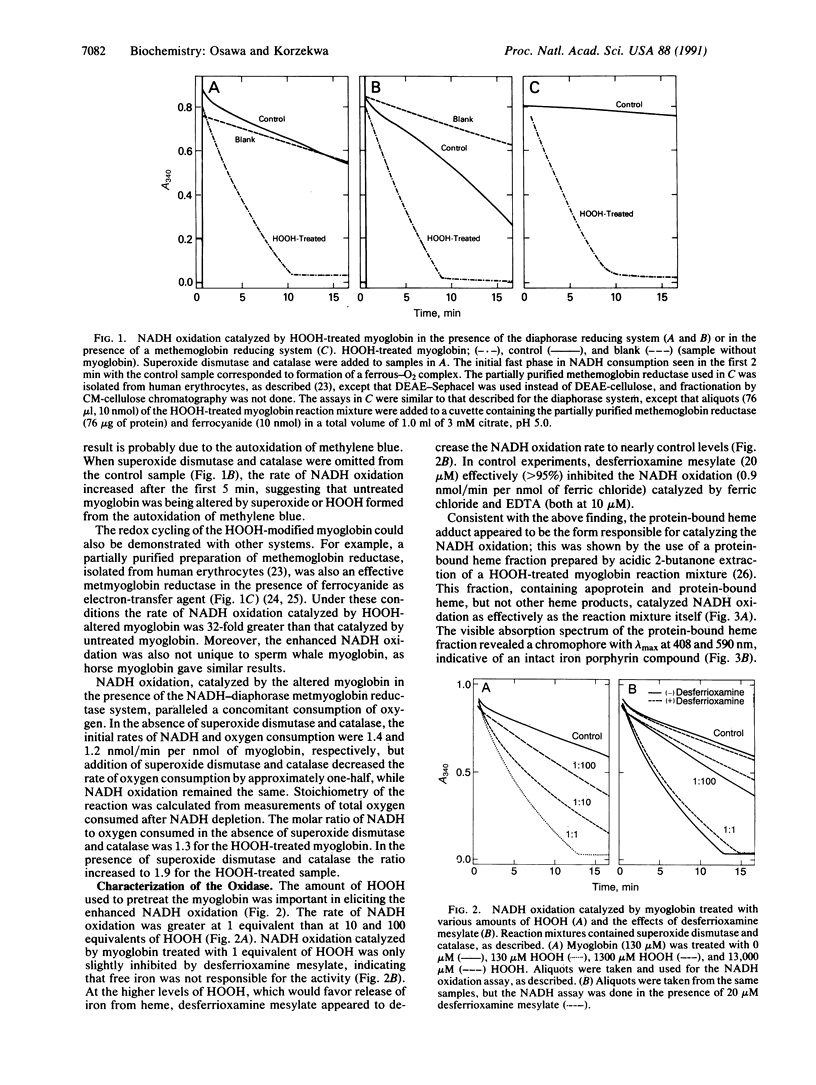
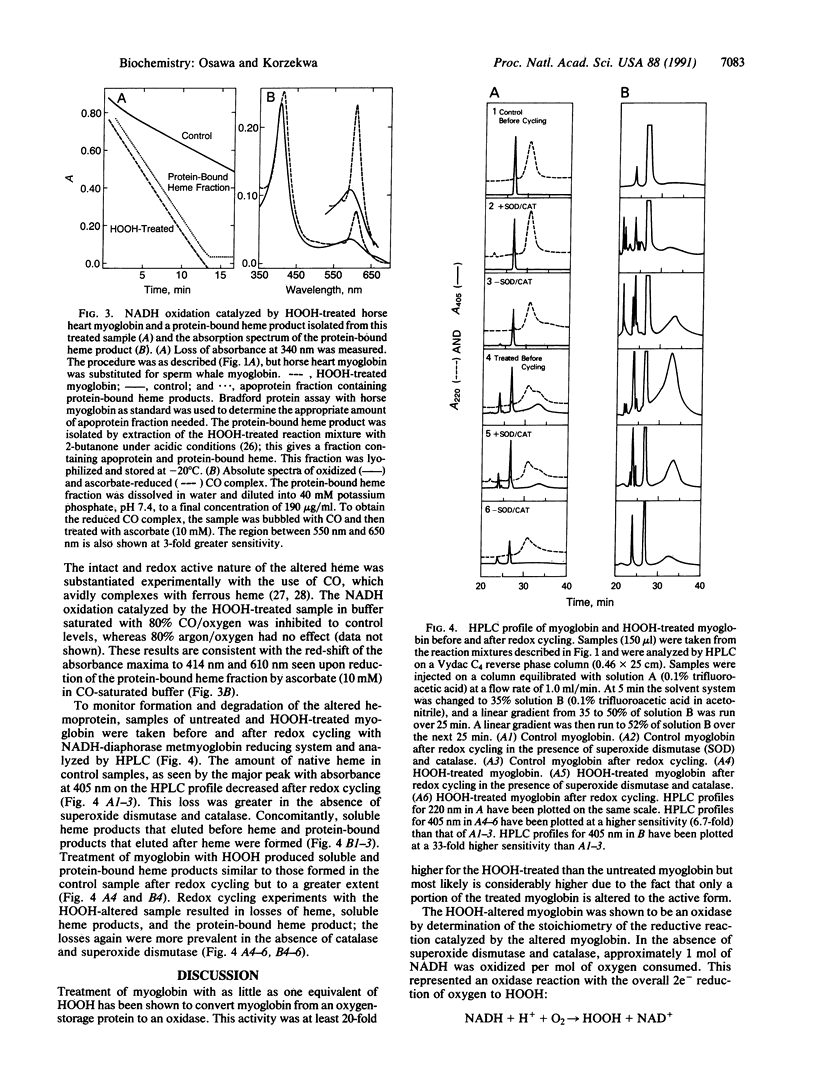
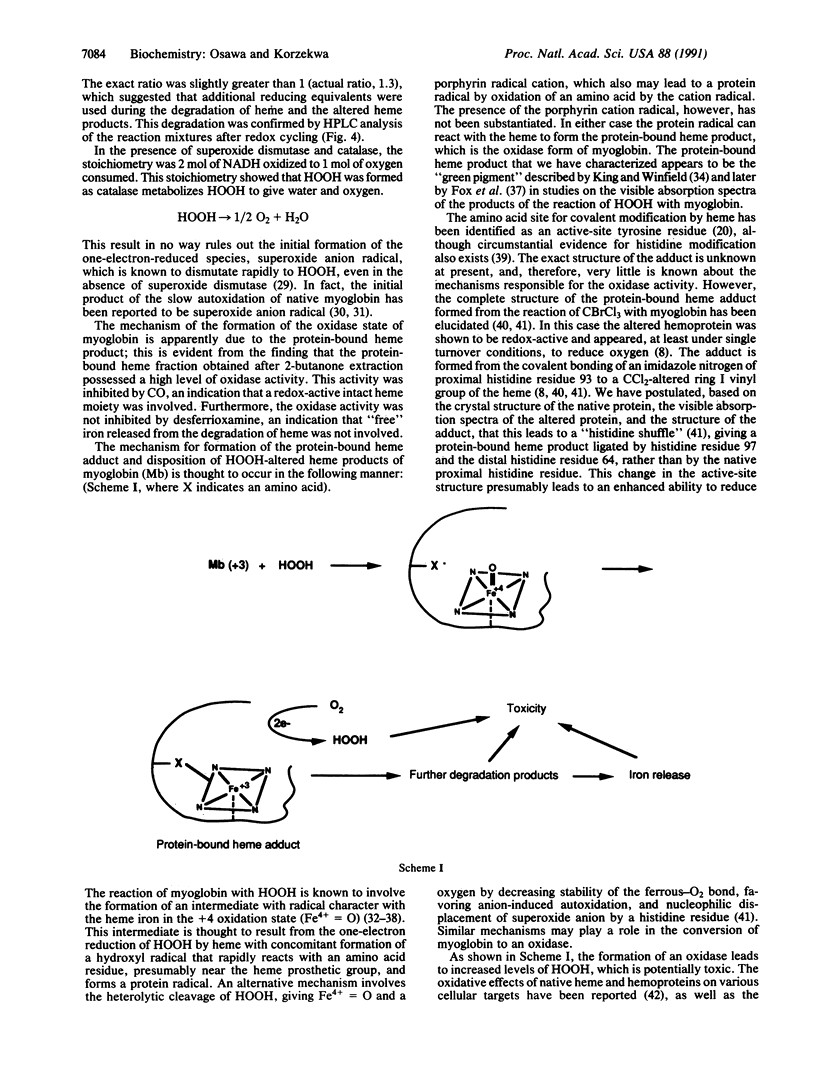
Abstract
It is generally thought that the oxidative modification of hemoproteins leads to their inactivation. In the current study, however, a transiently activated form of myoglobin was shown to be formed when the prosthetic heme group became covalently bound to the polypeptide during the reaction of myoglobin with low levels of HOOH. In the presence of an enzymatic metmyoglobin reducing system containing diaphorase and methylene blue with excess NADH, this HOOH-altered myoglobin catalyzed NADH oxidation and oxygen consumption; the overall stoichiometry indicated a two-electron reduction of oxygen to HOOH. This reaction was not catalyzed by iron released from heme, as desferrioxamine had no effect on the activity. Stoichiometric amounts of HOOH were sufficient to produce the activated oxidase state of myoglobin, whereas larger amounts of HOOH lead to heme destruction, iron release, and inactivation of the oxidase activity. The alteration of myoglobin to an enzyme that can form toxic oxygen metabolites may have pathological importance, especially in myocardial injury caused by ischemia and reperfusion, where myoglobin is present in large amounts and HOOH is formed. Furthermore, the oxidase form may be involved in the mechanism of destruction of the heme seen with oxidative treatment of myoglobin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnao M. B., Acosta M., del Río J. A., Varón R., García-Cánovas F. A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide. Biochim Biophys Acta. 1990 Oct 18;1041(1):43–47. doi: 10.1016/0167-4838(90)90120-5. [DOI] [PubMed] [Google Scholar]
- Brown W. D., Snyder H. E. Nonenzymatic reduction and oxidation of myoglobin and hemoglobin by nicotinamide adenine dinucleotides and flavins. J Biol Chem. 1969 Dec 25;244(24):6702–6706. [PubMed] [Google Scholar]
- Cantoni L., Gibbs A. H., De Matteis F. Loss of haem and haemoproteins during the generation of superoxide anion and hydrogen peroxide: a pathway not involving production of carbon monoxide. Int J Biochem. 1981;13(7):823–830. doi: 10.1016/0020-711x(81)90102-6. [DOI] [PubMed] [Google Scholar]
- Catalano C. E., Choe Y. S., Ortiz de Montellano P. R. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J Biol Chem. 1989 Jun 25;264(18):10534–10541. [PubMed] [Google Scholar]
- Chen Y. N., Bienkowski M. J., Marnett L. J. Controlled tryptic digestion of prostaglandin H synthase. Characterization of protein fragments and enhanced rate of proteolysis of oxidatively inactivated enzyme. J Biol Chem. 1987 Dec 15;262(35):16892–16899. [PubMed] [Google Scholar]
- Davies M. J. Detection of myoglobin-derived radicals on reaction of metmyoglobin with hydrogen peroxide and other peroxidic compounds. Free Radic Res Commun. 1990;10(6):361–370. doi: 10.3109/10715769009149905. [DOI] [PubMed] [Google Scholar]
- De Matteis F., Gibbs A. H., Unseld A. Loss of haem from cytochrome P-450 caused by lipid peroxidation and 2-allyl-2-isoprophylacetamide. An abnormal pathway not involving production of carbon monoxide. Biochem J. 1977 Dec 15;168(3):417–422. doi: 10.1042/bj1680417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. W., Nurcombe H. L., Hart C. A. Oxidative inactivation of myeloperoxidase released from human neutrophils. Biochem J. 1987 Aug 1;245(3):925–928. doi: 10.1042/bj2450925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrali M., Ciccoli L., Comporti M. Allyl alcohol-induced hemolysis and its relation to iron release and lipid peroxidation. Biochem Pharmacol. 1989 Jun 1;38(11):1819–1825. doi: 10.1016/0006-2952(89)90417-6. [DOI] [PubMed] [Google Scholar]
- Fox J. B., Jr, Nicholas R. A., Ackerman S. A., Swift C. E. A multiple wavelength analysis of the reaction between hydrogen peroxide and metmyoglobin. Biochemistry. 1974 Dec 3;13(25):5178–5186. doi: 10.1021/bi00722a020. [DOI] [PubMed] [Google Scholar]
- GEORGE P., IRVINE D. H. The reaction between metmyoglobin and hydrogen peroxide. Biochem J. 1952 Nov;52(3):511–517. doi: 10.1042/bj0520511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P. Covalent binding to apoprotein is a major fate of heme in a variety of reactions in which cytochrome P-450 is destroyed. Biochem Biophys Res Commun. 1986 Jul 16;138(1):193–198. doi: 10.1016/0006-291x(86)90265-2. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P. Destruction of heme and hemoproteins mediated by liver microsomal reduced nicotinamide adenine dinucleotide phosphate-cytochrome P-450 reductase. Biochemistry. 1978 Aug 22;17(17):3633–3639. doi: 10.1021/bi00610a033. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Avron M. The enzymatic reduction of ferrihemoglobin. I. The reduction of ferrihemoglobin in red blood cells and hemolysates. Biochim Biophys Acta. 1967 Sep 12;146(1):91–101. doi: 10.1016/0005-2744(67)90075-7. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Avron M. The enzymatic reduction of ferrihemoglobin. II. Purification of a ferrihemoglobin reductase from human erythrocytes. Biochim Biophys Acta. 1967;146(2):397–408. doi: 10.1016/0005-2744(67)90224-0. [DOI] [PubMed] [Google Scholar]
- Hildebrandt A. G., Roots I., Tjoe M., Heinemeyer G. Hydrogen peroxide in hepatic microsomes. Methods Enzymol. 1978;52:342–350. doi: 10.1016/s0076-6879(78)52037-5. [DOI] [PubMed] [Google Scholar]
- Iba M. M., Mannering G. J. NADPH- and linoleic acid hydroperoxide-induced lipid peroxidation and destruction of cytochrome P-450 in hepatic microsomes. Biochem Pharmacol. 1987 May 1;36(9):1447–1455. doi: 10.1016/0006-2952(87)90109-2. [DOI] [PubMed] [Google Scholar]
- Jenzer H., Kohler H., Broger C. The role of hydroxyl radicals in irreversible inactivation of lactoperoxidase by excess H2O2. A spin-trapping/ESR and absorption spectroscopy study. Arch Biochem Biophys. 1987 Nov 1;258(2):381–390. doi: 10.1016/0003-9861(87)90359-6. [DOI] [PubMed] [Google Scholar]
- Jenzer H., Kohler H. The role of superoxide radicals in lactoperoxidase-catalysed H2O2-metabolism and in irreversible enzyme inactivation. Biochem Biophys Res Commun. 1986 Aug 29;139(1):327–332. doi: 10.1016/s0006-291x(86)80117-6. [DOI] [PubMed] [Google Scholar]
- KING N. K., WINFIELD M. E. The mechanism of metmyoglobin oxidation. J Biol Chem. 1963 Apr;238:1520–1528. [PubMed] [Google Scholar]
- King N. K., Winfield M. E. Products of methemoglobin oxidation at acid pH. Aust J Biol Sci. 1966 Feb;19(1):211–217. [PubMed] [Google Scholar]
- Kulmacz R. J. Prostaglandin H synthase and hydroperoxides: peroxidase reaction and inactivation kinetics. Arch Biochem Biophys. 1986 Sep;249(2):273–285. doi: 10.1016/0003-9861(86)90003-2. [DOI] [PubMed] [Google Scholar]
- Levin W., Lu A. Y., Jacobson M., Kuntzman R., Poyer J. L., McCay P. B. Lipid peroxidation and the degradation of cytochrome P-450 heme. Arch Biochem Biophys. 1973 Oct;158(2):842–852. doi: 10.1016/0003-9861(73)90580-8. [DOI] [PubMed] [Google Scholar]
- Mitsos S. E., Kim D., Lucchesi B. R., Fantone J. C. Modulation of myoglobin-H2O2-mediated peroxidation reactions by sulfhydryl compounds. Lab Invest. 1988 Dec;59(6):824–830. [PubMed] [Google Scholar]
- Osawa Y., Highet R. J., Bax A., Pohl L. R. Characterization by NMR of the heme-myoglobin adduct formed during the reductive metabolism of BrCCl3. Covalent bonding of the proximal histidine to the ring I vinyl group. J Biol Chem. 1991 Feb 15;266(5):3208–3214. [PubMed] [Google Scholar]
- Osawa Y., Martin B. M., Griffin P. R., Yates J. R., 3rd, Shabanowitz J., Hunt D. F., Murphy A. C., Chen L., Cotter R. J., Pohl L. R. Metabolism-based covalent bonding of the heme prosthetic group to its apoprotein during the reductive debromination of BrCCl3 by myoglobin. J Biol Chem. 1990 Jun 25;265(18):10340–10346. [PubMed] [Google Scholar]
- Osawa Y., Pohl L. R. Covalent bonding of the prosthetic heme to protein: a potential mechanism for the suicide inactivation or activation of hemoproteins. Chem Res Toxicol. 1989 May-Jun;2(3):131–141. doi: 10.1021/tx00009a001. [DOI] [PubMed] [Google Scholar]
- Paller M. S. Hemoglobin- and myoglobin-induced acute renal failure in rats: role of iron in nephrotoxicity. Am J Physiol. 1988 Sep;255(3 Pt 2):F539–F544. doi: 10.1152/ajprenal.1988.255.3.F539. [DOI] [PubMed] [Google Scholar]
- Pieper G. M., Todd G. L., Wu S. T., Salhany J. M., Clayton F. C., Eliot R. S. Attenuation of myocardial acidosis by propranolol during ischaemic arrest and reperfusion: evidence with 31P nuclear magnetic resonance. Cardiovasc Res. 1980 Dec;14(11):646–653. doi: 10.1093/cvr/14.11.646. [DOI] [PubMed] [Google Scholar]
- Prasad M. R., Engelman R. M., Jones R. M., Das D. K. Effects of oxyradicals on oxymyoglobin. Deoxygenation, haem removal and iron release. Biochem J. 1989 Nov 1;263(3):731–736. doi: 10.1042/bj2630731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals in biological systems. Does myoglobin stimulate hydroxyl radical formation from hydrogen peroxide? Free Radic Res Commun. 1988;4(6):415–422. doi: 10.3109/10715768809066910. [DOI] [PubMed] [Google Scholar]
- Reddy B. R., Kloner R. A., Przyklenk K. Early treatment with deferoxamine limits myocardial ischemic/reperfusion injury. Free Radic Biol Med. 1989;7(1):45–52. doi: 10.1016/0891-5849(89)90099-3. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Lee Y. M., Brown W. D. Interactions of heme proteins with hydrogen peroxide: protein crosslinking and covalent binding of benzo[a]pyrene and 17 beta-estradiol. Arch Biochem Biophys. 1983 Mar;221(2):417–427. doi: 10.1016/0003-9861(83)90160-1. [DOI] [PubMed] [Google Scholar]
- Sakaida I., Kyle M. E., Farber J. L. Autophagic degradation of protein generates a pool of ferric iron required for the killing of cultured hepatocytes by an oxidative stress. Mol Pharmacol. 1990 Mar;37(3):435–442. [PubMed] [Google Scholar]
- Schaefer W. H., Harris T. M., Guengerich F. P. Characterization of the enzymatic and nonenzymatic peroxidative degradation of iron porphyrins and cytochrome P-450 heme. Biochemistry. 1985 Jun 18;24(13):3254–3263. doi: 10.1021/bi00334a027. [DOI] [PubMed] [Google Scholar]
- Sullivan J. L. The iron paradigm of ischemic heart disease. Am Heart J. 1989 May;117(5):1177–1188. doi: 10.1016/0002-8703(89)90887-9. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Tajima G., Shikama K. Autoxidation of oxymyoglobin. An overall stoichiometry including subsequent side reactions. J Biol Chem. 1987 Sep 15;262(26):12603–12606. [PubMed] [Google Scholar]
- Trotta R. J., Sullivan S. G., Stern A. Lipid peroxidation and haemoglobin degradation in red blood cells exposed to t-butyl hydroperoxide. The relative roles of haem- and glutathione-dependent decomposition of t-butyl hydroperoxide and membrane lipid hydroperoxides in lipid peroxidation and haemolysis. Biochem J. 1983 Jun 15;212(3):759–772. doi: 10.1042/bj2120759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. H. Oxidative effects of heme and porphyrins on proteins and lipids. Semin Hematol. 1989 Apr;26(2):105–113. [PubMed] [Google Scholar]
- Wallace W. J., Houtchens R. A., Maxwell J. C., Caughey W. S. Mechanism of autooxidation for hemoglobins and myoglobins. Promotion of superoxide production by protons and anions. J Biol Chem. 1982 May 10;257(9):4966–4977. [PubMed] [Google Scholar]
- Yonetani T., Schleyer H. Studies on cytochrome c peroxidase. IX. The reaction of ferrimyoglobin with hydroperoxides and a comparison of peroxide-induced compounds of ferrimyoglobin and cytochrome c peroxidase. J Biol Chem. 1967 Apr 25;242(8):1974–1979. [PubMed] [Google Scholar]



