Abstract
The purpose of the present study was to examine the effects of the phenylalkylamine class of the L-type voltage-dependent calcium channel antagonist, verapamil (1.0, 2.5, 5.0, or 10 mg/kg i.p.), administered immediately after the acquisition task, on memory consolidation of the open field habituation task, in male Wistar rats. On the 48 h retested trial, all tested parameters (ambulation in the side wall and in the central areas, number of rearing, time spent grooming and defecation rate) significantly decreased in the saline treated animals. A significant decrease of rearing was observed in all verapamil treated groups. On the retention day, the ambulation in the side wall and central areas significantly decreased in the animals treated with 1 mg/kg and 10 mg/kg of verapamil, while the time spent grooming and the defecation rate significantly decreased only in the group treated with 1 mg/kg of verapamil. According to the change ratio scores that correct the individual behavioral baseline differences during initial and final sessions, habituation deficit was found in animals treated with verapamil as follows: ambulation along the side wall area (1, 2.5, and 5 mg/kg), number of rearing (all used dose) and time spent grooming (2.5, 5, and 10 mg/kg). In conclusion, the present data suggest that the post-training administration of verapamil, parameter- and dose-dependently, impairs the habituation to a novel environment.
Keywords: habituation, memory consolidation, open field, verapamil, rat
Introduction
Three different subfamilies of calcium (Cav) channels have, thus far, been defined and named Cav1, Cav2, and Cav3 (Ertel et al., 2000; Catterall et al., 2005; Hofmann et al., 2014). The Cav1 subfamily (Cavl.l–Cavl.4) includes channels which mediate L-type Ca2+currents, whereas the Cav2 subfamily (Cav2.1–Cav2.3) comprises channels which originate P/Q-, N-, and R-type Ca2+currents, respectively. Lastly, the Cav3 subfamily (Cav3.1–Cav3.3) includes channels which mediate T-type Ca2+ currents (Ertel et al., 2000; Catterall et al., 2005; Hofmann et al., 2014).
Molecular genetics approaches clearly suggest essential implication of the Cav1.3 but not the Cav1.2 subunit of the L-type voltage-gated calcium channels (LVGCCs), in the process of memory consolidation of contextual fear conditioning in mice (McKinney and Murphy, 2006; McKinney et al., 2008). However, the effects of several classes of LVGCC antagonists, such as dihydropyridines (e.g., nimodipine, nicardipine, and nifedipine), benzothiazepines (e.g., diltiazem), phenylalkylamines (e.g., verapamil), and diphenylalkylamines (e.g., flunarizine), on memory consolidation in young mice and rats, are controversial. It has been found that nimodipine does not affect the memory consolidation of rats tested in the simple learning association (Isaacson et al., 1989), as well as of mice tested in contextual fear conditioning (Suzuki et al., 2004). Calcium channel blockers that bind principally to dihydropyridine receptors (nimodipine, nifedipine, and amlodipine) do facilitate memory consolidation of mice tested in passive avoidance, linear maze, and elevated plus-maze tasks (Quartermain et al., 1993, 2001; Biala et al., 2013). On the other hand, diltiazem and flunarizine improve memory consolidation of mice tested in passive avoidance and linear maze (Quartermain et al., 1993, 2001) but not in the elevated plus-maze task (Biala et al., 2013). Studies in mice demonstrated that systemic post-training treatment with verapamil does not affect memory consolidation in the passive avoidance task (Quartermain et al., 1993; Masoudian et al., 2015), but improves retention in linear maze and elevated plus maze tasks (Biala et al., 2013). Noting the lack of data related to the effect of LVGCC antagonists on memory consolidation in rats, the aim of the present study was to evaluate the effects of verapamil post-training treatment on memory retention, in the open field habituation task.
Materials and Methods
Experimental Animals
Experiments were carried out on male Wistar rats, weighing 200–250 g. The animals were housed in standard Makrolon cages on sawdust bedding. They were kept in an air-conditioned room (20 ± 1°C), at 30% humidity and under a 12 h light/12 h dark cycle (lights on from 08:00 to 20:00 h). Food and tap water were available ad libitum. One week before the experimental procedure, the rats were handled daily for 5 min each. The behavioral tests were performed during the light period (16:00–20:00 h).
All procedures related to the animal maintenance and experimentation were in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and the guidelines issued by the Spanish Ministry of Agriculture, Fishing and Feeding (Royal Decree 1201/2005 of October 21, 2005) and were approved by the Animal Ethics Committee of the University of Murcia. Efforts were made to minimize the number of animals used, as well as their suffering.
Drugs
The saline solution of verapamil (Sigma, St. Louis, MO, USA) was administered intraperitoneally at the dose of 1, 2.5, 5, or 10 mg/kg. Control animals were treated with physiological saline at the dose of 1 ml/kg body weight. Eight animals were assigned in each tested group.
Open Field Test
The open field test was performed in a square white plywood box (100 cm × 100 cm × 40 cm). The floor was divided into 25 (20 × 20 cm) squares. On day 1, the rats were initially placed at one of the four corners of the box and their behavior was monitored during 10 min. After that, the rats were removed from the open field, drug administered and returned to their home cage. Forty-eight hours later, the retention test was given. The open field test was performed under 300 lux light intensity and recorded using a video camera to enable subsequent evaluation. The apparatus was cleaned with 70% ethanol before each animal was tested. Eight animals were assigned in each tested group.
In the open field test, the ambulation along the side wall area (number of outer squares entered), the ambulation in the central area (number of inner squares entered), the number of rearing (standing on the hind legs, with or without contact with the sides of the arena), the time spent frozen (time that the animal spent immobile), the time spent in grooming (time that the animal spend licking, scratching or cleaning any part of its head or body) and the defecation (number of fecal boli deposited) were recorded. With the aim to correct the individual baseline differences in the studied parameters, we used a change ratio score to compare behavior during initial and final sessions. This score is calculated according to the Bolivar (2009) as follows: day 2 parameter value/(day 1 parameter value + day 2 parameter value). Thus, the change ratio score will approach 0.5 if no change in behavior has occurred, i.e., no habituation. If the ratio approaches 0, there is evidence of habituation. If the ratio approaches 1.0, the tested behavioral parameter has actually increased from the initial to the final time periods.
Statistical Analysis
The statistical analysis was made using the SPSS 19.0 statistical package. The data were analyzed with the General Linear Model (GLM) repeated measures analysis and presented as mean ± standard error of the mean (SEM). If the GLM showed significant differences between groups, a post hoc analysis was performed. The group differences on the acquisition trial were analyzed by the two-tailed Student’s t-test for independent samples. The two-tailed Student’s t-test for paired-samples was used for comparison of the data between the acquisition and the retention trial. The habituation scores were analyzed by the one-way ANOVA test, followed by the two-tailed Student’s t-test for independent samples. Differences were considered statistically significant if p < 0.05.
Results
Open Field Test
Only one animal from the saline treated group and four animals from the verapamil treated groups displayed freezing behavior (data not showed). The rest of the data from the open field test are presented in the Figure 1.
FIGURE 1.
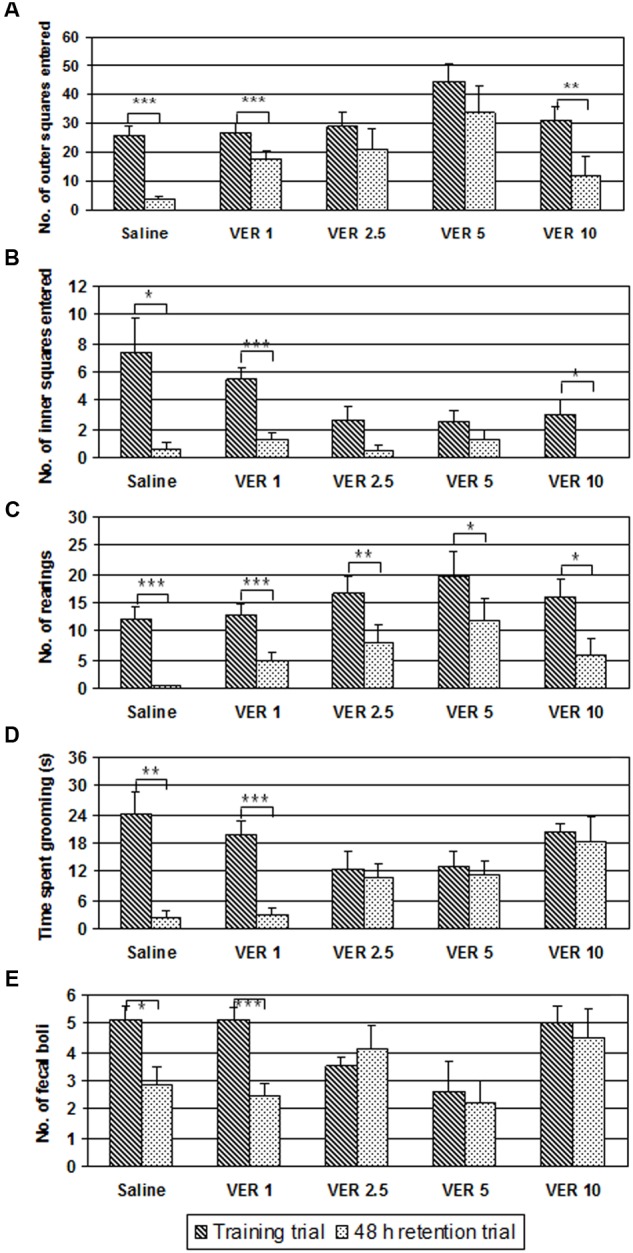
Effect of 1, 2.5, 5, and 10 mg/kg of verapamil (VER-1, VER-2.5, VER-5, and VER-10, respectively), administered intraperitoneally, immediately after the acquisition trial, on ambulation in the side wall area (A), ambulation in the central area (B), number of rearing (C), time spent grooming (D), and defecation (E), in the open field habituation task. The data are presented as mean ± standard error of the mean (SEM). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 vs. acquisition trial.
The GLM repeated measure analysis showed a significant effect of time on ambulation in the side wall area (F(1) = 47.577, p < 0.001), ambulation in the central area (F(1) = 42.976, p < 0.001), number of rearing (F(1) = 64.390, p < 0.001), time spent grooming (F(1) = 19.682, p < 0.001), and defecation (F(1) = 6.712, p < 0.05). There was significant effect of group on ambulation in the side wall area (F(4) = 3.207, p < 0.05) but not on ambulation in the central area (F(4) = 1.987, p > 0.05), number of rearing (F(4) = 2.013, p > 0.05), time spent grooming (F(4) = 2.006, p > 0.05), and defecation (F(4) = 2.454, p > 0.05). There was significant effect of interaction time × group on ambulation in the central area (F(4) = 3.261, p < 0.05) and on the time spent grooming (F(2) = 4.785, p < 0.01) but not on ambulation in the side wall area (F(4) = 2.128, p > 0.05), number of rearing (F(4) = 0.372, p > 0.05), and defecation (F(4) = 2.390, p > 0.05).
In the acquisition trial of the open field test, there were no significant differences between groups in the ambulation in the side wall area (F(4) = 2.591, p > 0.05), ambulation in the central area (F(4) = 2.542, p > 0.05), number of rearing (F(4) = 1.061, p > 0.05), time spent grooming (F(4) = 2.233, p > 0.05), and defecation rate (F(4) = 2.604, p > 0.05). The two-tailed Student’s t-test for paired-samples showed that ambulation in the side wall (Figure 1A) and in the central (Figure 1B) areas, significantly decreased on the retention day, in the saline group (t = 6.677, df = 7, p < 0.001; t = 3.441, df = 7, p < 0.05, respectively) and in the animals treated with verapamil at the dose of 1 mg/kg (t = 5.105, df = 7, p < 0.001; t = 6.298, df = 7, p < 0.001, respectively) and 10 mg/kg (t = 3.486, df = 7, p < 0.01; t = 2.722, df = 7, p < 0.05, respectively) but not in the animals treated with verapamil at the dose of 2.5 (t = 2.004, df = 7, p > 0.05; t = 1.945, df = 7, p > 0.05, respectively) and 5 mg/kg (t = 1.708, df = 7, p > 0.05; t = 2.236, df = 7, p > 0.05, respectively). The two-tailed Student’s t-test for paired-samples showed that the number of rearing (t = 5.276, df = 7, p < 0.001; t = 5.420, df = 7, p < 0.001; t = 3.804, df = 7, p < 0.01; t = 2.396, df = 7, p < 0.05; t = 3.155, df = 7, p < 0.05) (Figure 1C) significantly decreased on the retention day in both the saline- and verapamil-treated (1, 2.5, 5, and 10 mg/kg) animals, respectively. On the retention day, the time spent grooming (Figure 1D) and the defecation rate (Figure 1E) significantly decreased in the groups treated with saline (t = 4.376, df = 7, p < 0.01; t = 3.000, df = 7, p < 0.05, respectively) and verapamil at the dose of 1 mg/kg (t = 6.120, df = 7, p < 0.001; t = 8.104, df = 7, p < 0.001, respectively) but not in animals treated with verapamil at the doses of 2.5 mg/kg (t = 0.501, df = 7, p > 0.05; t = -0.804, df = 7, p > 0.05, respectively), 5 mg/kg (t = 0.367, df = 7, p > 0.05; t = 0.351, df = 7, p > 0.05, respectively), and 10 mg/kg (t = 0.346, df = 7, p > 0.05; t = 0.408, df = 7, p > 0.05, respectively).
The one-way ANOVA test revealed significant differences in the change ratio score for ambulation in the side wall area (F(4) = 7.097, p < 0.001), number of rearing (F(4) = 5.169, p < 0.01) and time spent grooming (F(4) = 5.616, p < 0.001) but not in ambulation in the central area (F(4) = 1.266, p > 0.05) and defecation rate (F(4) = 1.539, p > 0.05). The post hoc analysis showed that the change ratio score for ambulation in the side wall area was significantly higher in animals treated with verapamil in doses of 1, 2.5, and 5 mg/kg than in those treated with saline (t = -10.104, df = 14, p < 0.001; t = -4.042, df = 14, p < 0.001; t = -4.091, df = 14, p < 0.001, respectively) or 10 mg/kg of verapamil (t = 3.242, df = 14, p < 0.01; t = -2.154, df = 14, p < 0.05; t = 2.195, df = 14, p < 0.05, respectively) (Figure 2). The change ratio score for the number of rearing was significantly higher in the animals treated with verapamil (1, 2.5, 5, and 10 mg/kg), compared to those treated with saline (t = -6.216, df = 14, p < 0.001; t = -3.501, df = 14, p < 0.01; t = -4.219, df = 14, p < 0.001; t = -3.349, df = 14, p < 0.01, respectively) (Figure 2). The animals treated with verapamil in doses of 2.5, 5, and 10 mg/kg have significantly higher change score for time spent grooming than the animals treated with saline (t = -3.307, df = 14, p < 0.01; t = -3.289, df = 14, p < 0.01; t = -3.431, df = 14, p < 0.01, respectively) and verapamil in dose of 1 mg/kg (t = -3.129, df = 14, p < 0.01; t = -3.092, df = 14, p < 0.01; t = -3.206, df = 14, p < 0.01, respectively) (Figure 2).
FIGURE 2.
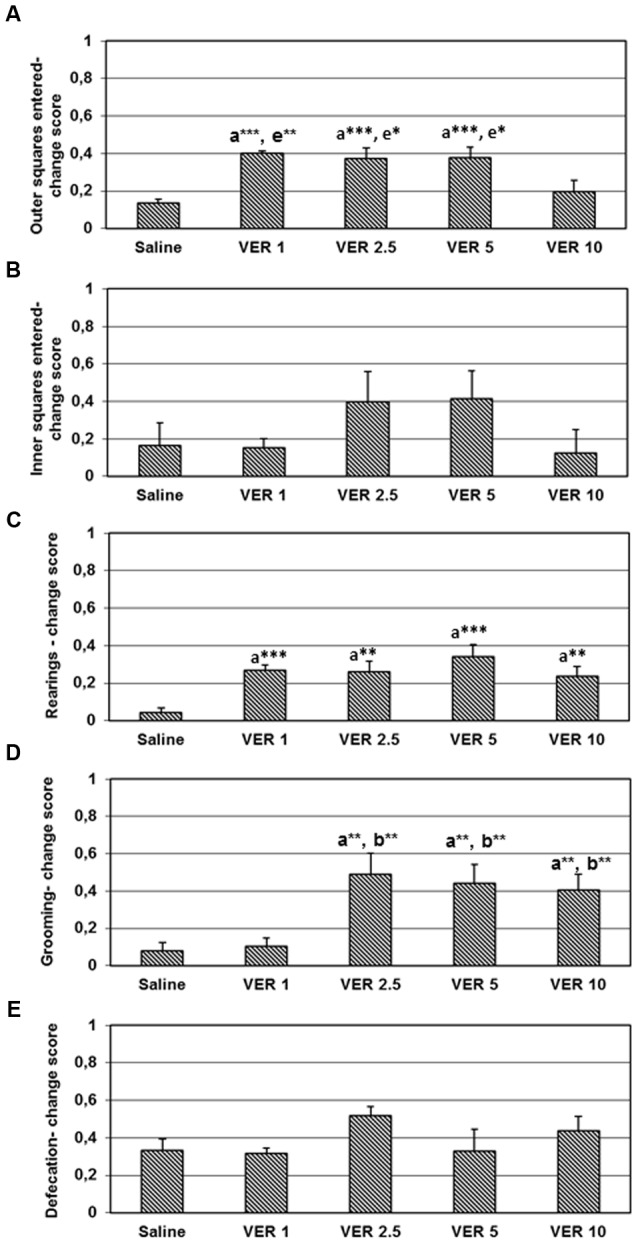
Effect of 1, 2.5, 5, and 10 mg/kg of verapamil (VER-1, VER-2.5, VER-5, and VER-10, respectively), administered i.p. immediately after the acquisition trial, on habituation score in ambulation in the side wall area (A), ambulation in the central area (B), number of rearing (C), time spent grooming (D), and defecation (E), in the open field habituation task. The data are presented as mean ± SEM. ∗∗p < 0.01, ∗∗∗p < 0.001 vs. acquisition trial. a,b,e vs. saline, VER-1 or VER-10 groups, respectively.
Discussion
In the present study, we demonstrated that verapamil, dose- and parameter-depending, affects habituation in the open field test, one of the most elementary forms of non-associative hippocampal-dependent learning (Leussis and Bolivar, 2006). Although, the animals treated with verapamil (in all tested doses), significantly reduced the rearing component of the exploratory behavior on the retention trial, the level of habituation was impaired compared to the saline treated animals. In contrast, the middle-used doses of verapamil (2.5 and 5 mg/kg) did not significantly decrease the ambulation component of the exploratory behavior, indicating, on this parameter, the inverted U-shape dose-response curve of its action. However, the habituation score of ambulation in the side wall area (verapamil 1, 2.5, and 5 mg/kg) was significantly impaired, but not the habituation score of ambulation in the central area. Regarding to grooming and defecation, a significant decrease in those parameters was found only in animals treated with verapamil in the dose of 1 mg/kg. In spite of this, the habituation score of grooming was significantly lower in animals treated with verapamil (2.5, 5, and 10 mg/kg) compared to the saline treated animals. The present data support our previous findings that mechanisms of habituation are complex and that each component could be differently affected by the drugs (Popović, 2014).
It has been demonstrated that verapamil treatment at the doses of 1, 2, and 10 mg/kg, but not at the dose of 20 mg/kg, significantly improves the consolidation of spatial memory in the linear maze task in mice (Quartermain et al., 2001). Similarly, verapamil at the doses of 5 and 10 mg/kg, but not at the doses of 2.5 and 20 mg/kg, displays an enhancement effect on the consolidation of spatial memory in the elevated plus maze task in mice (Biala et al., 2013), resembling the inverted U-shape dose-response curve of its action. It has been suggested that the inverted U-shape dose-response curve of the verapamil action, could be due to modulation rather than to a complete blockade of LVGCCs (Biala et al., 2013).
The mechanism by which verapamil facilitates or impairs memory consolidation in different tasks, is still unclear. Although classified as L-type calcium channel blocker (being Cav1.2 channels more sensitive than the Cav1.3 ones), verapamil blocks Cav2.1, Cav2.2, Cav2.3, and Cav3.2 channels too (Ishibashi et al., 1995; Cai et al., 1997; Dobrev et al., 1999; Tarabova et al., 2007; Kuryshev et al., 2014). Given that the systemic administration of verapamil (range dose 1–10 mg/kg) does not affect the open field behavior (Rogério and Takahashi, 1990; Popović, 1997), the effect of verapamil on memory consolidation of the open field habituation, seems to be independent on the anxiety level. In this line, in Cav1.3 knockout mice the open field behavior was not changed (Busquet et al., 2010), while only severe reduction in the CaV1.2 activity, in the mouse forebrain, enhanced anxiety-like behaviors in open field (Lee et al., 2012). As far to our knowledge, there are no data analyzing the implication of these subunits in the consolidation of the open field habituation.
In view of the fact that verapamil can block large conductance calcium-activated potassium channels (BK channel) (Harper et al., 2001) and that habituation of the exploratory locomotor behavior in the open field is not affected in the BK knock-out mice (Typlt et al., 2013), it could be less probable that the effect of verapamil be partially due to the action on these channels. It is possible that verapamil, through its action as an antagonist of P-glycoprotein transporters or multidrug resistance proteins (Pajeva and Wiese, 2002), impairs memory consolidation in open field task, since P-glycoprotein knockout mice display lower level of habituation than the wild-type mice (Schoenfelder et al., 2012).
Conclusion
As far to our knowledge, the present data represent the first evidence that post-training verapamil administration produces parameter- and dose-dependent impairment of habituation in the open field task in rats. Moreover, the present findings resembling the inverted U-shape dose-response curve of verapamil action.
Ethics Statement
All procedures related to the animal maintenance and experimentation were in accordance with the European Communities Council Directive of November 24, 1986 (86/609/EEC) and the guidelines issued by the Spanish Ministry of Agriculture, Fishing and Feeding (Royal Decree 1201/2005 of October 21, 2005) and were approved by the Animal Ethics Committee of the University of Murcia. Efforts were made to minimize the number of animals used, as well as their suffering.
Author Contributions
All authors (NP, VGdB, MC-B, and MP) contributed to the design of the study, wrote the protocol and managed the literature searches. Authors NP, VGdB, and MP performed the experiments and undertook the statistical analysis. All authors (NP, VGdB, MC-B, and MP) contributed to drafting the work and have approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. Funding for this study was provided by the Health Council of Murcia Region, Spain (MC) and the Spanish Ministry of Economy and Competitiveness (BFU2014-57516-P; LPL, JLF). The funding source had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
References
- Biala G., Kruk-Slomka M., Jozwiak K. (2013). Influence of acute and chronic calcium channel antagonists on the acquisition and consolidation of memory and nicotine-induced cognitive effects in mice. Naunyn Schmiedebergs Arch. Pharmacol. 386 651–664. 10.1007/s00210-013-0866-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar V. J. (2009). Intrasession and intersession habituation in mice: from inbred strain variability to linkage analysis. Neurobiol. Learn. Mem. 92 206–214. 10.1016/j.nlm.2009.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busquet P., Nguyen N. K., Schmid E., Tanimoto N., Seeliger M. W., Ben-Yosef T., et al. (2010). Cav1.3 L-type Ca2+ channels modulate depression-like behaviour in mice independent of deaf phenotype. Int. J. Neuropsychopharmacol. 13 499–513. 10.1017/S1461145709990368 [DOI] [PubMed] [Google Scholar]
- Cai D., Mulle J. G., Yue D. T. (1997). Inhibition of recombinant Ca2+ channels by benzothiazepines and phenylalkylamines: class-specific pharmacology and underlying molecular determinants. Mol. Pharmacol. 51 872–881. [PubMed] [Google Scholar]
- Catterall W. A., Perez-Reyes E., Snutch T. P., Striessnig J. (2005). International Union of Pharmacology. XLWI. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57 411–425. 10.1124/pr.57.4.4 [DOI] [PubMed] [Google Scholar]
- Dobrev D., Milde A. S., Andreas K., Ravens U. (1999). The effects of verapamil and diltiazem on N-, P- and Q-type calcium channels mediating dopamine release in rat striatum. Br. J. Pharmacol. 127 576–582. 10.1038/sj.bjp.0702574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ertel E. A., Campbell K. P., Harpold M. M., Hofmann F., Mori Y., Perez-Reyes E., et al. (2000). Nomenclature of voltage-gated calcium channels. Neuron 25 533–535. 10.1016/S0896-6273(00)81057-0 [DOI] [PubMed] [Google Scholar]
- Harper A. A., Catacuzzeno L., Trequattrini C., Petris A., Franciolini F. (2001). Verapamil block of large-conductance Ca-activated K channels in rat aortic myocytes. J. Membr. Biol. 179 103–111. 10.1007/s002320010041 [DOI] [PubMed] [Google Scholar]
- Hofmann F., Flockerzi V., Kahl S., Wegener J. W. (2014). L-Type CaV1.2 calcium channels: from in vitro findings to in vivo function. Physiol. Rev. 94 303–326. 10.1152/physrev.00016.2013 [DOI] [PubMed] [Google Scholar]
- Isaacson R. L., Maier D. L., Mandel A. H. (1989). Post training or pretest administration of nimodipine fails to affect retention of a simple learned association. Physiol. Behav. 46 191–193. 10.1016/0031-9384(89)90254-0 [DOI] [PubMed] [Google Scholar]
- Ishibashi H., Yatani A., Akaike N. (1995). Block of P-type Ca2+ channels in freshly dissociated rat cerebellar Pukinj neurons by diltiazem and verapamil. Brain Res. 695 88–91. 10.1016/0006-8993(95)00815-8 [DOI] [PubMed] [Google Scholar]
- Kuryshev Y. A., Brown A. M., Duzic E., Kirsch G. E. (2014). Evaluating state dependence and subtype selectivity of calcium channel modulators in automated electrophysiology assays. Assay Drug Dev. Technol. 12 110–119. 10.1089/adt.2013.552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Gonzales K. L., Lee A., Moosmang S., Hofmann F., Pieper A. A., et al. (2012). Selective genetic deletion of cacna1c in the mouse prefrontal cortex. Mol. Psychiatry 17 1054–1055. 10.1038/mp.2012.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis M. P., Bolivar V. J. (2006). Habituation in rodents: a review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 30 1045–1064. 10.1016/j.neubiorev.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Masoudian N., Masoudian N., Pour A. R., Vafaiee A. A., Andalib S., Vaseghi G. (2015). Evaluation effects of verapamil as a calcium channel blocker on acquisition, consolidation and retrieval of memory in mice. J. Chem. Health Risks 5 99–104. [Google Scholar]
- McKinney B. C., Murphy G. G. (2006). The L-type voltage-gated calcium channel Cav1.3 mediates consolidation, but not extinction, of contextually conditioned fear in mice. Learn. Mem. 13 584–589. 10.1101/lm.279006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney B. C., Sze W., White J. A., Murphy G. G. (2008). L-type voltage-gated calcium channels in conditioned fear: a genetic and pharmacological analysis. Learn. Mem. 15 326–334. 10.1101/lm.893808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajeva I. K., Wiese M. (2002). Pharmacophore model of drugs involved in P-glycoprotein multidrug resistance: explanation of structural variety (hypothesis). J. Med. Chem. 45 5671–5686. 10.1021/jm020941h [DOI] [PubMed] [Google Scholar]
- Popović M., Popović N., Jovanova-Nešić K., Bokonjić D., Dobrić S., Rosić N. (1997). Open field behavior in nucleus basalis magnocellularis-lesioned rats treated with physostigmine and verapamil. Int. J. Neurosci. 91 181–188. 10.3109/00207459708986375 [DOI] [PubMed] [Google Scholar]
- Popović N., Caballero-Bleda M., Popović M. (2014). Post-training scopolamine treatment induced maladaptive behavior in open field habituation task in rats. PLoS ONE 9:6 10.1371/journal.pone.0100348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartermain D., Garcia de Soria V., Kwan A. (2001). Calcium channel antagonists enhance retention of passive avoidance and maze learning in mice. Neurobiol. Learn. Mem. 75 77–90. 10.1006/nlme.1999.3958 [DOI] [PubMed] [Google Scholar]
- Quartermain D., Hawxhurst A., Ermita B., Puente J. (1993). Effect of the calcium channel blocker amlodipine on memory in mice. Behav. Neural. Biol. 60 211–219. 10.1016/0163-1047(93)90390-4 [DOI] [PubMed] [Google Scholar]
- Rogério R., Takahashi R. N. (1990). Some calcium channel antagonists have no effect on the open-field behavior of rats nor do they interact with cocaine. Braz. J. Med. Biol. Res. 23 1153–1155. [PubMed] [Google Scholar]
- Schoenfelder Y., Hiemke C., Schmitt U. (2012). Behavioural consequences of p-glycoprotein deficiency in mice, with special focus on stress-related mechanisms. J. Neuroendocrinol. 24 809–817. 10.1111/j.1365-2826.2012.02278.x [DOI] [PubMed] [Google Scholar]
- Suzuki A., Josselyn S. A., Frankland P. W., Masushige S., Silva A. J., Kida S. (2004). Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J. Neurosci. 24 4787–4795. 10.1523/JNEUROSCI.5491-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabova B., Lacinova L., Engel J. (2007). Effects of phyenilalkylamines and benzothiazepines on Cav1.3-mediated Ca2+ currents in neonatal mouse inner hair cells. Eur. J. Pharmacol. 573 39–48. 10.1016/j.ejphar.2007.06.050 [DOI] [PubMed] [Google Scholar]
- Typlt M., Mirkowski M., Azzopardi E., Ruth P., Pilz P. K. D., Schmid S. (2013). Habituation of reflexive and motivated behavior in mice with deficient BK channel function. Front. Integr. Neurosci. 7:79 10.3389/fnint.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]


