissue, embryonic stem cells, or induced pluripotent stem cells, capable of self-renewal and self-organization, and exhibiting similar organ functionality as the tissue of origin” (Fatehullah et al., 2016).
“The character and organization of tissues are determined by the spatial arrangement, the mutual relations, and the typical groupings of cells, which, together with the intercellular material, combine into developmental and functional patterns. To the structure and integrity of these cellular patterns are related the course of the prospective development…” Moscona and Moscona, 1952
To our minds, the first definition, provided by Shamir and Ewald (2014) is the most inclusive and accurate, given that it includes the different definitions the word “organoid” has been associated with. It avoids the specific restriction imposed by other definitions and includes organoids generated from induced or embryonic stem cells. Researchers indeed are able to generate organoids in laminin-rich gels from single cells of normal tissues or malignant tumors, or even cell lines, without necessarily starting from cells that express stem cell markers (Weaver and Bissell, 1999). This is especially relevant given that we still do not understand whether a stem cell can be defined independently of its niche (Schofield, 1978; Mesa et al., 2015). Niches are specialized microenvironments located within each tissue where stem cells reside. Niches exert a key influence over stem cell function and are defined as the sum of the cell–cell, cell–ECM, and cell–soluble factor interactions, in the context of the physical and geometric constraints that a cell may experience at a given time (Kaplan et al., 2007). The ability of the niche to determine the functional spectrum of stem cell activities leads to the hypothesis that stem cell niche microenvironments are critical in the definition of stem cell functions (Kaplan et al., 2007; LaBarge et al., 2007).
It should be acknowledged that the development of the culture conditions that were established by scientists working on organoids (as originally defined) has contributed to the significant advances reported in the stem cell field in the last 10 years. Independently of the methods used to generate the organoids and keep them in culture, these advances represent outstanding model systems to study human development and disease. For many organs, such as the brain, mouse and human development are not the same (Lancaster et al., 2013). Moreover, induced pluripotent stem cells derived from skin fibroblasts as well as 3D cultures of normal and diseased human organs offer models for human diseases that are not easy to study in animal models (Lancaster et al., 2013). Additionally, developing screening platforms based on human organoids may provide a more cost-effective and precise preclinical setting for drug discovery in the long term.
Interestingly, the word organoid initially had a different meaning from all of the above. In the 1950s and 1960s, papers referring to organoids often centered on intracellular structures (organelles), with titles such as “Quantitative cine analysis of cell organoid activity” (Pomerat et al., 1954) or “Nuclear and cytoplasmic organoids in the living cell” (Duryee and Doherty, 1954). The word organoid was used also for tumors (Gordienko, 1964) or abnormal cellular growths (Wolter, 1967). Many papers described cases of “Organoid Nevus,” a malformation of the skin, most commonly in the scalp (Pinkus, 1976). Other researchers seemed to use organoids for cellular clusters that maintained the structural characteristics of the tissue of origin. For instance, Schneider et al. (1963), in a paper titled “Some unusual observations of organoid tissues and blood elements in monolayer cultures,” observed organoids as 1-mm nodules attached to the flask or floating after mechanical and enzymatic digestion of mammalian tissues. From 1980 on, however, papers referring to 3D cultures included the use of the word organoids.
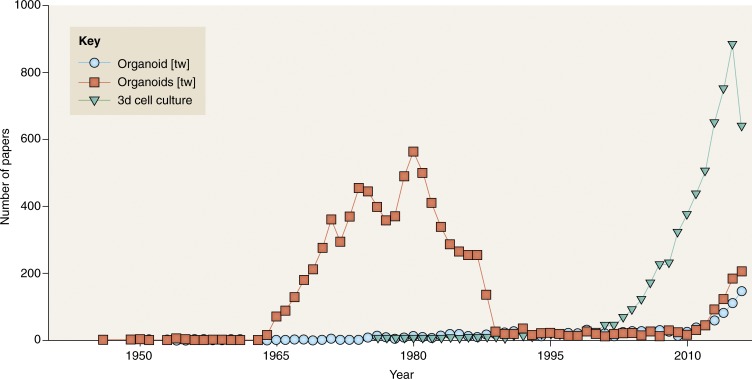
The aforementioned use and misuse of the word organoids appears to have contributed to significant divergence regarding when the organoid field began to develop. In a recent review, Clevers (2016) states that there was an initial increase in organoid research in the 1965 to 1985 period (Fig. 1, organoids, red squares), showing an astounding 563 papers in 1980. This number and the fact that the graph shows a sudden drop in 1985 surprised us and caught our attention. A close look at the papers referenced for this period shows that the PubMed search picked up many papers that included the word “organ,” but not necessarily “organoids.” A different search using “organoid” followed by the “text word” tag shows papers actually using the word “organoid” (Fig. 1, blue circles). This search, in a way, also overestimates the number of papers about organoids because the results encompass research on organoids as defined before 1980, which included small structures within the cells’ cytoplasm. From 1980, researchers began to use collagen and laminin-rich matrices to culture cells and organoids in 3D, and thus 3D included organoids as discussed above. The start of the dramatic increase in the number of published papers on organoids was in fact around 2011, with 150 papers published in 2016 up to the time this article was written. For comparison, we also show the search that referred to “3D cell culture” (Fig. 1, green triangles). In reality, as we have argued, the two terms are one and the same until such time that those scientists who are active in this field would subdivide and define the different types of 3D/organoids practiced in different laboratories. (Indeed, M.J. Bissell and H. Clever discussed this nomenclature problem in a meeting entitled “Organoids” organized by the European Molecular Biology Organization in October of 2016. The aim is to bring clarity in nomenclature and keep the focus on showing the overriding importance of context and architecture in how tissues and organs are formed and maintained.) Organoid cultures as models for the study of development and disease could not have occurred without the advances in what is now referred to as 3D cell cultures. Even though the first papers that were essentially doing 3D cultures/organoids started in the 1960s, the number of publications began to increase steadily from 2003 on, with a total of 640 publications up to the time this article was written in 2016. What the data in Fig. 2 show is that it took about half a century for the recognition that this way of thinking is not simply utilization of a technique but that form and function are fundamentally intertwined and that once the organism is formed, essentially, phenotype is dominant over genotype.
Figure 1.
Number of publications per year on organoids and 3D cell cultures according to PubMed. The number of publications per year is graphed for the following PubMed searches: “organoids [tw]” is shown in red squares, “organoid [tw]” is shown in blue circles, and “3D cell culture” is shown in green triangles. Figure courtesy of Neil Smith.
An adequate historical perspective is necessary to understand where the field as we know it comes from, and where it is going. Despite fancier cell culture systems and techniques, we are still trying to understand the same basic question as researchers decades ago: What are the cues that govern the formation and the stability of the differentiated state, and can we recapitulate morphogenesis in culture?
A historical timeline of organoid and 3D cell cultures
1906–1980: Developing the tools
When did 3D and organoid culture really start (Fig. 2)? Today’s methods are the product of what started in 1906 with the hanging drop tissue culture technique developed by Ross Harrison (Harrison, 1906). As early as 1900, researchers wanted to recapitulate organogenesis in culture and to do so they began by culturing tissue fragments. In these pioneering experiments, Harrison wanted to study the origin of nerve fibers. He took a fragment of embryo nerve cord and placed it on a drop of lymph on a coverslip, which was inverted and sealed over a hollowed slide (Harrison, 1906). This setting provided an adequate environment for the nerve fibers to grow into the medium. Other researchers further adapted this system to culture tissues of diverse origins for prolonged periods of time (Burrows, 1910; Carrel and Burrows, 1910; Fell, 1972). By 1914, Thomson (1914) defined cultures as “controlled” or “uncontrolled” in reference to the fact that in the former the histological and functional characteristics of the original organ were maintained, whereas in the latter tissue organization and functionality were lost. During the 1920s, research focused on embryology, especially limb morphogenesis, leading to the development of tube cultures (Strangeways and Fell, 1926) and the watch glass method, which consisted of a concave glass surface holding a plasma clot in its center over which the tissue fragment/organ rudiment was embedded and cultured. The watch glass was enclosed in a Petri dish carpeted with wet cotton wool (Fell and Robison, 1929). By the 1950s, many other organs had been cultured in vitro, but with the limitations imposed by these culture methods and the lens paper method, which allows the culture of thin organ slices (Trowell, 1954, 1955). Mostly researchers worked on avoiding the migration of cells from the tissue specimen, tried to optimize the gas exchange conditions, and reduce necrosis. In this same time frame, researchers started to analyze the regenerative capacity of dissociated cells. As early as 1907, Wilson (1907) showed that sponges could be broken down to single cells that were able to reassociate into tissue-like structures. The same was true for Coelenterates (De Morgan and Drew, 1914) and amphibian embryonic cells (Holtfreter, 1948). Moscona, in the early 1950s, established a method to enzymatically digest limb and kidney rudiments of early chick embryos. He cultured these cells in suspension and showed that they were able to reaggregate and reestablish the structural pattern of their tissue of origin (Moscona and Moscona, 1952; Moscona, 1959). Thus, by the middle of the twentieth century, researchers were working toward generating organs from dissociated cells, albeit mainly in suspension. Knowing that collagen was a universal component of connective tissues, as early as 1932, Huzella (1932) and collaborators had experimented culturing cells on fibrous collagen, a condition that is considered a 2D culture. However, it was in 1956 that Robert Ehrmann and George Gey published a method to reconstitute collagen extracted from rat-tail tendons as a transparent gel (Ehrmann and Gey, 1956). In their original paper, 29 cells lines and tissues were tested and, in most cases, cells grew and survived better on top of collagen dried on 2D dishes than on glass or plasma clots. At that time, Mandl et al. (1953) isolated collagenase from Clostridium histolyticum and, four years later, Lasfargues (1957) established a method using collagenase to dissociate adult mouse mammary gland tissue, generating mammary organoids (duct fragments) devoid of fibroblasts and adipocytes. This was the rationale that led to the establishment of a method to produce millions of viable hepatocytes by Berry and Friend (1969) by perfusing livers with this same collagenase. Adult and embryonic hepatocytes, as well as other cell types, were shown to grow better on collagen, but in most cases cells were losing their differentiation functions after one or more days in culture (Bissell and Tilles, 1971). Michalopoulos and Pitot (1975) determined in 1975 that it was possible to trigger differentiation of specific epithelial cells, such as hepatocytes, by modifying the behavior of the substratum to which they were attached; in particular, these researchers observed that, when cells exerted pressure on the thick collagen gels on which they were growing, the gels detached and floated on the top of the medium. Thus, floatation itself created a more permissive environment for differentiation, although they did not report a mechanism. When these researchers imitated the phenomena by rimming the gels to allow them to float, adult liver cells expressed tissue-specific markers of differentiation. Emerman and Pitelka (1977) performed the same experimental condition with dissociated normal mammary epithelial cells derived from pregnant mice in the presence of lactogenic hormones and showed that when gels were made to float, cells maintained some milk protein expression for a month in culture, a phenomenon that was not observed on plastic, glass, or attached collagen gels (Emerman and Pitelka, 1977).
Meanwhile, in New Jersey, Richard Swarm and his group were working on the characterization of the ECM of chondrosarcomas, unraveling the interactions between collagen, hyaluronic acid, and associated proteins. In doing so, they isolated a gel with characteristics of the basement membrane and named it EHS sarcoma using the initials of the three investigators—Engelbreth, Holm, and Swarm (Swarm, 1963; Orkin et al., 1977)—who discovered and defined it. This was the discovery of what we know today as Matrigel (Kleinman and Martin, 2005), or otherwise, more accurately laminin-rich gel. In the seminal paper of Orkin et al. (1977), the researchers determined that collagen IV is a major constituent of the matrix isolated from the tumor. Laminin was characterized two years later, also extracted from the matrix of the EHS sarcoma (Timpl et al., 1979). Antibodies raised against laminin determined that it was a constituent of basement membranes in normal tissues. Fibronectin had been discovered in 1973 in the context of cultured cells (Gahmberg and Hakomori, 1973; Hynes, 1973; Ruoslahti et al., 1973) and was found to be a major component of basement membrane (Stenman and Vaheri, 1978).
1980–2016: Understanding the mechanisms of morphogenesis
The early period described above set the stage for the next era in the development of organoid and 3D cultures. By 1980, researchers had the tools (both material and conceptual) to start unraveling the mysteries that govern tissue-specific function and morphogenesis. In 1981, Bissell et al. submitted a paper to the Journal of Theoretical Biology hypothesizing that ECM regulates gene expression. The article titled “How does extracellular matrix regulate gene expression” (Bissell et al., 1982) was published the same year Hall et al. (1982) determined that if MDCK or normal murine mammary gland cells cultured on top of collagen I gels, once confluent, were overlaid with another collagen layer (the epithelium in response to a collagen cocoon), cells rearranged to form tissue-like structures, which is compatible with the notion that it is the matrix that pulls the strings. A similar phenomena was described for follicle formation from isolated thyroid cells (Chambard et al., 1981). Using floating collagen gels as described by Emerman and Pitelka (1977) and radioactive carbon, Lee et al. (1984, 1985) showed that the milk that was produced and secreted indeed was produced endogenously. Evidence emerged that de novo synthesis of milk proteins was under the control of the ECM (Lee et al., 1984, 1985). The idea that the ECM influences gene expression was further supported by culturing mouse mammary cells on an EHS matrix where synthesis of β-casein was observed in more than 90% of the cells, together with the formation of glandular structures (Li et al., 1987; Barcellos-Hoff et al., 1989). Other cell types such as rat Sertoli cells cultured either on, or inside, an EHS matrix were shown to undergo striking changes in morphology and secretory activity (Hadley et al., 1985), and so were rat hepatocytes (Bissell et al., 1987; Schuetz et al., 1988), avian neural crest cell cultures (Maxwell and Forbes, 1987), and exocrine acinar epithelial cells (Oliver et al., 1987). Similar results were obtained when primary normal human prostate cells were cultured in EHS (Fong et al., 1991). The concept that certain components of the matrix were regulating cell morphogenesis and differentiation was palpable; the question that remained was, what is the exact mechanism? In 1990, further evidence suggested that matrix components were interacting with response elements in the nucleus (Schmidhauser et al., 1990, 1992; Myers et al., 1998). Simultaneously Streuli and Bissell (1990) demonstrated that the appearance of milk protein after collagen gels were floated was concomitant with formation of an endogenous basement membrane in primary mammary cells. The first evidence that the ECM, through direct interaction with integrins, was regulating gene expression and differentiation came in 1991 when Streuli et al. (1991) embedded single mammary cells inside a laminin-rich ECM. They showed that these cells were able to synthsize β-casein when laminin was present, but treatment with an anti–β1 integrin antibody would prevent expression of the milk protein. Further studies determined that pure laminin-111, but not other ECM components, directs the expression of the β-casein gene through reporter assays (Streuli et al., 1995) and that both α6β4 and β1 integrins are involved (Muschler et al., 1999).
As evidence accumulated showing the critical role of the ECM in orchestrating tissue morphogenesis and function using animal models, primary human breast cells and tumor biopsied carcinoma cells were cultured in an EHS matrix, showing that this culture system recapitulates the growth behavior and the structural and functional differentiation of these cells in vivo. This work established a culture method that allowed the distinction of normal cells from cancer cells (Petersen et al., 1992).
As the 1990s progressed, other researchers started testing 3D organoid cultures of different origin. Mammary gland is not confined only to acini. To dissect the mechanisms underlying branching morphogenesis of the mammary gland we used small pieces of the gland of virgin mice that we referred to as organoids, within collagen gels. We showed that morphogenesis depends on the interplay of growth factors, morphogens, and matrix metalloproteinases (MMP; Simian et al., 2001). Previously, Montesano and collaborators had described the roles of hepatocyte growth factor and TGF-β on branching morphogenesis of clonally derived mammary epithelial cells in collagen I gels (Soriano et al., 1995, 1996). Both epimorphin (Hirai et al., 1992) and MMPs were required for morphogenesis, but neither was required for proliferation. These results provided the first direct evidence for a crucial role of MMPs in branching in mammary epithelium. A study in 2006 showed that tissue geometry determines the site of branching morphogenesis (Nelson et al., 2006). Using a micropatterning approach to control the initial 3D structure of mouse mammary epithelial tubules, it was determined that tubules dictate the position of the branches by defining the local concentration of TGF-β that acts in this context as an inhibitory morphogen. Subsequently, a modified 3D culture system for primary mouse mammary organoids using laminin-rich ECM in 96-well plates to culture the organoids was established (Fata et al., 2007). This new system that enables the simultaneous examination of multiple treatments allowed the authors to establish that the interplay between growth factors, their spatial localization, and the duration of their activation as well as downstream effectors cooperate and regulate whether mammary branches initiate and/or elongate. To assess in real time the cellular behavior that leads to branching morphogenesis, the system described above was used to carry out long-term confocal time-lapse analysis (Ewald et al., 2008; Huebner et al., 2016). The behavior of both the luminal and the myoepithelial cells was followed in real time, showing that mammary ducts elongate through a distinct type of collective epithelial migration, with no leading cell extensions or leading actin-rich protrusions (Ewald et al., 2008). On the whole, these experiments showed that 3D culture systems are an excellent platform to unravel the underlying mechanisms of development, providing the opportunity to understand the context in which they remain normal or go awry and have an impact on disease development.
Stem cells versus organoids
As the classical organoid field developed together with 3D cultures, much progress was being made in stem cell biology. In 1981, Evans and Kaufman (1981) established cultures of pluripotent stem cells from in vitro cultures of mouse blastocyts. In 1996, the first neurosphere cultures were characterized from mouse embryos (Reynolds and Weiss, 1996). In 1998, Thomson et al. (1998) established human blastocyst-derived pluripotent cell lines. In the mammary gland field, Max Wicha and Gabriela Dontu based methodologically on Reynold’s work (Reynolds and Weiss, 1996) designed an in vitro cultivation system that allowed the propagation of stem cells from human mammary gland and referred to them as mammospheres (Dontu et al., 2003). They showed the previously hypothesized existence of mammary progenitors capable of differentiating into luminal, myoepithelial, or both cell lineages. When single progenitor cells were cultured at low density in Matrigel, they developed into functional ductal/acinar structures. Thus, stem cells isolated either from embryos or from adult tissues could give rise to organoids. In 2009, Sato et al. (2009) used stem cells that express Leu-rich repeat–containing G protein–coupled receptor 5 isolated from primary intestinal tissue and showed that these stem cells could clonally generate crypt–villus architecture in 3D culture. Based on the mammary gland literature, these authors also used Matrigel to carry out their 3D cultures and supplemented them with factors required for the growth of intestinal epithelium. Crypt organoids were generated, consisting of a central lumen lined by villus-like epithelium and several surrounding crypt-like domains (Sato et al., 2009). This methodology was then successfully used in cultures of stomach (Barker et al., 2010), pancreas (Huch et al., 2013a), colon (Sato et al., 2011), and liver (Huch et al., 2013b). Mouse and human embryonic stem cells have also been used to generate organoids in a dish, such as polarized cortical brain tissues (Eiraku et al., 2008) and optic cups (Eiraku et al., 2011; Nakano et al., 2012). Induced pluripotent stem cells, a breakthrough that took place in 2007, have provided an additional tool to study morphogenesis (Takahashi et al., 2007; Yu et al., 2007; Park et al., 2008). Lancaster et al. (2013) established a culture method that allowed the generation of cerebral organoids from induced pluripotent stem cells derived from skin fibroblasts from a patient with microcephaly. In a recent review, Kelava and Lancaster (2016) outlined the importance of the ECM and the microenvironment in the recent advances in organoid cultures of brain tissues, implicitly demonstrating the relevance the last century has had in the current advances. In the context of the methodological and experimental journey we have revisited in this article, we define an organoid as a unit of function of a given organ that is able to reproduce, in culture, a biological structure similar in architecture and function to its counterpart in vivo. The origin of this unit is today multiple, as it can come from a fragment of tissue, a stem cell located in an adult organ, an embryonic stem cell, or an induced pluripotent stem cell. However, it is critical to remember that the relevance or necessity of stem cells to production of organoids may be overstated to say the least. Even unselected single human breast cells were shown to be capable of making beautiful clonal acini, which of course are organoids when in 3D cultures, as early as 1992 (Petersen et al., 1992). Even more astonishing, single malignant breast cells under de right conditions can make phenotypically normal organoids that do not make tumors in animals (Weaver et al., 1997; Bissell and Hines, 2011). We should be mindful of claims of “stemness” being necessary for formation of organoids. The moral here is that when the right context is created, a single cell derived from a frozen mammary gland of a sheep can become a dolly! The complexity and paradoxes of biology are also its beauty, which never ceases to beckon us to go deeply in search of anwers.
“While it is true that the usefulness of a culture system is increased by how far it is developed to mimic the in vivo situation, we use cultured cells because the in vivo events, in fact, are not well understood.” Bissell, 1981
Exploiting the power of organoids: Future directions
Many of the discoveries described here have been overlooked in the oganoid literature in the past six years or so. Many scientists have contributed to the technological development of systems allowing the culture of organoids from practically any mouse or human organ: It behooves those newcomers who publish under the title of organoids to familiarize themselves with the history and accomplishments of past pioneers, many of whom are no longer with us. This will allow a depth of appreciation that is needed to solve problems in developmental biology, differentiation, mechanisms of the maintenance of tissue-specific function, aging, and cancer. There is of course a lot more to do in this field. Exciting new platforms are currently being developed. The advances in biotechnology areas, such as tissue engineering, biomaterials, and micro- and biofabrication, have set the stage for the development of devices where microfabrication and microfluidic technology are combined to support organoid culture and fluid flow, enabling high-throughput testing, environmental sampling, and biosensing (Skardal et al., 2016). These technologies are currently being explored in a range of tissue types and could have significant impact in medicine if attention is paid to functional differentiation and integrity of form and function maintainance. At such time, these will be ready to be implemented not only in drug discovery but also in patient treatment. The future will improve multi-organoid systems, also referred to as “body on a chip,” developing systems of increased biological complexity, where multiple organoids derived from different tissues are brought together and allowed to integrate (Maschmeyer et al., 2015a,b).
The future is certainly going to have many ways to study organs in correct context. Whether we call these 3D cultures or organoids is like calling a rose by any other name. What we should keep in mind is that the essence is the same. The field has arrived and scientists now appreciate that “dimensionality” and context hugely matter. This field and this way of thinking will remain central in the development of new therapeutic strategies and in the advancement of personalized medicine. In studying 3D cultures and organoids, revisiting the past allows us to understand where we should be going.
Acknowledgments
The authors of this perspective apologize to the many colleagues whose work they were not able to cite because of space limitations.
The writing of this article is supported by a grant awarded by the Instituto Nacional del Cáncer, Ministerio de Salud de la Nación, Argentina, to M. Simian. M.J. Bissell’s research is supported by the National Cancer Institute, the U.S. Department of Energy, the U.S. Department of Defense, and the Breast Cancer Research Foundation.
The authors declare no competing financial interests.
References
- Barcellos-Hoff M.H., Aggeler J., Ram T.G., and Bissell M.J.. 1989. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 105:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M., et al. 2010. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 6:25–36. 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- Berry M.N., and Friend D.S.. 1969. High-yield preparation of isolated rat liver parenchymal cells: A biochemical and fine structural study. J. Cell Biol. 43:506–520. 10.1083/jcb.43.3.506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M.J. 1981. The differentiated state of normal and malignant cells or how to define a “normal” cell in culture. Int. Rev. Cytol. 70:27–100. 10.1016/S0074-7696(08)61130-4 [DOI] [PubMed] [Google Scholar]
- Bissell D.M., and Tilles J.G.. 1971. Morphology and function of cells of human embryonic liver in monolayer culture. J. Cell Biol. 50:222–231. 10.1083/jcb.50.1.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M.J., and Hines W.C.. 2011. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 17:320–329. 10.1038/nm.2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D.M., Arenson D.M., Maher J.J., and Roll F.J.. 1987. Support of cultured hepatocytes by a laminin-rich gel. Evidence for a functionally significant subendothelial matrix in normal rat liver. J. Clin. Invest. 79:801–812. 10.1172/JCI112887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M.J., Hall H.G., and Parry G.. 1982. How does the extracellular matrix direct gene expression?. J. Theor. Biol. 99:31–68. 10.1016/0022-5193(82)90388-5 [DOI] [PubMed] [Google Scholar]
- Burrows M.T. 1910. The cultivation of tissues of the chick-embryo outside the body. J. Am. Med. Assoc. 55:2057–2058. 10.1001/jama.1910.04330240035009 [DOI] [Google Scholar]
- Carrel A., and Burrows M.T.. 1910. Cultivation of adult tissues and organs outside of the body. J. Am. Med. Assoc. 55:1379–1381. 10.1001/jama.1910.04330160047018 [DOI] [Google Scholar]
- Chambard M., Gabrion J., and Mauchamp J.. 1981. Influence of collagen gel on the orientation of epithelial cell polarity: Follicle formation from isolated thyroid cells and from preformed monolayers. J. Cell Biol. 91:157–166. 10.1083/jcb.91.1.157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. 2016. Modeling development and disease with organoids. Cell. 165:1586–1597. 10.1016/j.cell.2016.05.082 [DOI] [PubMed] [Google Scholar]
- De Morgan W., and Drew G.. 1914. A study of the restitution masses formed by the dissociated cells of the hydroids Antennularia ramosa and A. antennina. J. Mar. Biol. Assoc. U. K. 10:440–463. 10.1017/S0025315400008237 [DOI] [Google Scholar]
- Dontu G., Abdallah W.M., Foley J.M., Jackson K.W., Clarke M.F., Kawamura M.J., and Wicha M.S.. 2003. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17:1253–1270. 10.1101/gad.1061803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duryee W.R., and Doherty J.K.. 1954. Nuclear and cytoplasmic organoids in the living cell. Ann. NY Acad. Sci. 58:1210–1231. 10.1111/j.1749-6632.1954.tb45904.x [DOI] [PubMed] [Google Scholar]
- Ehrmann R.L., and Gey G.O.. 1956. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J. Natl. Cancer Inst. 16:1375–1403. [PubMed] [Google Scholar]
- Eiraku M., Watanabe K., Matsuo-Takasaki M., Kawada M., Yonemura S., Matsumura M., Wataya T., Nishiyama A., Muguruma K., and Sasai Y.. 2008. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 3:519–532. 10.1016/j.stem.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T., and Sasai Y.. 2011. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature. 472:51–56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Emerman J.T., and Pitelka D.R.. 1977. Maintenance and induction of morphological differentiation in dissociated mammary epithelium on floating collagen membranes. In Vitro. 13:316–328. 10.1007/BF02616178 [DOI] [PubMed] [Google Scholar]
- Evans M.J., and Kaufman M.H.. 1981. Establishment in culture of pluripotential cells from mouse embryos. Nature. 292:154–156. 10.1038/292154a0 [DOI] [PubMed] [Google Scholar]
- Ewald A.J., Brenot A., Duong M., Chan B.S., and Werb Z.. 2008. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell. 14:570–581. 10.1016/j.devcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fata J.E., Mori H., Ewald A.J., Zhang H., Yao E., Werb Z., and Bissell M.J.. 2007. The MAPKERK-1,2 pathway integrates distinct and antagonistic signals from TGFα and FGF7 in morphogenesis of mouse mammary epithelium. Dev. Biol. 306:193–207. 10.1016/j.ydbio.2007.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehullah A., Tan S.H., and Barker N.. 2016. Organoids as an in vitro model of human development and disease. Nat. Cell Biol. 18:246–254. 10.1038/ncb3312 [DOI] [PubMed] [Google Scholar]
- Fell H.B. 1972. Tissue culture and its contribution to biology and medicine. J. Exp. Biol. 57:1–13. [DOI] [PubMed] [Google Scholar]
- Fell H.B., and Robison R.. 1929. The growth, development and phosphatase activity of embryonic avian femora and limb-buds cultivated in vitro. Biochem. J. 23:767–784. 10.1042/bj0230767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong C.J., Sherwood E.R., Sutkowski D.M., Abu-Jawdeh G.M., Yokoo H., Bauer K.D., Kozlowski J.M., and Lee C.. 1991. Reconstituted basement membrane promotes morphological and functional differentiation of primary human prostatic epithelial cells. Prostate. 19:221–235. 10.1002/pros.2990190304 [DOI] [PubMed] [Google Scholar]
- Gahmberg C.G., and Hakomori S.I.. 1973. Altered growth behavior of malignant cells associated with changes in externally labeled glycoprotein and glycolipid. Proc. Natl. Acad. Sci. USA. 70:3329–3333. 10.1073/pnas.70.12.3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko S.M. 1964. [Organoid teratoma of the nose in an infant]. Vestn. Otorinolaringol. 26:92–94. [PubMed] [Google Scholar]
- Hadley M.A., Byers S.W., Suárez-Quian C.A., Kleinman H.K., and Dym M.. 1985. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 101:1511–1522. 10.1083/jcb.101.4.1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall H.G., Farson D.A., and Bissell M.J.. 1982. Lumen formation by epithelial cell lines in response to collagen overlay: A morphogenetic model in culture. Proc. Natl. Acad. Sci. USA. 79:4672–4676. 10.1073/pnas.79.15.4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.G. 1906. Observations on the living developing nerve fiber. Exp. Biol. Med. 4:140–143. 10.3181/00379727-4-98 [DOI] [Google Scholar]
- Hirai Y., Takebe K., Takashina M., Kobayashi S., and Takeichi M.. 1992. Epimorphin: A mesenchymal protein essential for epithelial morphogenesis. Cell. 69:471–481. 10.1016/0092-8674(92)90448-L [DOI] [PubMed] [Google Scholar]
- Holtfreter J. 1948. The mechanism of embryonic induction and its relation to parthenogenesis and malignancy. In Symposia of the Society for Experimental Biology. Cambridge University Press, Cambridge, England, UK. 17. [Google Scholar]
- Huch M., Bonfanti P., Boj S.F., Sato T., Loomans C.J., van de Wetering M., Sojoodi M., Li V.S., Schuijers J., Gracanin A., et al. 2013a Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 32:2708–2721. 10.1038/emboj.2013.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huch M., Dorrell C., Boj S.F., van Es J.H., Li V.S., van de Wetering M., Sato T., Hamer K., Sasaki N., Finegold M.J., et al. 2013b In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature. 494:247–250. 10.1038/nature11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner R.J., Neumann N.M., and Ewald A.J.. 2016. Mammary epithelial tubes elongate through MAPK-dependent coordination of cell migration. Development. 143:983–993. 10.1242/dev.127944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huzella T. 1932. Orientation de la croissance des cultures de tissus sur la trame fibrillaire artificielle coagulée de la solution de collagène. SAC r. Soc. Biol. Paris. 109:515. [Google Scholar]
- Hynes R.O. 1973. Alteration of cell-surface proteins by viral transformation and by proteolysis. Proc. Natl. Acad. Sci. USA. 70:3170–3174. 10.1073/pnas.70.11.3170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.N., Psaila B., and Lyden D.. 2007. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol. Med. 13:72–81. 10.1016/j.molmed.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Kelava I., and Lancaster M.A.. 2016. Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol. 420:199–209. 10.1016/j.ydbio.2016.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H.K., and Martin G.R.. 2005. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 15:378–386. 10.1016/j.semcancer.2005.05.004 [DOI] [PubMed] [Google Scholar]
- LaBarge M.A., Petersen O.W., and Bissell M.J.. 2007. Of microenvironments and mammary stem cells. Stem Cell Rev. 3:137–146. 10.1007/s12015-007-0024-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster M.A., and Knoblich J.A.. 2014. Organogenesis in a dish: Modeling development and disease using organoid technologies. Science. 345:1247125 10.1126/science.1247125 [DOI] [PubMed] [Google Scholar]
- Lancaster M.A., Renner M., Martin C.A., Wenzel D., Bicknell L.S., Hurles M.E., Homfray T., Penninger J.M., Jackson A.P., and Knoblich J.A.. 2013. Cerebral organoids model human brain development and microcephaly. Nature. 501:373–379. 10.1038/nature12517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasfargues E.Y. 1957. Cultivation and behavior in vitro of the normal mammary epithelium of the adult mouse. Anat. Rec. 127:117–129. 10.1002/ar.1091270111 [DOI] [PubMed] [Google Scholar]
- Lee E.Y., Parry G., and Bissell M.J.. 1984. Modulation of secreted proteins of mouse mammary epithelial cells by the collagenous substrata. J. Cell Biol. 98:146–155. 10.1083/jcb.98.1.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.Y., Lee W.H., Kaetzel C.S., Parry G., and Bissell M.J.. 1985. Interaction of mouse mammary epithelial cells with collagen substrata: Regulation of casein gene expression and secretion. Proc. Natl. Acad. Sci. USA. 82:1419–1423. 10.1073/pnas.82.5.1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M.L., Aggeler J., Farson D.A., Hatier C., Hassell J., and Bissell M.J.. 1987. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc. Natl. Acad. Sci. USA. 84:136–140. 10.1073/pnas.84.1.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon D., McKay T.B., Sarkar-Nag A., Priyadarsini S., and Karamichos D.. 2015. Human keratoconus cell contractility is mediated by transforming growth factor-β isoforms. J. Funct. Biomater. 6:422–438. 10.3390/jfb6020422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandl I., MacLennan J.D., and Howes E.L.. 1953. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J. Clin. Invest. 32:1323–1329. 10.1172/JCI102861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschmeyer I., Hasenberg T., Jaenicke A., Lindner M., Lorenz A.K., Zech J., Garbe L.A., Sonntag F., Hayden P., Ayehunie S., et al. 2015a Chip-based human liver–intestine and liver–skin co-cultures—A first step toward systemic repeated dose substance testing in vitro. Eur. J. Pharm. Biopharm. 95:77–87. 10.1016/j.ejpb.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschmeyer I., Lorenz A.K., Schimek K., Hasenberg T., Ramme A.P., Hübner J., Lindner M., Drewell C., Bauer S., Thomas A., et al. 2015b A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip. 15:2688–2699. 10.1039/C5LC00392J [DOI] [PubMed] [Google Scholar]
- Maxwell G.D., and Forbes M.E.. 1987. Exogenous basement-membrane-like matrix stimulates adrenergic development in avian neural crest cultures. Development. 101:767–776. [DOI] [PubMed] [Google Scholar]
- Mesa K.R., Rompolas P., and Greco V.. 2015. The dynamic duo: Niche/stem cell interdependency. Stem Cell Reports. 4:961–966. 10.1016/j.stemcr.2015.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G., and Pitot H.C.. 1975. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp. Cell Res. 94:70–78. 10.1016/0014-4827(75)90532-7 [DOI] [PubMed] [Google Scholar]
- Moscona A.A. 1959. Tissues from dissociated cells. Sci. Am. 200:132–134. 10.1038/scientificamerican0559-132 [DOI] [PubMed] [Google Scholar]
- Moscona A., and Moscona H.. 1952. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J. Anat. 86:287–301. [PMC free article] [PubMed] [Google Scholar]
- Muschler J., Lochter A., Roskelley C.D., Yurchenco P., and Bissell M.J.. 1999. Division of labor among the α6β4 integrin, β1 integrins, and an E3 laminin receptor to signal morphogenesis and β-casein expression in mammary epithelial cells. Mol. Biol. Cell. 10:2817–2828. 10.1091/mbc.10.9.2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers C.A., Schmidhauser C., Mellentin-Michelotti J., Fragoso G., Roskelley C.D., Casperson G., Mossi R., Pujuguet P., Hager G., and Bissell M.J.. 1998. Characterization of BCE-1, a transcriptional enhancer regulated by prolactin and extracellular matrix and modulated by the state of histone acetylation. Mol. Cell. Biol. 18:2184–2195. 10.1128/MCB.18.4.2184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano T., Ando S., Takata N., Kawada M., Muguruma K., Sekiguchi K., Saito K., Yonemura S., Eiraku M., and Sasai Y.. 2012. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 10:771–785. 10.1016/j.stem.2012.05.009 [DOI] [PubMed] [Google Scholar]
- Nelson C.M., Vanduijn M.M., Inman J.L., Fletcher D.A., and Bissell M.J.. 2006. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science. 314:298–300. 10.1126/science.1131000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C., Waters J.F., Tolbert C.L., and Kleinman H.K.. 1987. Growth of exocrine acinar cells on a reconstituted basement membrane gel. In Vitro Cell. Dev. Biol. 23:465–473. 10.1007/BF02628416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin R.W., Gehron P., McGoodwin E.B., Martin G.R., Valentine T., and Swarm R.. 1977. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 145:204–220. 10.1084/jem.145.1.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park I.H., Lerou P.H., Zhao R., Huo H., and Daley G.Q.. 2008. Generation of human-induced pluripotent stem cells. Nat. Protoc. 3:1180–1186. 10.1038/nprot.2008.92 [DOI] [PubMed] [Google Scholar]
- Petersen O.W., Rønnov-Jessen L., Howlett A.R., and Bissell M.J.. 1992. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc. Natl. Acad. Sci. USA. 89:9064–9068. 10.1073/pnas.89.19.9064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkus H. 1976. Organoid nevus. Mod. Probl. Paediatr. 20:50–57. [PubMed] [Google Scholar]
- Pomerat C.M., Lefeber C.G., and Smith M.. 1954. Quantitative cine analysis of cell organoid activity. Ann. NY Acad. Sci. 58:1311–1321. 10.1111/j.1749-6632.1954.tb45911.x [DOI] [PubMed] [Google Scholar]
- Reynolds B.A., and Weiss S.. 1996. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev. Biol. 175:1–13. 10.1006/dbio.1996.0090 [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A., Kuusela P., and Linder E.. 1973. Fibroblast surface antigen: A new serum protein. Biochim. Biophys. Acta. 322:352–358. 10.1016/0005-2795(73)90310-3 [DOI] [PubMed] [Google Scholar]
- Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., and Clevers H.. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459:262–265. 10.1038/nature07935 [DOI] [PubMed] [Google Scholar]
- Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Van den Brink S., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., and Clevers H.. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 141:1762–1772. 10.1053/j.gastro.2011.07.050 10.1053/j.gastro.2011.07.050 [DOI] [PubMed] [Google Scholar]
- Schmidhauser C., Bissell M.J., Myers C.A., and Casperson G.F.. 1990. Extracellular matrix and hormones transcriptionally regulate bovine beta-casein 5′ sequences in stably transfected mouse mammary cells. Proc. Natl. Acad. Sci. USA. 87:9118–9122. 10.1073/pnas.87.23.9118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C., Casperson G.F., Myers C.A., Sanzo K.T., Bolten S., and Bissell M.J.. 1992. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of beta-casein gene expression. Mol. Biol. Cell. 3:699–709. 10.1091/mbc.3.6.699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Muirhead E.E., and Zydeck F.A.. 1963. Some unusual observations of organoid tissues and blood elements in monolayer cultures. Exp. Cell Res. 30:449–459. 10.1016/0014-4827(63)90322-7 [DOI] [PubMed] [Google Scholar]
- Schofield R. 1978. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 4:7–25. [PubMed] [Google Scholar]
- Schuetz E.G., Li D., Omiecinski C.J., Muller-Eberhard U., Kleinman H.K., Elswick B., and Guzelian P.S.. 1988. Regulation of gene expression in adult rat hepatocytes cultured on a basement membrane matrix. J. Cell. Physiol. 134:309–323. 10.1002/jcp.1041340302 [DOI] [PubMed] [Google Scholar]
- Shamir E.R., and Ewald A.J.. 2014. Three-dimensional organotypic culture: Experimental models of mammalian biology and disease. Nat. Rev. Mol. Cell Biol. 15:647–664. 10.1038/nrm3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simian M., Hirai Y., Navre M., Werb Z., Lochter A., and Bissell M.J.. 2001. The interplay of matrix metalloproteinases, morphogens and growth factors is necessary for branching of mammary epithelial cells. Development. 128:3117–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skardal A., Shupe T., and Atala A.. 2016. Organoid-on-a-chip and body-on-a-chip systems for drug screening and disease modeling. Drug Discov. Today. 21:1399–1411. 10.1016/j.drudis.2016.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J.V., Pepper M.S., Nakamura T., Orci L., and Montesano R.. 1995. Hepatocyte growth factor stimulates extensive development of branching duct-like structures by cloned mammary gland epithelial cells. J. Cell Sci. 108:413–430. [DOI] [PubMed] [Google Scholar]
- Soriano J.V., Orci L., and Montesano R.. 1996. TGF-β1 induces morphogenesis of branching cords by cloned mammary epithelial cells at subpicomolar concentrations. Biochem. Biophys. Res. Commun. 220:879–885. 10.1006/bbrc.1996.0499 [DOI] [PubMed] [Google Scholar]
- Stenman S., and Vaheri A.. 1978. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J. Exp. Med. 147:1054–1064. 10.1084/jem.147.4.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strangeways T., and Fell H.B.. 1926. Experimental studies on the differentiation of embryonic tissues growing in vivo and in vitro.–II. The development of the isolated early embryonic eye of the fowl when cultivated in vitro. Proc. R. Soc. Lond., B. 100:273–283. 10.1098/rspb.1926.0049 [DOI] [Google Scholar]
- Streuli C.H., and Bissell M.J.. 1990. Expression of extracellular matrix components is regulated by substratum. J. Cell Biol. 110:1405–1415. 10.1083/jcb.110.4.1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C.H., Bailey N., and Bissell M.J.. 1991. Control of mammary epithelial differentiation: Basement membrane induces tissue-specific gene expression in the absence of cell–cell interaction and morphological polarity. J. Cell Biol. 115:1383–1395. 10.1083/jcb.115.5.1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli C.H., Schmidhauser C., Bailey N., Yurchenco P., Skubitz A.P., Roskelley C., and Bissell M.J.. 1995. Laminin mediates tissue-specific gene expression in mammary epithelia. J. Cell Biol. 129:591–603. 10.1083/jcb.129.3.591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarm R.L. 1963. Transplantation of a murine chondrosarcoma in mice of different inbred strains. J. Natl. Cancer Inst. 31:953–975. [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., and Yamanaka S.. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. 10.1016/j.cell.2007.11.019 [DOI] [PubMed] [Google Scholar]
- Thomson D. 1914. Controlled growth en masse (somatic growth) of embryonic chick tissue in vitro. Proc. R. Soc. Med. 7:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson J.A., Itskovitz-Eldor J., Shapiro S.S., Waknitz M.A., Swiergiel J.J., Marshall V.S., and Jones J.M.. 1998. Embryonic stem cell lines derived from human blastocysts. Science. 282:1145–1147. 10.1126/science.282.5391.1145 [DOI] [PubMed] [Google Scholar]
- Timpl R., Rohde H., Robey P.G., Rennard S.I., Foidart J.M., and Martin G.R.. 1979. Laminin—a glycoprotein from basement membranes. J. Biol. Chem. 254:9933–9937. [PubMed] [Google Scholar]
- Trowell O.A. 1954. A modified technique for organ culture in vitro. Exp. Cell Res. 6:246–248. 10.1016/0014-4827(54)90169-X [DOI] [PubMed] [Google Scholar]
- Trowell O.A. 1955. Experiments on lymph nodes cultured in vitro. Ann. NY Acad. Sci. 59:1066–1069. 10.1111/j.1749-6632.1955.tb46002.x [DOI] [PubMed] [Google Scholar]
- Wang F., Weaver V.M., Petersen O.W., Larabell C.A., Dedhar S., Briand P., Lupu R., and Bissell M.J.. 1998. Reciprocal interactions between β1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: A different perspective in epithelial biology. Proc. Natl. Acad. Sci. USA. 95:14821–14826. 10.1073/pnas.95.25.14821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver V.M., and Bissell M.J.. 1999. Functional culture models to study mechanisms governing apoptosis in normal and malignant mammary epithelial cells. J. Mammary Gland Biol. Neoplasia. 4:193–201. 10.1023/A:1018781325716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver V.M., Petersen O.W., Wang F., Larabell C.A., Briand P., Damsky C., and Bissell M.J.. 1997. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J. Cell Biol. 137:231–245. 10.1083/jcb.137.1.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver V.M., Lelièvre S., Lakins J.N., Chrenek M.A., Jones J.C., Giancotti F., Werb Z., and Bissell M.J.. 2002. β4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2:205–216. 10.1016/S1535-6108(02)00125-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H. 1907. On some phenomena of coalescence and regeneration in sponges. J. Exp. Zool. 5:245–258. 10.1002/jez.1400050204 [DOI] [Google Scholar]
- Wolter J.R. 1967. Proliferating pigment epithelium. Producing a simple organoid structure in the subrentinal space of a human eye. Arch. Ophthalmol. 77:651–654. 10.1001/archopht.1967.00980020653016 [DOI] [PubMed] [Google Scholar]
- Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S., Nie J., Jonsdottir G.A., Ruotti V., Stewart R., et al. 2007. Induced pluripotent stem cell lines derived from human somatic cells. Science. 318:1917–1920. 10.1126/science.1151526 [DOI] [PubMed] [Google Scholar]