Abstract
Streptococcus pneumoniae and Haemophilus influenzae carriage is a useful index for measuring the emergence of resistance and outcome in vaccination trials. We performed a study to determine which sampling site, nasopharynx (NP) or oropharynx (OP), yields the highest rate of S. pneumoniae and H. influenzae isolation at different ages. Both NP and OP cultures were obtained from 216 children aged <60 months and their mothers. The total S. pneumoniae carriage rate was 68% among children and 15% among mothers (P < 0.001). Using NP alone for the isolation of S. pneumoniae would have missed 2, 2, and 42% and using OP alone would have missed 77, 66, and 45% of S. pneumoniae in children aged 0 to 23 months, 24 to 59 months, and mothers, respectively. Using NP cultures alone for H. influenzae would have missed 23, 24, and 81% of the isolates, respectively. The respective figures for H. influenzae isolation from OP alone are 38, 29, and 9%. In children, S. pneumoniae was carried mainly in the NP while H. influenzae was equally carried in the NP and OP. In mothers, S. pneumoniae was carried equally in the NP and OP while H. influenzae was carried significantly more often in the OP. In children, H. influenzae colonization increased during illness, mainly in the NP. Culturing only one site significantly reduced the recovery of H. influenzae at all ages. NP cultures for S. pneumoniae detected close to 100% of isolates in children but only 58% of isolates in mothers.
Streptococcus pneumoniae and Haemophilus influenzae are important bacterial pathogens causing infections in young children and in elderly patients (1, 22, 23). Both organisms commonly colonize the mucosal membrane of the nasopharynx and throat of healthy children, and most children are colonized at some point during the first 2 years of life (10, 15). Information on the nasopharyngeal (NP) and oropharyngeal (OP) carriage rate of both organisms is crucial to understanding disease epidemiology, since these organisms can spread from colonized sites to adjacent mucosal tissues to cause mucosal infections or, in the case of S. pneumoniae, invade the bloodstream to cause bacteremia and meningitis. In addition to carriage in the upper respiratory tract, the organism can spread from person to person (16, 17, 24). Furthermore, studying the organisms carried in the upper respiratory tract provides useful information on the emergence of resistance in clinical isolates (18, 27). It is also important to evaluate the mucosal carriage of the organisms in vaccine trials as an index of potential herd protection.
Nasopharyngeal sampling is considered superior to OP sampling for detecting S. pneumoniae, especially in young children (25), but there are other contradictory results showing that in adults OP sampling yields higher isolation rates (4, 16). Only a few carriage studies have directly compared different sampling methods for the detection of S. pneumoniae and H. influenzae (4, 6, 25; R. Dagan, O. Zamir, M. Sikuler-Cohen, P. Yagupsky, P. Peeters, and M. Hohenboken, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-1526, 2001). In one study looking at carriage rate in all age groups, S. pneumoniae was isolated significantly more often from the nasal site than from the oropharyngeal site while H. influenzae was equally found in both sites (6). In another study conducted on adults, S. pneumoniae isolation rate was 29% from OP but only 8% from NP and 16% from saliva (4). In these two studies, the relative importance of NP and OP for the recovery of both S. pneumoniae and H. influenzae varied with age (4, 16). On the other hand, NP cultures were found to be more sensitive than OP cultures for detecting S. pneumoniae carriage in Navajo and White Mountain Apache adults (J. P. Watt, K. L. O'Brien, S. Katz, M. A. Bronsdon, J. A. Elliott, J. Dallas, M. J. Perilla, R. R. Facklam, R. Reid, M. Santosham, and C. G. Whitney, 3rd Int. Symp. Pneumococci Pneumococcal Dis., p. 25, 2002). In these studies, different sampling and laboratory techniques were used, such as antigen detection by latex agglutination, mouse inoculation (4, 16), and skim milk-tryptone-glucose-glycerin medium (Watt et al., 3rd Int. Symp. Pneumococci Pneumococcal Dis.).
In addition to age and sampling site, other factors—especially health reasons—have been shown to affect carriage of respiratory pathogens. Acute respiratory illnesses were shown to increase colonization rates of both pathogens (2, 13). Recent antibiotic treatment decreased total carriage while increasing carriage rates of antibiotic-resistant pathogens (28).
The aims of our study were (i) to determine the contribution of each of the sites, NP and OP, to the isolation of S. pneumoniae and H. influenzae in children and their mothers and (ii) to determine the influence of ethnic origin, acute respiratory infection, and recent antibiotic use on S. pneumoniae and H. influenzae recovery rates from NP and OP.
MATERIALS AND METHODS
The study was conducted between 1 November 2001 and 30 August 2002 in two primary health care clinics in Jewish and Bedouin towns in southern Israel. Samples were also obtained from children evaluated 1 month after recovery from pneumonia, at the Pediatric Emergency Room of the Soroka University Medical Center in Beer-Sheva, Israel. The study was approved by the Soroka University Medical Center Ethics Committee.
In the Negev region in southern Israel, two different populations, Jews and Bedouins, live side by side. The socioeconomic conditions and lifestyles of these two groups differ. The Jewish population is largely urban, whereas the Bedouin population, formerly composed of desert nomads, is in transition to a Western lifestyle (7). The two pediatric populations also differ in disease patterns and rates. Hospitalization rates for respiratory and other infectious diseases are higher among Bedouin infants, and the seasonal patterns of disease differ between the two groups (11, 19). The infectious agents responsible for these clinical manifestations are also distributed differently (14, 26). There is no financial barrier to the use of health care services in the region, and all Jewish and Bedouin children in the area visit the same emergency department, are hospitalized in the same wards, and are treated by identical diagnostic and treatment protocols. The pneumococcal conjugate vaccine has only recently been licensed in Israel and is currently not being used at all.
Study population.
Children aged 1 to 59 months and their mothers were enrolled in the study. In all cases, informed consent was given by the mothers to obtain NP and OP cultures from them and from their offspring. Children or mothers with severe respiratory problems, chronic illness (i.e., malignancies), or bleeding disturbances or those with anatomical abnormalities of the mouth and nose (i.e., cleft palate) were excluded from the study. Based on the literature and on our own experience, children younger than 2 years of age are at a higher risk of infections from S. pneumoniae and H. influenzae (e.g., acute otitis media). The subjects were enrolled by their physicians in the primary clinics during the regular working hours.
Data collection.
A structured interview was conducted to document the children's and mothers' demographic data (age, gender, and ethnic origin), health status, daycare attendance, recent antibiotic exposures, and medical history. Acutely ill children were defined as children with upper or lower respiratory infection and a temperature of more than 38°C at the time of the sampling. Recent antibiotic treatment was defined as any antibiotic treatment during the last month (30 days) before NP and OP samples were taken.
Sampling.
Two samples from each individual (mother and child) were obtained at the same visit: (i) an NP sample was obtained by use of flexible swab stick with a Dacron-rayon tip, inserted into the nostril until resistance was found, and (ii) OP samples were taken by direct inspection of the posterior wall and tonsillar regions by using rigid cotton-tipped applicators. The swabs were inoculated into modified Stewart transport medium (Medical Wire/Equipment, Corsham, Wiltshire, United Kingdom) and were all processed within 12 h at the Clinical Microbiology Laboratory of the Soroka University Medical Center, Beer Sheva.
Bacteriology.
For the detection of S. pneumoniae, swabs were inoculated onto Colombia agar with 5% sheep blood and 5.0 μg of gentamicin/ml and were incubated aerobically at 35°C for 48 h. S. pneumoniae was identified by colony morphology, alpha-hemolysis, and inhibition by optochin, which were confirmed by a positive slide agglutination test (Phadebact; Pharmacia Diagnostics, Uppsala, Sweden).
Serogrouping and serotyping were performed on the basis of capsular swelling (quellung reaction) with antisera provided by the Statens Serum Institut, Copenhagen, Denmark (3). Currently, a seven-valent pneumococcal conjugate vaccine is being used in Western countries but not yet in Israel. The following serotypes are included in the pneumococcal seven-valent conjugate vaccine: 4, 6B, 9V, 14, 18C, 19F, and 23F.
Identification of H. influenzae was based on Gram staining, growth on chocolate agar medium, failure to grow on Trypticase agar with added sheep blood, and nutritional requirement of both hemin and nicotine adenine nucleotide. Organisms that failed to agglutinate with polyvalent antisera to H. influenzae groups a, c to f, and b (Phadebact; Pharmacia Diagnostics) were considered untypeable.
Statistical analysis.
Based on the primary hypothesis, sample size calculations were analyzed for S. pneumoniae total carriage and carriage by the different sites, according to McNemar's test formula for paired samples, with an alpha level of 0.05 and a power of 90%. According to these calculations, the highest sample size needed was 92 pairs. Since no data were available for carriage of H. influenzae (for children versus adults) we decided to enlarge the study group by more than double and enrolled 216 pairs.
Data were recorded by using Microsoft Office Access software. Statistical analysis was performed with SPSS, version 10.0, software. Contingency table analysis for comparing rates between unmatched samples was performed by using the χ2 test or Fisher's exact test, as appropriate, and for comparing rates between matched samples (child-mother pairs), McNemar's test was performed. Ninety-five percent confidence intervals (95% CIs) were calculated for the nondetected proportions of NP or OP S. pneumoniae or H. influenzae carriage. Multiple logistic regression models were used to examine the effects of multiple risk factors on S. pneumoniae and H. influenzae carriage. The explanatory variables in the regression models included those previously hypothesized to effect the outcome: gender, antibiotic treatment during the last month, day care attendance, age group, place of enrollment (emergency room or community), and ethnicity. Statistical significance was defined as a P value of <0.05.
RESULTS
NP and OP samples were obtained from 432 subjects: 216 mothers and their 216 offspring. All swabs were plated for the detection of both S. pneumoniae and H. influenzae. Forty percent of the children were females. The mean age of the children was 20.2 months (standard deviation, ±15.2), and the mean age of the mothers was 29.4 years (standard deviation, ±5.4). All H. influenzae isolates were non-type b isolates. Differences were found neither in the total carriage of S. pneumoniae and H. influenzae nor in NP or OP carriage between Jewish and Bedouin groups.
S. pneumoniae carriage.
A total of 180 of 432 (42%) subjects were positive for S. pneumoniae, of which 147 (82%) were children and 33 (18%) were mothers. A significant difference was found in the S. pneumoniae carriage rate between children (147 of 216 [68%]) and mothers (33 of 216 [15%]) (P < 0.001).
Of 144 children aged 0 to 23 months, S. pneumoniae was carried alone in 25 (17%) children, H. influenzae was carried alone in 23 (16%) children, and both were carried in 78 (54%) children and 18 children (13%) were negative for both pathogens. Of 72 children aged 24 to 59 months, S. pneumoniae was carried alone in 11 (15%) children, H. influenzae was carried in 18 (25%) children, and both were carried in 37 (51%) children and 6 (8%) children were negative for both pathogens. No significant differences were found between the two pediatric age groups. In contrast, of the mothers, only 8 (4%) carried S. pneumoniae alone, 53 (24%) carried H. influenzae alone, 25 (12%) carried both organisms simultaneously, and 130 (60%) were negative for both organisms. In each age group, the pathogen distributions between children and mothers were significantly different (P < 0.001).
In children, S. pneumoniae was carried in 147 subjects, 144 (98%) of whom had positive NP cultures (with or without OP), and in only 2% of children, S. pneumoniae was isolated from the OP alone (Fig. 1). The relative part of OP carriage increased with age. In the younger age group, the OP carriage rate was 24 of 144 (17%), in older children, the carriage rate was 12 of 72 (21%), and in mothers, it was 18 of 55 (55%). No differences were found between the two children's age groups with regard to the carriage rates in the NP or OP. However, significant differences were found between each group of children and the mothers' group (Fig. 1).
FIG. 1.
Relative importance of NP versus OP sampling in S. pneumoniae carriage in children and their mothers. NS, not significant.
H. influenzae carriage.
A total of 230 of 432 (53%) subjects were positive for H. influenzae: 152 (66%) children and 78 (34%) mothers. Among children, the total carriage rate was 70%, and among mothers, the total carriage rate was 36% (Table 1). While in children H. influenzae was carried similarly in different sites (NP samples alone, 34%; both NP and OP samples, 43%; OP samples alone, 23%), in mothers it was mainly carried in the OP samples (81%) (Fig. 2).
TABLE 1.
S. pneumoniae and H. influenzae non-type b carriage in children and mothersa
| Organism | No. (%) of infected:
|
||
|---|---|---|---|
| Children aged:
|
Mothers (n = 216) | ||
| 0-23 mo (n = 144) | 24-59 mo (n = 72) | ||
| S. pneumoniae | 103 (72) | 44 (61) | 33 (15) |
| H. influenzae | 101 (70) | 51 (71) | 78 (36) |
A P value of <0.001 was found between mothers and children (in each group) for S. pneumoniae and H. influenzae.
FIG. 2.
Relative importance of NP versus OP sampling in H. influenzae carriage in children and their mothers. NS, not significant.
Usefulness of NP versus OP sampling in evaluation of carriage.
Using only NP for the isolation of S. pneumoniae would have missed 2% (2 of 103; 95% CI, 0 to 5), 2% (1 of 44; 95% CI, 0 to 7), and 42% (14 of 33; 95% CI, 26 to 59) of the isolates in children aged 0 to 23 months, children aged 24 to 59 months, and mothers, respectively. Likewise, using only OP would have missed 77% (79 of 103; 95% CI, 69 to 85), 66% (29 of 44; 95% CI, 52 to 80), and 45% (15 of 33; 95% CI, 28 to 62) of S. pneumoniae in children aged 0 to 23 months, children aged 24 to 59 months, and mothers, respectively. Using NP cultures only for H. influenzae would have missed 23% (23 of 101; 95% CI, 15 to 31), 24% (12 of 51; 95% CI, 12 to 35), and 81% (63 of 78; 95% CI, 72 to 90) of the isolates in children aged 0 to 23 months, children aged 24 to 59 months, and mothers, respectively. The respective figures for H. influenzae isolation from OP alone are 38% (37 of 101; 95% CI, 27 to 46), 29% (15 to 51; 95% CI, 17 to 42), and 9% (7 of 78; 95% CI, 3 to 15).
Healthy versus sick children and antibiotic use.
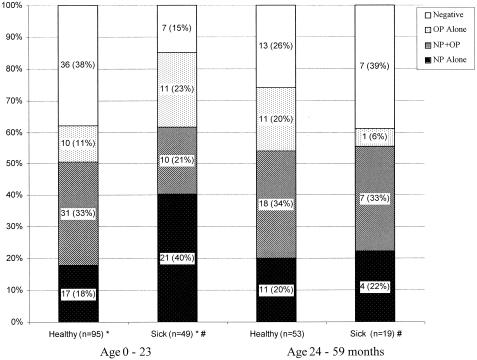
No differences in the overall carriage or carriage in any specific site were noted between healthy and sick children in different age groups with regard to S. pneumoniae. In contrast, H. influenzae total carriage was significantly higher in sick children (40 of 47 [89%]) than in healthy children (58 of 94 [62%]) younger than 24 months (P = 0.004). This increase was mainly due to the increase in carriage rate in the NP alone 40% (19 of 44) versus 18% (17 of 94) in sick and healthy young children, respectively (Fig. 3). Comparison between sick children younger than 24 months and sick children older than 24 months of age showed differences in the total carriage rate of 40 of 47 (85%) versus 11 of 18 (61%) (P = 0.047). No differences were found between healthy children in the different age groups or in healthy versus sick children older than 24 months.
FIG. 3.
Carriage rate of H. influenzae in NP, OP, and NP plus OP samples from sick and healthy children. *, P value of <0.001 between NP-only sampling of H. influenzae carriage in sick and healthy children; #, P value of 0.052 between sick children aged 0 to 23 months and sick children aged 24 to 59 months.
Children who were treated previously with antibiotics had a significantly lower carriage rate of S. pneumoniae (59 of 102 [58%] versus 85 of 109 [78%], respectively; P = 0.002), but no differences were found between sites regardless of the place of enrollment (in a multiple logistic regression analysis). With regard to H. influenzae carriage, no differences were found between the overall carriage and carriage in a specific site (OP or NP) between children receiving and not receiving antibiotic treatment, 75 of 102 (74%) and 74 of 109 (68%), respectively.
Usefulness of NP versus OP sampling in evaluation of S. pneumoniae carriage regarding serotypes.
No significant differences were found between NP and OP with regard to S. pneumoniae serotypes included in the seven-valent conjugate pneumococcal vaccine (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) and those not included in the vaccine. In children, the carriage of vaccine types was 42% for both sites, and in mothers, it was 5 of 17 (29%) and 8 of 18 (44%) for NP and OP, respectively.
DISCUSSION
In the present study two different but adjacent biological niches were evaluated in different age groups for the recovery of S. pneumoniae and H. influenzae. While the NP recovery rates of S. pneumoniae in children younger than 5 years old were significantly higher than the OP rates, the relative contribution of the OP carriage significantly increased after the second year of life and was significantly higher in mothers than in children. The OP site was found to be the only positive site in 42% of all positive cultures in mothers.
There is some evidence from previous studies that in children in their first year of life, NP sampling is significantly more often positive for S. pneumoniae than OP sampling (6, 25). However, previous studies with adults yielded inconclusive results. In one small study, higher isolation rates from the OP site (29%) was observed compared to only 8% from the NP site in adult patients with asthma and chronic obstructive lung disease (4). In contrast, NP culture was superior to OP culture for detecting S. pneumoniae carriage among Navajo and White Mountain Apache adults (Watt et al., 3rd Int. Symp. Pneumococci Pneumococcal Dis.). However, even in this study, 106 (38%) of 282 positive cultures were from the OP site only. These results are similar to the present study, where 42% of all positive cultures were from the OP site only, which accounted for 7% of all cultures taken. In another study from our region looking at the spread of S. pneumoniae in a closed community, the NP isolation rate was 7% in adults, similar to our results in the present study where the NP carriage (with or without OP carriage) was 9% (5). During an outbreak of conjunctivitis caused by S. pneumoniae in the United States (20), OP cultures were positive in 12% of patients, a rate in the same order of magnitude as that found in our study. From the data presented above it may be concluded that up to one-half of all S. pneumoniae isolates can be missed if only one sample site (OP or NP) is screened.
The H. influenzae carriage rate was significantly higher in children than in their mothers. Although the total carriage rates did not change with age in children, the OP carriage rate increased after the second year of life, although the NP carriage rate was not reduced in this age group. In contrast, in mothers, the OP site became the dominant site, since 81% of all positive cultures were positive in the OP site alone. The samples from the NP site alone contributed to positive results from 38 and 29% of children but only 9% of mothers. The contribution of the OP site alone to H. influenzae detection in the present study was higher than that reported in other studies, in which the OP site alone was responsible for only 10 to 20% of the isolation rates in infants (6). Thus, even in young children and infants, in studies looking at H. influenzae carriage rates, both OP and NP samples should be obtained for accurate carriage estimates. However, studies of adults should take into consideration the relative contribution of each site, since NP alone contributed less than 10% of the H. influenzae isolates and added only 3% of positive cultures.
It was previously suggested that respiratory infections do not change the total carriage rates or the ratio between NP and OP carriage among children with respiratory infection (6). In the present study, acutely ill children did not differ from healthy children with regard to S. pneumoniae carriage rate and NP versus OP distribution. In contrast, the total carriage rates of H. influenzae were significantly higher in sick children mainly in the NP in the first 2 years of life but not in older children.
Although in Israel the present seven-valent pneumococcal conjugate vaccine is not being used, it has recently been licensed. This vaccine is being used in North America and in several Western European countries. Therefore, the seven serotypes included in the pneumococcal conjugate vaccine were evaluated separately. The finding that OP or NP did not differ in terms of prevalence of S. pneumoniae of the serotypes that are included in the seven-valent pneumococcal conjugate vaccine (vaccine types) versus the nonvaccine types is important. The use of pneumococcal conjugate vaccines is associated with an extensive replacement of the vaccine serotypes by nonvaccine serotypes both in carriage and in acute otitis media (12) (Dagan, 41st ICAAC), and thus, in areas with actual or potential widespread vaccination, this replacement may potentially not be detected by culturing one site only. However, our findings that the two culture sites do not differ in the distribution of vaccine and nonvaccine types enable us to assume that even studies conducted with cultures of one niche only will accurately reflect the extent of replacement. We did not attempt to determine whether H. influenzae organisms isolated from the NP site differed from those isolated from the OP site.
Several speculations can be made for the higher carriage of S. pneumoniae and H. influenzae in the younger age group than in the older age group and, in particular, in adults. There may be technical problems such as difficulties in obtaining the sample properly, which may happen with noncooperative subjects. However, in our case, all samples were obtained by physicians well acquainted with this technique. Other technical problems may arise, such as (i) different processing may be used, although in our case all specimens were processed within 24 h at the same laboratory following the same procedures; (ii) factors such as the age of the patient, nutritional status, and underlying disease have a major impact on microbial etiology associated with lower respiratory tract infections (8, 9, 21); and (iii) the immune response to various S. pneumoniae and H. influenzae antigens increases with age. In any case, none of these factors should have affected the sampling sites.
In conclusion, in children, S. pneumoniae was carried mainly in the NP site while H. influenzae was equally carried in the NP and OP site. In mothers, S. pneumoniae was carried equally in the NP and OP sites while H. influenzae was carried significantly more in the OP site. In children, H. influenzae colonization increased during illness, mainly in the NP site. Culturing only one site significantly reduced the recovery of H. influenzae in all age groups. NP cultures for S. pneumoniae detected close to 100% of isolates in children but only 58% of isolates in mothers.
REFERENCES
- 1.Ahman, H., H. Kayhty, P. Tamminen, A. Vuorela, F. Malinoski, and J. Eskola. 1996. Pentavalent pneumococcal oligosaccharide conjugate vaccine PncCRM is well-tolerated and able to induce an antibody response in infants. Pediatr. Infect. Dis. J. 15:134-139. [DOI] [PubMed] [Google Scholar]
- 2.Aran, A., D. Fraser, and R. Dagan. 2001. Characteristics of nasopharyngeal carriage of Streptococcus pneumoniae in children during acute respiratory disease. Harefuah 140:300-305, 368. [PubMed] [Google Scholar]
- 3.Austrian, R. 1976. The quellung reaction, a neglected microbiologic technique. Mt. Sinai J. Med. 43:699-709. [PubMed] [Google Scholar]
- 4.Boersma, W. G., A. Lowenberg, Y. Holloway, H. Kuttschrutter, J. A. Snijder, and H. Koeter. 1993. The role of antigen detection in pneumococcal carriers: a comparison between cultures and capsular antigen detection in upper respiratory tract secretions. Scand. J. Infect. Dis. 25:51-56. [PubMed] [Google Scholar]
- 5.Borer, A., H. Meirson, N. Peled, N. Porat, R. Dagan, D. Fraser, J. Gilad, N. Zehavi, and P. Yagupsky. 2001. Antibiotic-resistant pneumococci carried by young children do not appear to disseminate to adult members of a closed community. Clin. Infect. Dis. 33:436-444. [DOI] [PubMed] [Google Scholar]
- 6.Capeding, M. R., H. Nohynek, L. T. Sombrero, L. G. Pascual, E. S. Sunico, G. A. Esparar, E. Esko, M. Leinonen, and P. Ruutu. 1995. Evaluation of sampling sites for detection of upper respiratory tract carriage of Streptococcus pneumoniae and Haemophilus influenzae among healthy Filipino infants. J. Clin. Microbiol. 33:3077-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Central Bureau of Statistics. 2001. Statistical abstracts of Israel, no. 52. The State of Israel, Jerusalem, Israel.
- 8.Chandra, R. K. 1988. Increased bacterial binding to respiratory epithelial cells in vitamin A deficiency. BMJ 297:834-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coles, C. L., R. Kanungo, L. Rahmathullah, R. D. Thulasiraj, J. Katz, M. Santosham, and J. M. Tielsch. 2001. Pneumococcal nasopharyngeal colonization in young South Indian infants. Pediatr. Infect. Dis. J. 20:289-295. [DOI] [PubMed] [Google Scholar]
- 10.Dabernat, H., M. A. Plisson-Saune, C. Delmas, M. Seguy, G. Faucon, R. Pelissier, H. Carsenti, C. Pradier, M. Roussel-Delvallez, J. Leroy, M. J. Dupont, F. De Bels, and P. Dellamonica. 2003. Haemophilus influenzae carriage in children attending French day care centers: a molecular epidemiological study. J. Clin. Microbiol. 41:1664-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan, R., D. Landau, H. Haikin, and A. Tal. 1993. Hospitalization of Jewish and Bedouin infants in southern Israel for bronchiolitis caused by respiratory syncytial virus. Pediatr. Infect. Dis. J. 12:381-386. [DOI] [PubMed] [Google Scholar]
- 12.Eskola, J., T. Kilpi, A. Palmu, J. Jokinen, J. Haapakoski, E. Herva, A. Takala, H. Kayhty, P. Karma, R. Kohberger, G. Siber, and P. H. Makela. 2001. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N. Engl. J. Med. 344:403-409. [DOI] [PubMed] [Google Scholar]
- 13.Faden, H., M. J. Waz, J. M. Bernstein, L. Brodsky, J. Stanievich, and P. L. Ogra. 1991. Nasopharyngeal flora in the first three years of life in normal and otitis-prone children. Ann. Otol. Rhinol. Laryngol. 100:612-615. [DOI] [PubMed] [Google Scholar]
- 14.Finkelman, Y., P. Yagupsky, D. Fraser, and R. Dagan. 1994. Epidemiology of Shigella infections in two ethnic groups in a geographic region in southern Israel. Eur. J. Clin. Microbiol. Infect. Dis. 13:367-373. [DOI] [PubMed] [Google Scholar]
- 15.Gray, B. M., M. E. Turner, and H. C. Dillon, Jr. 1982. Epidemiologic studies of Streptococcus pneumoniae in infants. The effects of season and age on pneumococcal acquisition and carriage in the first 24 months of life. Am. J. Epidemiol. 116:692-703. [DOI] [PubMed] [Google Scholar]
- 16.Hendley, J. O., M. A. Sande, P. M. Stewart, and J. M. Gwaltney, Jr. 1975. Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J. Infect. Dis. 132:55-61. [DOI] [PubMed] [Google Scholar]
- 17.Hoshino, K., H. Watanabe, R. Sugita, N. Asoh, S. A. Ntabaguzi, K. Watanabe, K. Oishi, and T. Nagatake. 2002. High rate of transmission of penicillin-resistant Streptococcus pneumoniae between parents and children. J. Clin. Microbiol. 40:4357-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klugman, K. P., H. J. Koornhof, A. Wasas, K. Storey, and I. Gilbertson. 1986. Carriage of penicillin resistant pneumococci. Arch. Dis. Child. 61:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy, A., D. Fraser, H. Vardi, and R. Dagan. 1998. Hospitalizations for infectious diseases in Jewish and Bedouin children in southern Israel. Eur. J. Epidemiol. 14:179-186. [DOI] [PubMed] [Google Scholar]
- 20.Martin, M., J. H. Turco, M. E. Zegans, R. R. Facklam, S. Sodha, J. A. Elliott, J. H. Pryor, B. Beall, D. D. Erdman, Y. Y. Baumgartner, P. A. Sanchez, J. D. Schwartzman, J. Montero, A. Schuchat, and C. G. Whitney. 2003. An outbreak of conjunctivitis due to atypical Streptococcus pneumoniae. N. Engl. J. Med. 348:1112-1121. [DOI] [PubMed] [Google Scholar]
- 21.McCracken, G. H., Jr. 2000. Etiology and treatment of pneumonia. Pediatr. Infect. Dis. J. 19:373-377. [DOI] [PubMed] [Google Scholar]
- 22.Moxon, E. R., and R. Wilson. 1991. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev. Infect. Dis. 13(Suppl. 6):S518-S527. [DOI] [PubMed] [Google Scholar]
- 23.Musher, D. M. 1992. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin. Infect. Dis. 14:801-807. [DOI] [PubMed] [Google Scholar]
- 24.Peerbooms, P. G., M. N. Engelen, D. A. Stokman, B. H. van Benthem, M. L. van Weert, S. M. Bruisten, A. van Belkum, and R. A. Coutinho. 2002. Nasopharyngeal carriage of potential bacterial pathogens related to day care attendance, with special reference to the molecular epidemiology of Haemophilus influenzae. J. Clin. Microbiol. 40:2832-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapola, S., E. Salo, P. Kiiski, M. Leinonen, and A. K. Takala. 1997. Comparison of four different sampling methods for detecting pharyngeal carriage of Streptococcus pneumoniae and Haemophilus influenzae in children. J. Clin. Microbiol. 35:1077-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenthal, J., R. Dagan, J. Press, and S. Sofer. 1988. Differences in the epidemiology of childhood community-acquired bacterial meningitis between two ethnic populations cohabiting in one geographic area. Pediatr. Infect. Dis. J. 7:630-633. [DOI] [PubMed] [Google Scholar]
- 27.Talon, D., J. Leroy, M. J. Dupont, X. Bertrand, F. Mermet, M. Thouverez, and J. M. Estavoyer. 2000. Antibiotic susceptibility and genotypic characterization of Haemophilus influenzae strains isolated from nasopharyngeal specimens from children in day-care centers in eastern France. Clin. Microbiol. Infect. 6:519-524. [DOI] [PubMed] [Google Scholar]
- 28.Varon, E., C. Levy, F. De La Rocque, M. Boucherat, D. Deforche, I. Podglajen, M. Navel, and R. Cohen. 2000. Impact of antimicrobial therapy on nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, and Branhamella catarrhalis in children with respiratory tract infections. Clin. Infect. Dis. 31:477-481. [DOI] [PubMed] [Google Scholar]