Abstract
Background
BCG and measles vaccine (MV) may have beneficial non-specific effects (NSEs). If an unplanned intervention with a vaccine (a natural experiment) modifies the estimated effect in a randomised controlled trial (RCT), this suggests NSEs. We used this approach to test NSEs of triple oral polio vaccine (OPV).
Methods
During an RCT of 2 doses of MV at 4.5 and 9 months versus 1 dose of MV at 9 months of age, we experienced 2 natural experiments with OPV. We assessed whether these OPV experiments modified the effect of 2-dose MV in the MV trial.
Setting
MV RCT conducted in urban Guinea-Bissau 2003–2009.
Interventions
Natural experiments with OPV due to missing vaccine and the implementation of OPV campaigns.
Main outcome measure
Changes in the mortality rate ratio (MRR) for 2-dose MV versus 1-dose MV.
Results
First, the MRR (2-dose/1-dose MV) overall was 0.70 (0.52 to 0.94), but the MRR was 1.04 (0.53 to 2.04) when OPV at birth (OPV0) was not given, suggesting that early priming with OPV was important for the effect of 2-dose MV. The effect of OPV0 depended on age of administration; the MRR (2-dose/1-dose MV) was 0.45 (0.29 to 0.71) for children receiving OPV0 in the first week of life, but 3.63 (0.87 to 15.2) for those receiving OPV0 after the first month of life (p=0.007, test of no interaction). Second, campaign-OPV may have reduced the difference between the randomisation groups since the MRR (2-dose/1-dose MV) was 0.60 (0.42 to 0.85) for children who had not received campaign-OPV before RCT-enrolment versus 0.72 (0.23 to 2.31) and 1.42 (0.70 to 2.90) for children who had received 1 or 2 doses of campaign-OPV-before-enrolment, respectively.
Conclusions
Bissau had no polio infection during this trial, so OPV0 and campaign-OPV may have NSEs since they modified the effect of 2-dose MV in an RCT. Different interventions may interact to a much larger effect than usually assumed.
Keywords: age of administration,; measles vaccine,; natural experiment,; OPV,; oral polio vaccine,; non-specific effect of vaccines
Strengths and limitations of this study.
This is one of the first studies to examine whether oral polio vaccine (OPV) has an effect on child survival and whether OPV interacts with other interventions.
We analysed whether two natural experiments with OPV at birth and OPV campaigns taking place during a trial of two doses of measles vaccine (MV) at 4.5 and 9 months compared with one dose of MV at 9 months of age affected the overall outcome in the trial.
The natural experiments were observational but control for background factors which varied between groups being compared in the trial did not modify the results.
There has been no polio infection in Guinea-Bissau during the study period, and since OPV modified the effect of the specific MV interventions, OPV may have non-specific effects.
Introduction
The WHO's Strategic Advisory Group of Experts (SAGE) on immunisation recently conducted a review of the potential non-specific effects of BCG, diphtheria-tetanus-pertussis (DTP) and measles vaccine (MV). The review concluded that BCG and MV approximately halved the mortality risk.1 2 The studies of MV in which measles infection or measles deaths were censored in the survival analyses3–5 suggest that the effects of MV on mortality are not fully explained by the prevention of measles infection. Likewise, there was no indication that prevention of tuberculosis (TB) explained the beneficial effect of BCG vaccination.1 Hence, these two vaccines may have NSEs. Several immunological studies supports that vaccines can induce non-specific or heterologous effects by inducing cross-reactive T-cells or by training of innate immunity.6 7 Hence, we need to examine the potential NSEs of other routine vaccines than those initially reviewed by SAGE.
We have therefore examined the potential NSEs of oral polio vaccine (OPV).8–10 Investigations of NSEs of routine vaccines are complex because it is usually not possible to randomise children to vaccines already recommended if it means that the vaccine is withheld or delayed for some children. OPV is additionally difficult to study because it is routinely given together with DTP in three doses in the first months of life, and it is therefore nearly impossible to assess the separate effects of OPV. Hence, ‘natural experiments’ where the national immunisation programme are missing vaccine or implement a new vaccine campaign are useful to assess the effect of OPV.8 9
Vaccine randomised controlled trials (RCTs) with focus on NSEs examine whether the study vaccine modifies susceptibility to unrelated infections with implications for mortality.3 10 If another vaccine given in both intervention groups has NSEs, it is likely to alter the difference between the randomisation groups within the RCT. A potential way to assess NSEs therefore arises if a natural experiment with a vaccine occurs during the conduct of a vaccine RCT. It is then possible to assess if the estimated effect of the RCT vaccine changes as a result of the natural experiments. If that happens, it would be evidence of NSEs of the natural experiment vaccine.
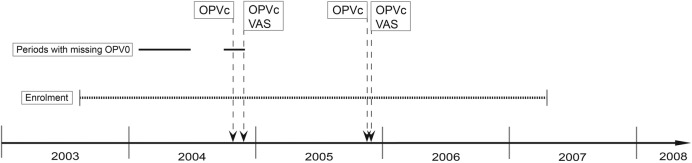
We used this approach in connection with an RCT of two doses of MV at 4.5 and 9 months versus one dose of MV at 9 months of age (figure 1). We experienced two natural experiments with OPV during the trial: (1) OPV at birth (OPV0) was not provided during several months; and (2) additional doses of OPV were administered in several national OPV campaigns (figure 2).
Figure 1.
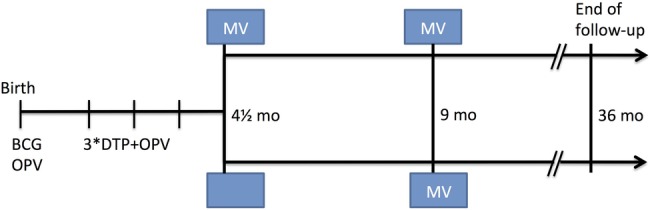
Design of the two-dose versus one-dose MV randomised controlled trial. DTP, diphtheria-tetanus-pertussis; OPV, oral polio vaccine; mo, month; MV, measles vaccine.
Figure 2.
Events during this study. Enrolment took place between 2003 and 2007. In 2004 and 2005, there were two oral polio vaccine (OPV) campaigns with a 1 month interval. In the first campaign, campaign-OPV (OPVc) was given to all children aged 0–59 months and in the second campaign OPVc was given to the same age group together with vitamin A supplementation (VAS) to children aged 6–59 months. During 2004, there were two periods, marked with a solid black line, in which children did not received OPV at birth (OPV0).
Methods
Two-dose MV trial
The two-dose MV trial recruited children between 2003 and 2007 and followed the children to 36 months of age in an urban area in Guinea-Bissau, which has been followed with a Health and Demographic Surveillance System (HDSS) since 1978.3 The two-dose MV trial design and randomisation procedures have been described in detail elsewhere.3 Briefly, at 4.5 months of age, 6417 children, who had received all three doses of DTP recommended at 6, 10 and 14 weeks of age, were randomised 1:2 to receive standard dose Edmonston-Zagreb (EZ) MV at enrolment or no vaccine. At 9 months of age, all children were invited back to receive the standard MV recommended by the WHO. The two-dose group received EZ MV; the one-dose group had been randomised at 4.5 months to receive either EZ or Schwarz MV at 9 months of age. Hence, between 4.5 and 9 months of age, the trial compared the effect on mortality of one early dose of MV versus no MV (ie, having the third doses of DTP and OPV as the most recent vaccination); between 9 and 36 months of age, the trial compared two doses of MV versus one dose of MV (figure 1). The project registered all routine vaccines that the children had received.
The results of the two-dose MV trial on child mortality between 4.5 and 36 months of age were published in 2010.3 The main analysis was based on 6417 participants. The majority of children in the MV trial3 had taken part in trials of neonatal vitamin A supplementation (NVAS)11 and since NVAS is not official policy and is unlikely to become policy,12 we tested the effect of two doses of MV for the 3402 children who had not received NVAS.
Natural experiment I: missing OPV0 in 2004. Nearly all children in the two-dose MV trial received BCG at birth or shortly thereafter. More than 80% of the children enrolled in the two-dose MV trial had previously taken part in trials3 in which the dates of BCG and OPV vaccinations after birth were documented. OPV was missing in Guinea-Bissau for several months in 2004 (figure 2) and many children did therefore not receive OPV0.13 Whether the children had received OPV0 or not was registered as part of these trials in which the date of BCG and OPV vaccinations was noted. We examined whether receiving OPV0 and the age at which BCG and OPV0 was given modified the effect of a two-dose versus one-dose MV in the RCT.
Natural experiment II: OPV campaigns in 2004 and 2005. There were two national campaigns with trivalent OPV with 1 month's interval in 2004 and in 2005 (see figure 2). In both years, OPV was first provided alone, and then 1 month later OPV was administered together with vitamin A supplementation (VAS). OPV was provided to all children between birth and 5 years of age, whereas VAS was only administered to children who were 6 months and older. In 2004, the campaigns took place in the end of October (OPV only) and November (OPV+VAS); in 2005, campaigns were in November (OPV only) and December (OPV+VAS).
The OPV campaigns were conducted by staff from the three health centres in the study area. They went from house to house. The nurses were accompanied by trained HDSS fieldworkers who had lists of all children in the study area generated from the HDSS database. Fieldworkers were followed by a supervisor for at least 1 day during the 4-day long campaigns. The fieldworkers registered the presence of the children, and whether they received OPV (and VAS) on that day, or whether they had received it elsewhere.
Patient involvement
The timing of the campaigns was decided by national public health authorities. The authors decided after the campaigns had occurred to examine whether the campaigns had an impact on the RCT. Hence, guardians of patients were not involved in the planning of the study. The results will be disseminated to the general public through medical journals and specifically to the WHO, which determines global vaccination policies, and to the Ministry of Health if there is scientific evidence to support a change in policy or practice.
Statistical methods
We used the previously presented RCT data set to compare mortality of children randomised to two doses of MV at 4.5 and 9 months or one dose of MV at 9 months of age, providing mortality rate ratios (MRRs) (two-dose/one-dose MV) from 4.5 months to 36 months of age.3 We conducted all analyses including all 6417 children, and also conducted all analyses in the subgroup of 3402 children who had not received NVAS at birth. The conclusions with respect to OPV were essentially similar, and therefore only the overall results are presented.
The original analysis stratified for the health centre area (N=3) but did not adjust for background factors since this was a randomised trial and the background factors were balanced between the two randomisation groups (3, table 1). Since the key point of the present analysis is to see whether the campaigns changed the effect measured in the RCT, we are not adjusting for other background factors in the main analyses. However, since the allocation to the subgroups in the two natural experiments could have been influenced by background factors, we analysed the distribution of background factors in both experiments (see online supplementary tables S1 and S2) and present analyses where we adjusted for background factors which differed significantly at the time of group allocation or before (see online supplementary tables S3 and S4).
Table 1.
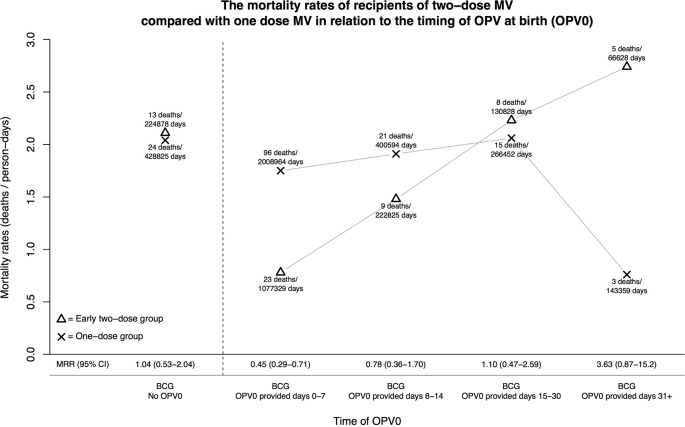
The mortality rates and mortality rate ratio (MRR) of recipients of two-dose MV compared with one-dose MV in relation to the timing of OPV at birth (OPV0)
| Mortality rates (deaths/person-days) (N) |
||||
|---|---|---|---|---|
| Timing of administration of OPV0 | Early 2-dose group | 1-dose group | MRR (2-dose/1-dose MV) (95% CI) | P for trend with age* |
| All children (main result of trial) N=6417 |
1.23 (58/1 722 488) (2129) | 1.79 (159/3 248 194) (4288) | 0.70 (0.52 to 0.94) | |
| BCG, no OPV0 | 2.11 (13/224 878) (284) | 2.04 (24/428 825) (570) | 1.04 (0.53 to 2.04) | |
| BCG+OPV0 provided Days 0–7 |
0.78 (23/1 077 329) (1317) | 1.75 (96/2 008 964) (2661) | 0.45 (0.29 to 0.71) | p=0.02 |
| BCG+OPV0 provided Days 8–14 |
1.48 (9/222 825) (277) | 1.91 (21/400 594) (523) | 0.78 (0.36 to 1.70) | |
| BCG+OPV0 provided Days 15–30 |
2.23 (8/130 828) (165) | 2.06 (15/266 452) (349) | 1.10 (0.47 to 2.59) | |
| BCG+OPV0 provided Days 31+ |
2.74 (5/66 628) (86) | 0.76 (3/143 359) (185) | 3.63 (0.87 to 15.2) | |
Notes: The estimates are based on a Cox proportional hazards model; to be precise, we have reported person-days and not person-years.
*The age trend tested for a significant linear increase in the effect across the four groups.
MV, measles vaccine; OPV, oral polio vaccine; OPV0, OPV at birth.
bmjopen-2016-013335supp_tables.pdf (60.3KB, pdf)
Natural experiment I: missing OPV0. We first examined whether the MRR (two-dose/one-dose MV) was different in children who received or did not receive OPV0. We have found in an RCT that early administration of OPV gave a stronger beneficial effect,10 and we therefore also assessed whether the age of administration of OPV0 and BCG had a modifying effect.
Natural experiment II: campaign-OPV. In the analysis of campaign-OPV, we examined whether the MRR (two-dose/one-dose MV) was different depending on whether the children had received campaign-OPV or not before enrolment in the trial. Thus, we calculated the MRR (two-dose/one-dose MV) separately for children who received campaign-OPV-before-enrolment in the trial (not given with VAS, which was only given after 6 months of age), and for those who did not receive campaign-OPV-before-enrolment. Of the 1380 children receiving campaign-OPV-before-enrolment, only 2% (N=25) had received OPV with VAS; we are only showing the results for all children since excluding the 25 children who got VAS with OPV did not change the results.
We also assessed if campaign-OPV-after-enrolment in the trial (usually given with VAS) modified the MRR (two-dose/one-dose MV). Since nearly all children receive campaign-OPV and we would not be able to document that absent and travelling older children had received campaign-OPV, this analysis was based on the assumption that all children still living in the community got the campaign-OPV on the first day of the campaign.
During the conduct of the present RCT, there was a national MV campaign (5/2006). The children in the two-dose trial were exempted from taking part in the MV campaign.3 In addition, there were national VAS campaigns in November 2003, and VAS and mebendazole campaigns in May and December 2006, July and December 2007, July 2008, and January and July of 2009. These VAS campaigns did not affect the mortality rate within the MV trial; the mortality rate after VAS campaigns versus before VAS campaigns was 0.92 (0.63 to 1.35).
Outcome measures and models: We present deaths and observation time together with MRR (two-dose/one-dose MV) from a Cox model with age as the underlying time scale and stratified by district as in the original analysis.3 The model assumptions were assessed graphically and tested using Schoenfeld residuals. Since mortality levels could have changed over time, we also conducted the analyses with calendar time as the underlying time scale adjusting for age as a linear predictor. Results were essentially similar, not changing any of the estimates by more than ‘0.02’. Thus, we only show results from the analyses with age as the underlying time scale. To test for no interaction, we compared the effect of two-dose vs one-dose MV in strata of the suspected effect modifier using Wald statistics.
Results
The two-dose MV trial of child mortality between 4.5 and 36 months of age included 6417 participants in the main analysis. Background factors were equally distributed between the two randomisation groups (3, table 1). In the survival analysis, the children were followed until they moved out of the area, died or attained 3 years of age, whichever came first. As described previously, the per-protocol MRR (two-dose/one-dose MV) between 4.5 and 36 months for children who did get two doses of MV was 0.70 (0.52 to 0.94).3 We have used the per-protocol data set for the main analyses in the present paper but results for the intention-to-treat data set are presented in online supplementary tables S5 and S6.
Natural experiment I: missing OPV0
All 6417 children in the trial were included in the analysis; 854 (13%) did not receive OPV0 (table 1). For children who had not received OPV0, the MRR (two-dose/one-dose MV) was 1.04 (0.53 to 2.04) (table 1, figure 3). The beneficial effect of two-dose MV increased the earlier OPV0 and BCG had been given; the MRR was 0.45 (0.29 to 0.71) for children receiving OPV0 in the first week of life but 3.63 (0.87 to 15.2) for those receiving OPV0 after the first month of life (p=0.007, test of no interaction). The trend for increasing benefit of two-dose MV with earlier OPV0 administration was given was statistically significant (p=0.02) (table 1). If age at OPV0 was analysed as a continuous variable, the MRR (two-dose/one-dose MV) was reduced by 4% (1–6%) for each additional day of age at OPV0 vaccination. There were minor but statistically significant differences in sex distribution and mother's mid-upper arm circumference (MUAC) between those who received OPV0 and those who did not (see online supplementary table S1); adjustment for these factors did not modify the results (see online supplementary table S3).
Figure 3.
The mortality rates and MRR for recipients of two-dose MV compared with one dose MV in relation to the timing of OPV at birth. MRR, mortality rate ratio; MV, measles vaccine; OPV, oral polio vaccine; OPV0, OPV at birth.
Results were essentially similar in the intention-to-treat analysis (see online supplementary table S5).
Natural experiment II: campaign-OPV
Campaign-OPV-before-enrolment: All 6417 children in the trial were included in the analysis. The proportion of children who had received campaign-OPV prior to enrolment was similar in the two-dose (21.5% (458/2129)) and one-dose (21.3% (912/4288)) groups. Among the children who were or became participants in the RCT and lived in the community in 2004 and 2005, 92% (2319/2512) and 87% (3599/4123) were registered to have received at least one campaign-OPV, respectively.
Among children who had not received any campaign-OPV-before-enrolment, the MRR (two-dose/one-dose MV) was 0.60 (0.42 to 0.85), whereas the MRR (two-dose/one-dose MV) was 1.16 (0.64 to 2.12) for those who received campaign-OPV-before-enrolment (p=0.06, test of no interaction) (table 2). If two doses of campaign-OPV had been received before enrolment, the MRR (two-dose/one-dose MV) was 1.42 (0.70 to 2.90), significantly different from the effect among children receiving no campaign-OPV-before-enrolment (p=0.03, test of no interaction) (table 2). The analyses gave similar results for boys and girls (data not shown).
Table 2.
The mortality rates and mortality rate ratio (MRR) of recipients of two-dose MV compared with one-dose MV in relation to the administration of campaign-OPV-before-enrolment, overall and by number of doses
| Mortality rates (deaths/person-days) |
MRR (2-dose/1-dose MV) (95% CI) | ||
|---|---|---|---|
| Early 2-dose group | 1-dose group | ||
| No campaign-OPV-before-enrolment | 1.11 (41/1 354 117) | 1.88 (131/2 542 844) | 0.60 (0.42 to 0.85)* |
| Campaign-OPV-before-enrolment | 1.69 (17/368 371) | 1.45 (28/705 350) | 1.16 (0.64 to 2.13)* |
| 1-dose OPV | 1.23 (4/118 730) | 1.68 (10/217 279) | 0.72 (0.23 to 2.31) |
| 2-dose OPV | 1.90 (13/249 641) | 1.35 (18/488 071) | 1.42 (0.70 to 2.90) |
Notes: The estimates are based on a Cox proportional hazards model; to be precise, we have reported person-days and not person-years.
*Test for whether the effect of early two-dose MV is equal in those receiving no campaign-OPV-before-enrolment and those receiving campaign-OPV-before-enrolment, p=0.06.
MV, measles vaccine; OPV, oral polio vaccine.
There were minor but statistically significant differences in the number of persons sleeping per room and mother's MUAC between those who received campaign-OPV-before-enrolment and those who did not (see online supplementary table S2); adjustment for these factors did not modify the results (see online supplementary table S4).
Results were essentially similar in the intention-to-treat analysis (see online supplementary table S6).
Campaign-OPV-after-enrolment: In the group which did not receive campaign-OPV-before-enrolment and did not receive or had not yet received campaign-OPV-after-enrolment, early two-dose MV was associated with a beneficial effect the MMR being 0.53 (0.32 to 0.87) (table 3); the effect was similar for boys and girls (data not shown). For children who had received campaign-OPV-after-enrolment, the MRR (two-dose MV/one-dose MV) was significantly better for girls than for boys (p=0.05, test of no interaction). The effect of campaign-OPV-after-enrolment on the MRR (two-dose MV/one-dose MV) differed significantly for the children who had (MRR=2.90 (0.82 to 10.3)) and had not received campaign-OPV-before-enrolment (MRR=0.68 (0.41 to 1.12)) (p=0.04, test of no interaction) (table 3).
Table 3.
The mortality rates and mortality rate ratio (MRR) of recipients of two-dose MV compared with one-dose MV in relation to the administration of campaign-OPV-before-enrolment and if the child had received campaign after enrolment
| Received no campaign-OPV-before-enrolment |
Received campaign-OPV-before-enrolment |
Joined MRR(2-dose/1-dose MV) (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Mortality rates (deaths/person-days) |
MRR (2-dose/1-dose MV) (95% CI) | Mortality rates (deaths/person-days) |
MRR (2-dose/1-dose MV) | ||||
| Early 2-dose group | 1-dose group | Early 2-dose group | 1-dose group | ||||
| All children | |||||||
| Not received or not yet received campaign-OPV-after-enrolment | 1.15 (20/633 629) | 2.19 (74/1 234 945) | 0.53 (0.32 to 0.87) | 1.95 (11/205 532) | 2.23 (24/393 152) | 0.87 (0.43 to 1.78) | 0.62 (0.41 to 0.92) |
| Received campaign-OPV-after-enrolment | 1.06 (21/720 488) | 1.59 (57/1 307 899) | 0.68 (0.41 to 1.12)† | 1.35 (6/162 839) | 0.47 (4/312 198) | 2.90 (0.82 to 10.3)† | 0.82 (0.52 to 1.29) |
Notes: The estimates are based on a Cox proportional hazards model; to be precise, we have reported person-days and not person-years.
*It was assumed in the analysis that all eligible children during a campaign in fact received campaign-OPV.
†0.04, Test of no interaction.
MV, measles vaccine; OPV, oral polio vaccine.
Discussion
Main observations: OPV administered as either OPV0 or campaign-OPV had NSEs because it changed the MRRs (two-dose MV/one-dose MV) in the RCT. First, the beneficial effect of early MV administered at 4.5 months of age depended on early priming with OPV0. Second, the beneficial effect of early MV was strongest when the children had not received campaign-OPV before or after enrolment in the trial.
Strengths and weaknesses: Although the present study was not a planned study, its design limited the possibility of uncontrolled confounding because all children were enrolled in the two-dose MV trial, which used the same inclusion criteria throughout. The unavailability of OPV0 and the availability of OPV campaigns were natural experiments not dependent on the health status of the children.
The mortality rate between 4.5 and 36 months was significantly lower the earlier OPV0 (and BCG) had been given. The timing of OPV0 could have depended on the health status and the socioeconomic conditions of the child. For example, the best families or healthiest children might be delivered at the maternity ward or come first for vaccination at a health centre. Alternatively, sick and disadvantaged children may have been brought later for vaccination. However, such confounding would not explain why two doses of MV had the best effect among those vaccinated with OPV0 in the first week of life, as children from the best families presumably have the least to gain. Furthermore, we found in an RCT of OPV0 that the effect was considerably stronger when the OPV0 had been given within the first 2 days of life.10 The observation therefore suggests that OPV0 primes the immune system in ways which are subsequently enhanced by early MV at 4–5 months of age.
It should be noted, however, that the period during 2004 with missing OPV0 has previously produced the unexpected result that male infant mortality was lower in neonates who had not received OPV0 than in neonates who had received OPV0.13 This finding was contradicted in a subsequent RCT.10 The unexpected result may have been due partly to the 2004-OPV campaigns coming just after the period with missing OPV0, which lowered the general mortality rate and had a particularly beneficial effect for males (submitted).
The main potential confounding factor for the effect of campaign-OPV would be a change in mortality rate coinciding with the timing of the OPV campaigns. However, adjustment for calendar time had no impact on the estimates. The study had limited power to analyse the interactions between trial randomisation and the many combinations of campaign-OPV before and after enrolment (tables 2 and 3).
Single intervention paradigm: In the current epidemiology culture, an RCT measures the effect of an intervention in a controlled situation, that is, other interventions are not tested at the same time. Hence, the effect measured in the RCT is believed to be the ‘true’ effect. If the intervention becomes policy, it will be assumed in future modelling of programme impact that this ‘true’ effect will continue. For example, high-dose VAS was tested in eight trials in the 1980s and early 1990s and found to reduce mortality by 23–30% in two meta-analyses. This effect is still assumed to exist,14 even though the only two recent trials contradict the assumption.15 16 There are similar conflicting stories for other interventions including neonatal vitamin A (NVAS)12 17 18 and MV.19
There are probably several causes of these conflicting stories, but a main reason is the disease-specific perspective underlying preventive global childcare programmes. It is usually assumed that interventions have only one specific effect on the immune system preventing a specific disease or deficiency. However, a growing number of studies show that interventions may have much wider effects by reprogramming the immune system to enhanced protection or susceptibility to unrelated infections.6 7 From this perspective, the ‘true’ effect of an intervention is the measured reduction in overall mortality/morbidity under a certain set of specified conditions in relation to other interventions affecting mortality. For example, the optimal effect of early MV at 4.5 months of age was obtained when children were primed with OPV0 (and BCG) in the first weeks of life (this study), did not receive NVAS,3 did not receive campaign-OPV before (this study), did not receive DTP after early MV3 and did receive MV in the presence of maternal antibodies.20 When altering these conditions, and other potential priming conditions that we do not know about yet, the ‘true’ effect is no longer observed. However, departures from the expected effects may be a fruitful instrument for accumulating new knowledge about the limits or conditions under which an intervention will work or have deleterious effects.
Consistency or contradiction with previous observations: Our finding that OPV has NSEs is supported in the literature. Earlier studies from the 1960s when OPV was developed reported that OPV reduced diarrhoeal mortality in Chile and Brazil and Russian researchers claimed more general effects on health including reducing respiratory infection from stimulation with non-pathogenic enterovirus including OPV.21–23 These observations23 were not pursued outside the Soviet Union.
When the first OPV campaigns were implemented in Guinea-Bissau in 1998, we examined the effect on child survival of having participated versus not having participated in the campaign.9 OPV apparently had a beneficial effect for the youngest children <6 months of age; for older children, it was difficult to determine a separate effect due to the strongly beneficial effect of MV. We have also found in a natural experiment, when DTP was missing, that the case fatality at the hospital was much lower for children who had received OPV only and not OPV+DTP as currently recommended.8
We have also conducted two RCTs of OPV0 in Bissau; in one trial, OPV0 was compared with neonatal VAS among low birthweight boys who normally do not receive BCG at birth, and in another RCT BCG+OPV0 was compared with BCG only among normal birthweight children. OPV0 was associated with 32% lower infant mortality in both trials, an effect which was statistically significant in the larger trial among normal birthweight children.10 24
Studies from high income countries have likewise suggested that OPV may have beneficial NSEs. In Finland, children who received OPV in a trial had fewer episodes of otitis media at age 6–18 months than control children who received inactivated polio vaccine (IPV).25 In a nationwide study in Denmark, the routine OPV which used to be given at 2 years of age (until 2001) was associated with a 15% (5–23%) reduction in the risk of hospital admissions compared with children who had acellular DTP, Haemophilus influenzae type b and inactivated polio vaccine (DTaP-Hib-IPV) as their most recent vaccination, most of the effect coming from prevention of admissions for lower respiratory infections.26 The effect of OPV was similar to the effect of MMR.27 In Denmark, a second and third dose of OPV was recommended at 3 and 4 years of age. These additional doses were associated with significant reductions in admissions.
Interpretation: OPV produced different NSEs in this study and we have tried to summarise these in table 4.28–31 Apparently, OPV given early in life provided priming which subsequently enhanced the beneficial effects of early MV. In our RCT of OPV0+BCG versus BCG only, the effect on infant mortality was particularly strong when OPV0 was given in the first days of life.10 Studies of BCG have likewise indicated that the beneficial effect is particularly good when administered early.32 Although the effect in the present analysis is different since it is generated by a subsequent early MV, it does suggest that OPV0 is capable of inducing strong immune training. It is known that other live vaccines may induce an immune training effect which reduces susceptibility to unrelated infections. For example, BCG reprogrammes monocytes through epigenetic changes to a more proinflammatory response; in animal models, this response reduces mortality from challenge to unrelated infections.7 It would be indicated to conduct similar studies for OPV.
Table 4.
Possible modifiers of the effect of early measles vaccination on mortality
| Epidemiological observations | Comparable biological data |
|---|---|
| Early OPV enhances the beneficial effect of early MV at 4–5 months of age (this paper) | Cross-stimulation has been shown to enhance immunological responses, eg, early BCG primes for a stronger specific immune response to hepatitis B vaccine (HBV) and OPV vaccinations.6 7 On the other hand, we have also observed that OPV given with BCG at birth reduces the specific immune response to BCG. Effect of cross-stimulation on mortality has not been studied. |
| Campaign-OPV-before-early MV reduces the beneficial effect of early MV (this paper). Campaign-OPV may have both reduced the mortality level in the 1-dose group and increased the mortality rate slightly in the 2-dose MV group, resulting in an overall elimination of the differential (beneficial) effect of early MV (this paper). | The sequence or combination of early life vaccines, including BCG, OPV, DTP, and HBV, often have an impact on subsequent mortality.19 28 29 The underlying biological mechanisms have not been studied. |
| Campaign-OPV-after-early MV (and usually after a second dose of MV) following previous OPV before early MV reduces the benefit of early MV by reducing mortality more strongly in the 1-dose MV group or by increasing mortality in the 2-dose group (this paper). | Several studies have shown that re-exposure to the same antigen enhances the beneficial effect, eg, BCG, MV and Vaccinia. Such beneficial boosting effects of OPV could be more pronounced in the 1-dose group which had more to gain. The underlying biological mechanisms have not been studied. |
| Presence of maternal measles antibodies enhances the beneficial effect of early MV at 4.5 months of age20 | This has been shown in animal studies but has not been studied for other human pathogens.30 |
| Neonatal vitamin A (NVAS) reduced the beneficial effect of early MV at 4.5 months of age3. As with campaign-OPV, NVAS may have both reduced the mortality level in the one-dose group and increased the mortality rate slightly in the 2-dose MV group, resulting in an overall elimination of the differential (beneficial) effect of early MV.11 | In vitro studies have shown that vitamin A induces innate immune tolerance. Providing vitamin A can abrogate the innate immune training induced by BCG.31 |
| DTP after early MV associated with increased mortality for females19 | Numerous studies have shown that DTP and other inactivated vaccines administered after MV reduced or removed the beneficial non-specific effects of MV.19 29 The underlying biological mechanisms have not been studied. |
DTP, diphtheria-tetanus-pertussis; MV, measles vaccine; NVAS, neonatal vitamin A supplementation; OPV, oral polio vaccine.
We have found in several studies that subsequent boosting with a live vaccine with a beneficial effect enhanced the beneficial effect. The present analysis suggests that different live vaccines with beneficial effects may interact with each other. Both MV and OPV have been reported to have particularly strong protective effects against lower respiratory infection,26 27 33 so maybe the vaccines boost similar responses.
Although OPV0 and OPV campaigns may have lowered mortality, campaign-OPV given before and after administration of early MV at 4.5 months of age may have increased mortality in the early two-dose MV group, at least relative to the one-dose MV group (table 3). We have had a similar experience of negative interaction between interventions with NVAS which neutralised the beneficial effect of early two-dose MV (table 4).3 11 Children who received the two-dose regime and no NVAS had a 50% reduction in mortality between 4.5 and 36 months. However, if they had been randomised to receive NVAS, there was no effect from receiving MV at 4.5 months. NVAS apparently reduced mortality in the one-dose group and increased it in the two-dose group. This appears to be parallel to what happened to children who received campaign-OPV both before and after enrolment in the present trial. Both experiences suggest that interfering immune training may have occurred in the first months of life.
All children had received the three doses of OPV with DTP before enrolment into the MV trial. Hence, it is a challenge to explain why additional doses of OPV before enrolment should affect the immune system and result in a modification of the response to early MV. It is an additional challenge that it seemingly makes a difference whether this additional dose is given at birth as OPV0 (which primes for a beneficial response to early MV) or campaign-OPV (which primes for a negative response to early MV). One speculation could be that OPV has different NSEs depending on the age and the type of vaccine it is given with: OPV0 was often given at birth, into the sterile gut, where it may affect the microbiome, and it was given with BCG. Campaign-OPV-before-enrolment, on the other hand, was given alone, and campaign-OPV seems to be beneficial in its own right (A Andersen, et al. National immunisation campaigns with OPV reduce all-cause mortality: a natural experiment within seven randomised trials. (In review)). Campaign-OPV-after-enrolment was often given with VAS and may have had yet other NSEs.
Implications: An increasing number of studies suggest that OPV may be associated with beneficial NSEs. With the beneficial NSEs of OPV0 and of campaign-OPV for children who received the currently recommended vaccination schedule, it is possible that the repeated campaigns with OPV have been a driving force in the decline towards Millennium Development Goal 4 (MDG4) which has been going on in the past 15–20 years (A Andersen, et al. National immunisation campaigns with OPV reduce all-cause mortality: a natural experiment within seven randomised trials. (In review)). This needs to be explored now before OPV is stopped, phased out or replaced by IPV. We have previously documented that IPV was associated with significantly higher female than male mortality in randomised trials in which IPV was given as a comparator vaccine.34 Hence, stopping OPV campaigns or replacing OPV with IPV could lead to changes in mortality level.35 During the period we are studying here, it was triple OPV which was given in the campaigns. This vaccine was discontinued in April 2016 to be replaced by bivalent OPV. Subsequent analyses have shown no significant differences in their beneficial effects (A Andersen, et al. National immunisation campaigns with OPV reduce all-cause mortality: a natural experiment within seven randomised trials. (In review)).
This study also suggests that campaign-OPV-before-enrolment interfered with the otherwise beneficial effect of early MV. This negative interaction could potentially be important in the situations where early MV is recommended, for example to children with HIV infection who should receive a first dose of MV already at 6 months of age.
Many interventions which have been recommended based on RCTs showing a beneficial effect on children survival, for example, VAS, have turned out to present different effects in subsequent RCTs. Campaigns with OPV, MV, VAS or NVAS may be behind the situations in which effects change. This phenomenon of interactions between interventions with an early priming effect on the immune system needs to be understood better to be able to predict whether new interventions will have beneficial or deleterious effects on overall health. It is worth emphasising that among children who received OPV0 and BCG in the first weeks of life, received no NVAS, received no campaign-OPV and received no DTP after MV, early MV was associated with 85% (53% to 96%) reduction in mortality between 4.5 and 36 months of age (data not shown). This effect was not in relation to unvaccinated control children but compared with the current strategy of one dose of MV at 9 months of age. Hence, we might be able to do much more to reduce mortality if we fully understood early immune training and used this knowledge.
The specific disease perspective in global public health has generated the idea that once we have eradicated a disease, we can remove the preventive intervention and save resources. To the extent interventions have beneficial immune training effects, this may be a disastrous misunderstanding. Since both polio and measles are targeted for eradication within the next 10–20 years, there are good reasons to further explore the beneficial NSEs of OPV and MV and possibly be able to induce them in other ways.
Footnotes
Contributors: PA and CSB planned the analysis. AA conducted the statistical analyses. CLM, AR, HCW, CSB and PA organised the trial of early MV. CSB and ABF organised data collection in connection with the campaigns. The first draft was written by PA; all authors contributed to the final version of the paper. PA and AA will act as guarantors of the study.
Funding: The work on non-specific effects of vaccines has been supported by the Danish Council for Development Research, Ministry of Foreign Affairs, Denmark (grant number 104.Dan.8.f.), Novo Nordisk Foundation and European Union FP7 support for OPTIMUNISE (grant number Health-F3-2011-261375). CSB held a starting grant from the ERC (ERC-2009-StG-243149). CVIVA is supported by a grant from the Danish National Research Foundation (DNRF108). PA held a research professorship grant from the Novo Nordisk Foundation.
Disclaimer: The funding agencies had no role in the study design, data collection, data analysis, data interpretation or the writing of the report.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The protocol for the original trial8 was approved by the Guinean Ministry of Health's Research Coordination Committee, the Danish Central Ethical Committee, and the Gambia/MRC Scientific and Ethics Committees.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Higgins JPT, Soares-Weiser K, Reingold A. Systematic review of the non-specific effects of BCG, DTP and measles containing vaccines. http://www.who.int/immunization/sage/meetings/2014/april (accessed 1 Jun 2014).
- 2.Strategic Advisory Group of Experts on Immunization. Meeting of the Strategic Advisory Group of Experts on Immunization. April 2014 - conclusions and recommendations Week Epidemiol Rec 2014;89:221–36. [PubMed] [Google Scholar]
- 3.Aaby P, Martins CL, Garly ML et al. Non-specific effects of standard measles vaccine at 4.5 and 9 months of age on childhood mortality: randomised controlled trial. BMJ 2010;341:c6495 10.1136/bmj.c6495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aaby P, Samb B, Simondon F et al. Non-specific beneficial effect of measles immunisation: analysis of mortality studies from developing countries. BMJ 1995;311:481–5. 10.1136/bmj.311.7003.481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ 2000;321:1435–8. 10.1136/bmj.321.7274.1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benn CS, Netea MG, Selin LK et al. A small jab—a big effect: non-specific immunomodulation by vaccines. Trends Immunol 2013;34:431–9. 10.1016/j.it.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Kleinnijenhuis J, Quintin J, Preijers F et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci U S A 2012;109:17537–42. 10.1073/pnas.1202870109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aaby P, Rodrigues A, Biai S et al. Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine 2004;22:3014–17. 10.1016/j.vaccine.2004.02.009 [DOI] [PubMed] [Google Scholar]
- 9.Aaby P, Hedegaard K, Sodemann M et al. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine 2005;23:1746–51. 10.1016/j.vaccine.2004.02.054 [DOI] [PubMed] [Google Scholar]
- 10.Lund N, Andersen A, Hansen AS et al. The effect of oral polio vaccine at birth on mortality. A randomized trial. Clin Infect Dis 2015;61:1504–11. 10.1093/cid/civ617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benn CS, Martins CL, Fisker AB et al. Interaction between neonatal vitamin A supplementation and timing of measles vaccination: a retrospective analysis of three randomized trials from Guinea-Bissau. Vaccine 2014;32:5468–74. 10.1016/j.vaccine.2014.07.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benn CS, Aaby P, Fisker AB. Neonatal vitamin A: time to move on? Lancet 2015;386:132–3. 10.1016/S0140-6736(15)61235-1 [DOI] [PubMed] [Google Scholar]
- 13.Benn CS, Fisker AB, Rodrigues A et al. Sex-differential effect on infant mortality of oral polio vaccine administered with BCG at birth in Guinea-Bissau. A natural experiment. PLoS ONE 2008;3:e4056 10.1371/journal.pone.0004056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayo-Wilson E, Imdad A, Herzer K et al. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta-analysis. BMJ 2011;343:d5094 10.1136/bmj.d5094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Awasthi S, Peto R, Read S et al. Vitamin A supplementation every 6 months with retinol in 1 million pre-school children in north India: DEVTA, a cluster-randomised trial. Lancet 2013;381:1469–77. 10.1016/S0140-6736(12)62125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fisker AB, Bale C, Rodrigues A et al. High-dose vitamin A with vaccination after 6 months of age: a randomized trial. Pediatrics 2014;134:e739–48. 10.1542/peds.2014-0550 [DOI] [PubMed] [Google Scholar]
- 17.Benn CS, Diness BR, Roth A et al. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ 2008;336:1416–20. 10.1136/bmj.39542.509444.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benn CS, Fisker A, Napirna BM et al. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ 2010;340:c1101 10.1136/bmj.c1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aaby P, Jensen H, Samb B et al. Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: re-analysis of West African studies. Lancet 2003;361:2183–8. 10.1016/S0140-6736(03)13771-3 [DOI] [PubMed] [Google Scholar]
- 20.Aaby P, Martins CL, Garly ML et al. Measles vaccination in the presence or absence of maternal measles antibody: impact on child survival. Clin Infect Dis 2014;59:484–92. 10.1093/cid/ciu354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras G. Sabin's vaccine used for nonspecific prevention of infant diarrhea of viral etiology. Bull Pan Am Health Organ 1974;8:123–32. [PubMed] [Google Scholar]
- 22.Contreras G. Effect of the administration of oral poliovirus vaccine on infantile diarrhoea mortality. Vaccine 1989;7:211–12. 10.1016/0264-410X(89)90230-2 [DOI] [PubMed] [Google Scholar]
- 23.Voroshilova MK. Potential use of nonpathogenic enteroviruses for control of human disease. Prog Med Virol 1989;36:191–202. [PubMed] [Google Scholar]
- 24.Lund N, Biering-Sørensen S, Andersen A et al. Neonatal vitamin A supplementation associated with a cluster of deaths and poor early growth in a randomised trial among low-birth-weight boys of vitamin A versus oral polio vaccine at birth. BMC Pediatr 2014;14:214 10.1186/1471-2431-14-214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seppälä E, Viskari H, Hoppu S et al. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine 2011;29:8615–18. 10.1016/j.vaccine.2011.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sørup S, Stensballe LG, Krause TG et al. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis 2015;3:ofv204 10.1093/ofid/ofv204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sørup S, Benn CS, Poulsen A et al. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA 2014;311:826–35. 10.1001/jama.2014.470 [DOI] [PubMed] [Google Scholar]
- 28.Garly ML, Jensen H, Martins CL et al. Hepatitis B vaccination associated with higher female than male mortality in Guinea-Bissau: an observational study. Pediatr Infect Dis J 2004;23:1086–92. [PubMed] [Google Scholar]
- 29.Aaby P, Benn C, Nielsen J et al. Testing the hypothesis that diphtheria-tetanus-pertussis vaccine has negative non-specific and sex-differential effects on child survival in high-mortality countries. BMJ Open 2012;2:pii e000707 10.1136/bmjopen-2011-000707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent AL, Ma W, Lager KM et al. Live attenuated influenza vaccine provides superior protection from heterologous infection in pigs with maternal antibodies without inducing vaccine-associated enhanced respiratory disease. J Virol 2012;86:10597–605. 10.1128/JVI.01439-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arts RJ, Blok B, van Crevel R et al. Vitamin A induces inhibitory histone methylation modifications and down-regulates trained immunity in human monocytes. J Leukoc Biol 2015;98:129–36. 10.1189/jlb.6AB0914-416R [DOI] [PubMed] [Google Scholar]
- 32.Storgaard L, Rodrigues A, Martins C et al. Development of BCG scar and subsequent morbidity and mortality in rural Guinea-Bissau. Clin Infect Dis 2015;61:950–9. 10.1093/cid/civ452 [DOI] [PubMed] [Google Scholar]
- 33.Martins CL, Benn CS, Andersen A et al. A randomized trial of a standard dose of Edmonston-Zagreb measles vaccine given at 4.5 months of age: effect on total hospital admissions. J Infect Dis 2014;209:1731–8. 10.1093/infdis/jit804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aaby P, Garly ML, Nielsen J et al. Increased female-male mortality ratio associated with inactivated polio and diphtheria-tetanus-pertussis vaccines: observations from vaccination trials in Guinea-Bissau. Pediatr Infect Dis J 2007;26:247–52. [DOI] [PubMed] [Google Scholar]
- 35.Fish EN, Flanagan KL, Furman D et al. Changing oral to inactivated polio vaccine may increase mortality. Lancet 2016;387:1054–5. 10.1016/S0140-6736(16)00661-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2016-013335supp_tables.pdf (60.3KB, pdf)