Abstract
Introduction
Crohn's disease (CD) is a chronic inflammatory disease predominantly affecting the gastrointestinal tract. CD usually requires lifelong medication and is accompanied by severe complications, such as fistulae and strictures, resulting in surgery. Infliximab (IFX) is very effective for treating paediatric patients with CD, but is currently only registered for therapy refractory patients—the so-called step-up strategy. We hypothesise that using IFX first-line, that is, top-down, will give more mucosal healing, fewer relapses, less complications, need for surgery and hospitalisation.
Methods and analysis
This international multicentre open-label randomised controlled trial includes children, aged 3–17 years, with new-onset, untreated CD with moderate-to-severe disease activity (weighted Paediatric Crohn's Disease Activity Index (wPCDAI)>40). Eligible patients will be randomised to top-down or step-up treatment. Top-down treatment consists of 5 IFX infusions combined with azathioprine (AZA). After these 5 infusions, patients will continue AZA. Patients randomised to step-up will receive standard induction treatment, either oral prednisolone or exclusive enteral nutrition, combined with AZA as maintenance treatment. The primary outcome is clinical remission (wPCDAI<12.5) at 52 weeks without need for additional CD-related therapy or surgery. Total follow-up is 5 years. Secondary outcomes include clinical disease activity, mucosal healing by endoscopy (at week 10 and optionally week 52), faecal calprotectin, growth, quality of life, medication use and adverse events.
Ethics and dissemination
Conducted according to the Declaration of Helsinki and Good Clinical Practice. Medical-ethical approval will be obtained for each site.
Trial registration number
NCT02517684; Pre-results.
Keywords: CROHN'S DISEASE, STEROID-SPARING EFFICACY, INFLIXIMAB, PAEDIATRIC GASTROENTEROLOGY, COST-EFFECTIVENESS
Background
Crohn's disease (CD) is a chronic inflammatory disease predominantly affecting the gastrointestinal tract. The disease pathogenesis is not fully known, but involves an aberrant immune response to the patients' intestinal microbiota. Owing to the inflammation, patients may present with symptoms such as abdominal pain, diarrhoea, fatigue and weight loss, and further investigation may reveal increased inflammatory products in the patients' blood and faeces. The diagnosis is based on the patients' history, physical examination, endoscopic and radiological imaging of the bowel as well as microscopic evaluation of mucosal biopsies.1
Approximately 4 per 100 000 children develop CD during childhood or adolescence.2 Compared with adult onset CD, patients with childhood onset may present with more extensive and progressive disease, and generally require more intensive treatment.3 4 Paediatric CD treatment focuses on relieving symptoms, restoring longitudinal growth and pubertal development, and on suppressing the inflammatory immune response leading to macroscopically detectable repair of the mucosal surface, also known as mucosal healing.5 Acquiring mucosal healing is important since it predicts a favourable disease outcome, and reduces the need for steroids, the risk of complications, of hospitalisation and the need for surgery.6 Current paediatric CD guidelines instruct physicians to start treatment with exclusive enteral nutrition (EEN) or prednisolone to induce disease remission, and at the same time start with a thiopurine, such as azathioprine (AZA), or methotrexate (MTX) to maintain remission.5 Only patients refractory to these treatments can step up to anti-tumour necrosis factor (anti-TNF) antibody therapy. However, this so-called step-up treatment strategy has disadvantages. Although prednisolone and EEN both induce clinical remission effectively (in ∼80% of patients), prednisolone has considerable side effects, and EEN necessitates a complete refrain from normal food for a long period of time which is unpleasant and hard to comply with.5 Furthermore, prednisolone only rarely induces mucosal healing.5 7 8 Once in clinical remission, 60–70% of patients maintain remission during the first year of AZA treatment.5 One registry showed that 54% (55/102) of paediatric patients with CD had received either an additional corticosteroid course or had started infliximab (IFX) within the first year after diagnosis.9 Thus, a large proportion of paediatric patients require more intensive treatment in the first year after diagnosis. For these patients, the step-up strategy delays the initiation of effective treatment and increases the risk of CD progression and complications.
Since its introduction, IFX—the first anti-TNF antibody registered for CD—has been shown to be very effective for treating refractory paediatric patients with CD.10 In the REACHi trial—the pivotal IFX trial in paediatric patients with CD refractory to AZA treatment—88% of patients responded to IFX after 10 weeks of therapy, of whom the majority achieved and maintained remission on IFX throughout week 54. Subsequent research showed that IFX efficacy can be improved through individualised dose optimisation to ensure therapeutic levels and by combination therapy with AZA or MTX to avoid immunogenicity.11–14 Notably, IFX was also demonstrated to be more effective the sooner it is initiated after diagnosis. Three retrospective trials, assessing the efficacy of IFX, demonstrated that patients receiving IFX ‘early’ after diagnosis (either directly after diagnosis or <1 or 2 years afterwards) had longer remission duration and increased fistula closing rates than those receiving IFX ‘late’.15–17 Postponing IFX could thus reduce its efficacy. IFX has also been shown to induce mucosal healing in a large proportion of patients: In the ACCENT 1 trial in adult patients with CD, 31% (10/32) of the patients receiving IFX maintenance treatment had mucosal healing (absence of ulcers) at week 10 and 50% (13/26) had mucosal healing at week 54—a post-hoc analysis of week 2 IFX responders who had mucosal ulcerations at baseline.18 Giving IFX early as part of the top-down strategy may thus optimise IFX efficacy and offer a good chance for restoration of the gut's mucosa, which in turn can reduce risks of disease relapse, hospitalisation and the need for surgery.
Evidence on the efficacy of top-down treatment as compared with step-up treatment is, however, limited. Currently, two prospective trials compared both strategies in adult patients with CD. In the first trial,19 133 adult patients with CD were randomised to start with either step-up treatment (steroids only) or top-down therapy (three IFX infusions and AZA maintenance therapy). Top-down therapy resulted in higher remission rates (week 26: 39/65 (60%) vs 23/64 (36%); week 52: 40/65 (62%) vs 27/64 (42%)), and led more often to mucosal healing (absence of ulcers at week 104: 19/26 (73%) vs 7/23 (30%)). In the second trial,20 77 patients were randomised to receive either six IFX infusions and AZA or prednisone and AZA. At week 30, top-down treatment resulted in higher remission rates (26/38 (68%) vs 17/39 (44%)) and mucosal healing rates (17/38 (45%) vs 7/39 (18%)).
There are no prospective randomised controlled trials (RCTs) in paediatric patients with CD, only several retrospective, observational studies. The first retrospective study found that patients who—by either the patient's or physician's choice—had started top-down treatment had lower relapse rates at 1 year than those who had started with step-up treatment (3/13 (23%) vs 8/13 (62%)).21 A second cohort demonstrated that IFX is more effective in therapy naïve than refractory patients (relapse-free rates at 3 years: 36% vs 15% (survival curve, no absolute numbers)).15 22 23 Results from a third retrospective cohort, using propensity scores analysis to correct for baseline differences, showed that early IFX monotherapy resulted in higher remission rates at 1 year than early immunomodulator monotherapy (thiopurine or MTX) (58/68 (85%) vs 152/248 (55%)).24 The available literature thus suggests that starting IFX therapy early is more effective in paediatric patients with CD, but this needs to be confirmed in a prospective randomised trial. Also, the top-down strategy by definition aims at stopping IFX therapy and stepping down to immunomodulator monotherapy. This is to reduce risks associated with combination therapy,13 limit healthcare expenses while hopefully not compromising on efficacy. Whether this approach truly offers the best risk/cost/benefit balance still needs to be tested. This study therefore aims to compare the efficacy and safety of the top-down IFX treatment with conventional step-up treatment in paediatric patients with CD with moderate-to-severe disease.
Methods
Trial design
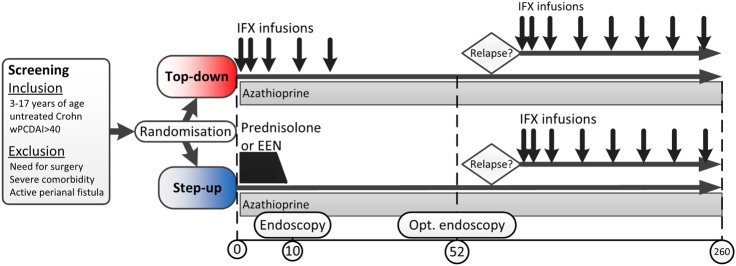
We designed an international multicentre open-label RCT with two parallel treatment arms (figure 1). In addition, one of these arms (step-up) contains two initial treatment options to choose from (prednisolone and EEN). This decision is made by the treating physician together with the patient and/or parents. This allocation based on choice was chosen over randomised allocation because of two reasons. First, this choice mimics the current clinical practice of the step-up strategy and is therefore a better comparator. Second, a strong aversion to one of the step-up treatments may prevent patients from participating in this trial.
Figure 1.
Study design. EEN, exclusive enteral nutrition; IFX, infliximab; opt, optional; wPCDAI, weighted Paediatric Crohn's Disease Activity Index.25
Eligible patients willing to participate in this trial will be randomised with concealed group allocation, resulting in two comparable groups. Although a double-blind design is considered ideal for treatment comparison, an open-label design was chosen instead, because the former was not feasible due to the use of three treatments with different routes of administration—IFX is given intravenously, prednisolone orally as tablets and EEN is a liquid formula either ingested by mouth or by nasogastric tube—which makes using placebos very complex and costly. As a consequence of the open-label design, our results could potentially be influenced by performance bias and detection bias. However, since a double-blind design is not feasible, our open-label RCT is the optimal design for this research question.
Eligibility criteria and recruitment
Newly diagnosed patients with CD, according to the revised Porto criteria,1 are eligible if untreated, aged 3 up to and including 17 years, with body weight above 10 kg, presenting with moderate-to-severe disease activity (weighted Paediatric Crohn's Disease activity Index (wPCDAI) above 40).25 Patients are excluded in case of a need for primary surgery, such as symptomatic bowel stenosis or stricture, active perianal fistulas, or if they have serious comorbidity, such as a severe infection, sepsis, opportunistic infection, positive stool culture (Salmonella enterica/Shigella spp./Yersinia enterocolitica/Campylobacter spp.), positive Clostridium difficile toxin assay, positive tuberculosis screening, or if they present with a suspected or definite pregnancy.
Patients suspected of having CD and undergoing routine diagnostic work-up are potential candidates and screened for this trial when presenting to one of the participating sites. After a CD diagnosis is established and eligibility criteria are met, patients and/or parents/guardians are informed about the trial and asked to consider participation. After a waiting period of a minimum of 2 days, written consent is asked for by the treating physician or researcher. Note that before the initial, diagnostic endoscopy and study consent, preliminary consent is sought for the collection of additional biopsies, which will be used for search for biomarkers predictive for treatment response, one of the additional study objectives.
Randomisation, blinding and treatment allocation
Eligible patients are equally (1:1 ratio) randomised by a computer-generated list into two treatment groups, stratified by centre. Randomisation is incorporated in the web-based Case Record Form (CRF) database used for this trial (Castor Electronic Data Capture (EDC)).26 Collaborators at each site have access to this database and can register and randomise their patients.
Treatment groups
Participants are randomised into two groups, either the experimental ‘top-down’ group or the control group named ‘step-up’, which is the current standard treatment strategy.5 The top-down group will receive five IFX infusions (Inflectra, IFX induction at weeks 0, 2 and 6, followed by two maintenance infusions every 8 weeks, dosed at 5 mg/kg) combined with oral AZA as maintenance treatment (once daily, dosed 2–3 mg/kg). Step-up treatment consists of standard induction treatment with either oral prednisolone (1 mg/kg daily with a maximum of 40 mg for 4 weeks, followed by tapering down 5 mg per week until stop) or EEN (polymeric feeding for 6–8 weeks after which normal diet is gradually reintroduced within 2–3 weeks).2 Similar to the top-down group, both prednisolone and EEN will be combined with oral AZA as maintenance treatment (2–3 mg/kg, once daily). AZA dosing may be altered based on thiopurine methyltransferase (TPMT) genotype, but TPMT testing is not obligatory. Following its initiation, routine complete blood counts are performed as part of routine clinical care (weekly in first month, monthly in second and third months, and thereafter once every 3 months) and AZA metabolites are measured about the time of induction treatment cessation. In both groups, MTX may be given instead of AZA, for instance in patients with low or absent TPMT activity. Screening for serum positivity to Varicella zoster virus—as well as Epstein-barr virus and hepatitis B—is part of routine clinical care, and if vaccination is required and if time allows, treatment initiation will be postponed.
The top-down and step-up groups differ in the type of induction treatment that is started after diagnosis, but may switch to similar treatments during follow-up. Treating physicians are allowed to change treatment or increase dosing during follow-up when clinically indicated, for instance in case of drug inefficacy (non-response or loss of response) or intolerance. IFX may thus also be given to a step-up patient, but only as second-line treatment. Note that the step-up group may thus include patients stepping up to IFX early after diagnosis, which, as explained in the introduction, was associated with better efficacy in retrospective trials than starting IFX late. Overall, this study thus compares two treatment strategies and not two different drugs, like in regular drug trials.
Study end points
Comparative efficacy and safety
In total, patients will be followed for 5 years from randomisation (figure 1). The primary end point is clinical remission at week 52 (defined as a wPCDAI score<12.5) without need for additional CD-related therapy or surgery, that is, additional to the treatment scheme described in the previous section (table 1).
Table 1.
Study end points
| Time (weeks) | |
|---|---|
| Primary end point | |
| Remission* without a need for additional CD-related therapy or surgery | 52 |
| Secondary efficacy end points | |
| Remission* and response† | 10, 52 |
| Endoscopic disease activity (presence of ulcers, SES-CD) | 10, optionally 52 |
| Faecal calprotectin | 10, 52 |
| Height and BMI Z-scores, bone age and pubertal development | 52 |
| Quality of life (IMPACT-III) | 14, 52 |
| Cumulative therapy use and therapy failure | 52 |
| Secondary safety end points | |
| Adverse events and complications | 52 |
| Long-term end points | |
| Remission* without a need for additional CD-related therapy or surgery | 104, 156, 208, 260 |
| Remission* and response† | 104, 156, 208, 260 |
| Faecal calprotectin | 104, 156, 208, 260 |
| Number of flares | 104, 156, 208, 260 |
| Quality of life (IMPACT-III) | 260 |
| Cumulative therapy use and therapy failure | 260 |
| Adverse events and complications | 260 |
| Subanalyses | |
| Comparing efficacy and safety of prednisolone plus AZA with EEN plus AZA | |
| Correlations between wPCDAI, faecal calprotectin and endoscopic disease severity (SES-CD) | |
| Additional objectives | |
| Comparing the cost-effectiveness of top-down with that of step-up | |
| Identifying predictive biomarkers for treatment response | |
| Assessing the pharmacokinetic and pharmacodynamic properties of IFX in children | |
Secondary end points include assessment of endoscopic disease activity, growth, quality of life and medication use. Endoscopic disease activity is an important outcome in this trial due to the expected difference between the two treatment strategies. To assess endoscopic disease activity, an endoscopic examination is scheduled at week 10, and another offered at week 52. The week 52 endoscopy is performed on a voluntary basis, as most patients may not benefit from this assessment while it does pose risk and discomfort. Endoscopic disease activity is also indirectly assessed via measuring the faecal marker calprotectin. To address longitudinal growth during follow-up, height and body mass index Z-scores will be calculated at baseline and during follow-up for all patients with use of age-specific and gender-specific anthropometric reference values (preferably country-specific, otherwise global reference values). Additionally, bone age will be measured with hand X-ray, and pubertal development will be assessed. Safety end points include the rate of adverse events and complications during follow-up.
Besides comparing the two treatment strategies top-down and step-up with each other, we planned two subanalyses. First, we aim to compare both the efficacy and safety of the two step-up treatment options, and second, to assess the correlation between clinical and endoscopic disease activity measures.
Healthcare costs, response prediction, and evaluation of the kinetic and dynamic properties of IFX
Three additional objectives are set. First, an additional objective is to compare the healthcare-related cost of both treatment strategies. This is an important outcome, because of the large difference in costs between biological and non-biological drugs. The recent introduction of an IFX biosimilar to the market has strongly reduced the costs of IFX therapy, while the costs of top-down therapy may be further reduced compared with step-up therapy by its hypothesised higher efficacy, which may reduce medication use, hospitalisation and surgery.6 We therefore hypothesise that after 5 years of follow-up healthcare-related costs of top-down therapy will be comparable to those of step-up therapy.
We will also look for biological markers that may predict treatment response. Additional biopsies and blood samples are collected from patients to measure RNA and protein expression, both before the start of treatment and during follow-up (additional biopsies are taken in pairs from affected and unaffected mucosal tissue in the ileum and colon with a maximum of eight biopsies). This may help unravel the underlying mechanisms of treatment response of both strategies and preferentially lead to markers predictive of treatment response. The ability to predict treatment response prior to its initiation would allow for tailored treatment, aimed at maximal effect and safety hereby decreasing healthcare cost.
Finally, the pharmacokinetic and pharmacodynamic properties of IFX in children will be assessed during follow-up. Currently, only a few controlled trials have assessed these properties of IFX in children and by gathering more, high-quality data, we expect to further optimise IFX dosing in children. On the basis of clinical experience, we hypothesise that fixed IFX dosing of 5 mg/kg will result in lower IFX trough levels in younger patients and as a consequence in lower drug efficacy.
Sample size calculation
Our sample size calculation was based on the week 52 remission ratios in three studies, two retrospective trials in paediatric patients with CD and one prospective RCT among adult patients with CD. The first retrospective trial compared top-down IFX use with conventional step-up IFX use in paediatric CD and found a remission difference at week 52 of 38% (15/18 (83%) vs 5/11 (45%)).23 The second trial compared early IFX use with early immunomodulator use and found a remission difference of 24% (58/68 (85%) vs 152/248 (61%)).24 The only prospective RCT, comparing top-down with step-up treatment in adult patients with CD, reported a remission difference of 19% at week 52 (40/65 (61.5%) vs 27/64 (42.2%)).19 On the basis of these data, we calculated a need for 100 inclusions (50 patients in each arm, considering a drop-out rate of 2%) to find a 25% difference in clinical remission at week 52 with a power of 80% (two-sided α 0.05). A low drop-out rate was considered appropriate, because there are only a few reasons for dropping out: only if the patient wishes to, or if after randomisation the assigned treatment is not started.
Data collection and monitoring
Data are collected in Castor EDC,26 a web-based CRF database enabling the central study coordinators to follow and check the CRF input of each of the collaborating centres online. Additionally, a certified monitor will visit each site every year. A Data Safety Monitoring Board was not appointed, as the risks of adverse events associated with this study are considered low, because only approved therapies are used and treatment is not blinded.
Statistic methods
Subject baseline and demographic data as well as baseline disease characteristics data will be summarised by treatment group. Parametric variables will be described by their mean and SD, and compared with use of the Student's t-test, and non-parametric variables will be described by their median and IQR and compared using the Mann-Whitney U test. Categorical variables will be summarised using counts and percentages, and compared using the χ2 test, or the Cox proportional hazard test in case of time-dependent categorical variables. Correlations will be assessed using either the Pearson correlation coefficient (parametric) or the Spearman's rank correlation coefficient (non-parametric). Analyses will be performed on an intention-to-treat basis. All statistical testing will be two-sided and significant at the 0.05 level. Missing data will be reported and left out of the analyses.
Discussion
Top-Down Infliximab Study in Kids with Crohn's disease (TISKids) is a unique study specifically designed to compare two treatment strategies. Two comparable groups are generated through randomisation so that each group only differs by the initial induction treatment started. During a 5-year follow-up period, the effects of these two strategies will be compared. Both major patient-related outcomes as well as other important healthcare-related outcomes will be addressed aiming to obtain as much information as possible concerning the benefits, risks and costs of both strategies.
In recent years, and because of increasing literature supporting early IFX use, IFX is being prescribed increasingly sooner after diagnosis. The guidelines for paediatric CD treatment were changed in their recommendations on this topic. They now advocate first-line IFX use for children with active perianal fistulising disease and state that first-line IFX may also be considered for patients with high risk of poor outcome.5 However, the data supporting this recommendation is not conclusive. The benefits and risks of this new strategy are not well studied, nor compared with those of the conventional step-up strategy. The comparative risks and costs of top-down treatment are not especially well known. This study will thus offer solid answers to these important and urgent clinical questions.
Acknowledgments
Collaborating sites and investigators ordered by country: Croatia: I. Hojsak and S. Kolacek, Children's Hospital Zagreb, Zagreb. England: C. Tzivinikos and M.K. Auth, Alder Hey Children's Hospital, Liverpool; R. Muhammed, Birmingham Children's Hospital, Birmingham. Finland: K-L Kolho, Helsinki University Children's Hospital, Helsinki. Italy: M. Aloi, Sapienza University Hospital, Rome; D. Knafelz, Bambino Gesu Hospital, Rome; P. Lionetti, Meyer Children's Hospital Florence, Florence; A. Staiano, University of Naples, Naples. Netherlands: T.G. de Meij and M. van Wijk, VU University Medical Centre, Amsterdam; G. Damen, Radboud University Medical Centre, Nijmegen; M. Groeneweg, Maasstad Hospital, Rotterdam; T. Hummel, Medisch Spectrum Twente, Enschede; O. Norbruis and S. Teklenburg, Isala Hospital, Zwolle; M.L. Mearin and C. Meijer, Leiden University Medical Centre, Leiden; H.M. van Wering, Amphia Hospital, Breda; V.M. Wolters, University Medical Centre Utrecht, Utrecht; J.M. Stapelbroek, Catharina Hospital, Eindhoven; C van der Feen, Jeroen Bosch Hospital, ‘s-Hertogenbosch; P.F. van Rheenen, Beatrix Children's Hospital—University Medical Centre Groningen, Groningen; A. van den Berg, HagaZiekenhuis, the Hague; A. Kindermann, Academic Medical Centre, Amsterdam. Poland: M. Sladek, Jagiellonian University MC, Cracow.
Contributors: MAC, JCE and LdR were involved in the protocol design. All authors are involved in the implementation of this trial.
Funding: The official sponsor is the Erasmus University Medical Centre, Rotterdam: sponsor contact information: l.deridder@erasmusmc.nl. This trial is sponsored by ZonMw (the Netherlands Organisation for Health Research and Development) project number 113202001, Crocokids (Dutch fundraising organisation to support research on IBD in children) and Hospira, now Pfizer. IFX (Inflectra) vials are provided by Hospira for the first year after randomisation.
Competing interests: JCE is part of the Scientific Advisory Committee of Janssen and Abbvie and received a research grant from Merck Sharp & Dohme (MSD). LdR reports a presentation on an Abbvie sponsored meeting.
Ethics approval: The study will be conducted according to the principles of the Declaration of Helsinki and Good Clinical Practice. Ethical approval is or will be obtained for each participating site.
Provenance and peer review: Not commissioned; externally peer reviewed.
A Randomized, multicenter, open-label study to Evaluate the safety and efficacy of Anti-TNF-a Chimeric monoclonal antibody in pediatric subjects with moderate to severe Crohn's disease.
References
- 1.Levine A, Koletzko S, Turner D, et al. . ESPGHAN revised Porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr 2014;58:795–806. doi:10.1097/MPG.0000000000000239 [DOI] [PubMed] [Google Scholar]
- 2.Benchimol EI, Fortinsky KJ, Gozdyra P, et al. . Epidemiology of pediatric inflammatory bowel disease: a systematic review of international trends. Inflamm Bowel Dis 2011;17:423–39. doi:10.1002/ibd.21349 [DOI] [PubMed] [Google Scholar]
- 3.Van Limbergen J, Russell RK, Drummond HE, et al. . Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology 2008;135:1114–22. doi:10.1053/j.gastro.2008.06.081 [DOI] [PubMed] [Google Scholar]
- 4.Pigneur B, Seksik P, Viola S, et al. . Natural history of Crohn's disease: comparison between childhood- and adult-onset disease. Inflamm Bowel Dis 2010;16:953–61. doi:10.1002/ibd.21152 [DOI] [PubMed] [Google Scholar]
- 5.Ruemmele FM, Veres G, Kolho KL, et al. . Consensus guidelines of ECCO/ESPGHAN on the medical management of pediatric Crohn's disease. J Crohns Colitis 2014;8:1179–207. doi:10.1016/j.crohns.2014.04.005 [DOI] [PubMed] [Google Scholar]
- 6.Vatn MH. Mucosal healing: impact on the natural course or therapeutic strategies. Dig Dis 2009;27:470–5. doi:10.1159/000233285 [DOI] [PubMed] [Google Scholar]
- 7.Lichtenstein GR, Feagan BG, Cohen RD, et al. . Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol 2006;4:621–30. doi:10.1016/j.cgh.2006.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Lichtenstein GR. Approach to steroid-dependent and steroid-refractory Crohn's disease. J Pediatr Gastroenterol Nutr 2001;33(Suppl 1):S27–35. doi:10.1097/00005176-200109001-00005 [DOI] [PubMed] [Google Scholar]
- 9.Markowitz J, Hyams J, Mack D, et al. . Corticosteroid therapy in the age of infliximab: acute and 1-year outcomes in newly diagnosed children with Crohn's disease. Clin Gastroenterol Hepatol 2006;4:1124–9. doi:10.1016/j.cgh.2006.05.011 [DOI] [PubMed] [Google Scholar]
- 10.Hyams J, Crandall W, Kugathasan S, et al. . Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn's disease in children. Gastroenterology 2007;132:863–73. doi:10.1053/j.gastro.2006.12.003 [DOI] [PubMed] [Google Scholar]
- 11.Vande Casteele N, Ferrante M, Van Assche G, et al. . Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 2015;148: 1320–9.e3 doi:10.1053/j.gastro.2015.02.031 [DOI] [PubMed] [Google Scholar]
- 12.Steenholdt C, Brynskov J, Thomsen OØ, et al. Individualised therapy is more cost-effective than dose intensification in patients with Crohn's disease who lose response to anti-TNF treatment: a randomised, controlled trial. Gut 2014;63:919–27. doi:10.1136/gutjnl-2013-305279 [DOI] [PubMed] [Google Scholar]
- 13.Cozijnsen MA, Escher JC, Griffiths A, et al. . Benefits and risks of combining anti-tumor necrosis factor with immunomodulator therapy in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2015;21:951–61. doi:10.1097/MIB.0000000000000245 [DOI] [PubMed] [Google Scholar]
- 14.Church PC, Guan J, Walters TD, et al. . Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn's disease. Inflamm Bowel Dis 2014;20:1177–86. doi:10.1097/MIB.0000000000000083 [DOI] [PubMed] [Google Scholar]
- 15.Cozijnsen MA, de Ridder L. Infliximab more effective in therapy-naive than in therapy-refractory patients. J Pediatr Gastroenterol Nutr 2015;61:e15 doi:10.1097/MPG.0000000000000884 [DOI] [PubMed] [Google Scholar]
- 16.Lionetti P, Bronzini F, Salvestrini C, et al. . Response to infliximab is related to disease duration in paediatric Crohn's disease. Aliment Pharmacol Ther 2003;18:425–31. doi:10.1046/j.1365-2036.2003.01672.x [DOI] [PubMed] [Google Scholar]
- 17.Kugathasan S, Werlin SL, Martinez A, et al. . Prolonged duration of response to infliximab in early but not late pediatric Crohn's disease. Am J Gastroenterol 2000;95:3189–94. doi:10.1111/j.1572-0241.2000.03263.x [DOI] [PubMed] [Google Scholar]
- 18.Rutgeerts P, Diamond RH, Bala M, et al. . Scheduled maintenance treatment with infliximab is superior to episodic treatment for the healing of mucosal ulceration associated with Crohn's disease. Gastrointest Endosc 2006;63:433–42. doi:10.1016/j.gie.2005.08.011 [DOI] [PubMed] [Google Scholar]
- 19.D'Haens G, Baert F, van Assche G, et al. . Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn's disease: an open randomised trial. Lancet 2008;371:660–7. doi:10.1016/S0140-6736(08)60304-9 [DOI] [PubMed] [Google Scholar]
- 20.Fan R, Zhong J, Wang ZT, et al. . Evaluation of “top-down” treatment of early Crohn's disease by double balloon enteroscopy. World J Gastroenterol 2014;20:14479–87. doi:10.3748/wjg.v20.i39.14479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JS, Lee JH, Lee JH, et al. . Efficacy of early treatment with infliximab in pediatric Crohn's disease. World J Gastroenterol 2010;16:1776–81. doi:10.3748/wjg.v16.i14.1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee YM, Kang B, Lee Y, et al. . Infliximab “top-down” strategy is superior to “step-up” in maintaining long-term remission in the treatment of pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2015;60:737–43. doi:10.1097/MPG.0000000000000711 [DOI] [PubMed] [Google Scholar]
- 23.Kim MJ, Lee JS, Lee JH, et al. . Infliximab therapy in children with Crohn's disease: a one-year evaluation of efficacy comparing ‘top-down’ and ‘step-up’ strategies. Acta Paediatr 2011;100: 451–5. doi:10.1111/j.1651-2227.2010.01938.x [DOI] [PubMed] [Google Scholar]
- 24.Walters TD, Kim MO, Denson LA, et al. . Increased effectiveness of early therapy with anti-tumor necrosis factor-α vs an immunomodulator in children with Crohn's disease. Gastroenterology 2014;146:383–91. doi:10.1053/j.gastro.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 25.Turner D, Griffiths AM, Walters TD, et al. . Mathematical weighting of the pediatric Crohn's disease activity index (PCDAI) and comparison with its other short versions. Inflamm Bowel Dis 2012;18:55–62. doi:10.1002/ibd.21649 [DOI] [PubMed] [Google Scholar]
- 26.Castor Electronic Data Capture, Ciwit BV, Amsterdam, The Netherlands, 2016.
- 27.Otley A, Smith C, Nicholas D, et al. . The IMPACT questionnaire: a valid measure of health-related quality of life in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2002;35:557–63. doi:10.1097/00005176-200210000-00018 [DOI] [PubMed] [Google Scholar]
- 28.Loonen HJ, Grootenhuis MA, Last BF, et al. . Measuring quality of life in children with inflammatory bowel disease: the impact-II (NL). Qual Life Res 2002;11:47–56. doi:10.1023/A:1014455702807 [DOI] [PubMed] [Google Scholar]
- 29.Daperno M, D'Haens G, Van Assche G, et al. . Development and validation of a new, simplified endoscopic activity score for Crohn's disease: the SES-CD. Gastrointest Endosc 2004;60:505–12. doi:10.1016/S0016-5107(04)01878-4 [DOI] [PubMed] [Google Scholar]