Abstract
We present here the first case in the People's Republic of China of human disease caused by the fungus Arthrographis kalrae. The male patient had fungal panophthalmitis and invasive sinusitis involving the maxillary and ethmoid sinuses. He was an apparently healthy man before receiving trauma to his left eye. He complained of pain and loss of visual acuity in the injured eye, which displayed redness and edema and eventually discharged pus. His symptoms became more severe after he was treated with steroids and several antibacterial agents. A computed tomography scan of the left eye revealed that the maxillary and ethmoid sinuses were involved. A smear of purulent material from the left eye orbit revealed fungal elements, and cultures of the material grew a fungus. The isolate was identified as A. kalrae based on gross and microscopic morphologies, biochemical assays, and DNA sequence analysis. The patient received amphotericin B intravenously, itraconazole orally, and atomized allitridum by nebulizing allitridum therapy. The patient's wound healed following surgical intervention, but the patient lost the use of his left eye. This case should remind ophthalmologists and other clinicians to consider the possibility of infections being fungal when antibacterial agents have no effect and the patient's condition worsens.
Fungal infections of the eye include keratitis (mycotic keratitis and keratomycosis) and endophthalmitis. Fungal keratitis occurs predominantly in young male patients who have no history of previous ocular disease but who do have a history of trauma, usually occurring outdoors in predominantly agricultural areas. It most commonly occurs in farmers, gardeners, and contact lens-wearing patients. Endophthalmitis, a severe infection of the eye, may be either endogenous or exogenous. Endogenous endophthalmitis may occur as a result of hematogenous spread or by direct spread from neighboring organs, such as the paranasal sinuses, as is the case with aspergillosis or zygomycosis. Fungi may infect the retina, the choroids, the vitreous humor, or the entire eye. Infection of the entire eye is termed panophthalmitis, signifying a purulent inflammation of all layers of the eye. Exogenous endophthalmitis may be a complication of surgery or trauma, in which organisms are inoculated directly into the eye, or it may result from extension of a fungal corneal ulcer when the cornea is perforated. Fungal endophthalmitis may be caused by either opportunistic or pathogenic fungi (as with fungal keratitis, and a broad range of fungal agents may be responsible for this entity). Candida species are the most common causative agents for both exogenous and endogenous endophthalmitis (6). Other fungi reported to cause endophthalmitis include Aspergillus spp., Fusarium spp., Zygomycetes spp., Trichosporon beigelii, Sarcopodium oculorum (10), Scedosporium apiospermum, and some saprophytic fungi (1, 5, 7).
Other causes of mycotic infections of the eye may be climate, preexisting diseases (either ophthalmic or systemic), and inappropriate use of topical or systemic antibiotics and steroids (7).
Invasive sinusitis can be caused by fungal or bacterial infections. Fungal sinusitis is rarely reported. Of the reported cases, most have occurred in immunocompromised patients, especially in patients with AIDS (12). Aspergillus species are the most commonly identified fungal causative agents, although Schizophyllum commune, Cryptococcus neoformans, Candida albicans, Rhizopus arrhizus, Pseudallescheria boydii (asexual stage, Scedosporium apiospermum), and Alternaria alternata have also been reported (2).
Here we report of a case of fungal panophthalmitis and invasive sinusitis, involving the maxillary and ethmoid sinuses, following eye trauma. Before the trauma occurred, the patient was an apparently healthy man without any underlying immunocompromising condition. Arthrographis kalrae was isolated repeatedly at different times from the purulent material obtained from the injured eye. To our knowledge, this is the first report of panophthalmitis and invasive sinusitis caused by A. kalrae in the People's Republic of China.
Patient data.
The patient was an apparently healthy 39-year-old Cantonese farmer who injured his left eye while working in the fields in August 2002. He was hit in the eye with yellow oil containing steel chips. He complained of pain, loss of vision in the left eye, and bleeding and edema of the upper eyelid. A local surgeon sutured the wound of his upper eyelid. However, the farmer's symptoms worsened. After 3 days, he was admitted to an ophthalmological hospital in the city of Guangzhou (Guangdong Province, Southeast China). Except for the left-eye findings, the physical examination was normal. The ophthalmological examination revealed a complete, normal right eye but no light perception or orientation in the left eye. The visual acuity of the left eye was zero, and the intraocular pressure was too high to be measured. The left eyelid had severe edema and petechial hemorrhaging in addition to the previously sutured wound and a horizontal wound. There was no secretion when the lacrimal sac was pressed. The sclera could not be examined. The posterior elastic corneal membrane was damaged, and there was evidence of keratic precipitate. The anterior chamber of the eye was turbid (Tyndall phenomenon), and there was a hyphema with 2 mm of blood accumulated. The iris of the injured eye could not be seen clearly, and the pupil was fixed and dilated. The lens and fundus of the eye could not be seen clearly. The left eyeball was extruded and fixed. An ultrasonic B scan showed that the left eye had vitreous flare, retinal detachment, and retinal hemorrhaging. In a routine blood examination, the white blood cell count was 14.6 × 109/liter, with a normal differential. The patient tested negative for AIDS.
The patient's symptoms did not improve after 4 days of therapy with an intramuscular injection of 80,000 U of tobramycin twice daily and an oral dose of 10 mg of dexamethasone once daily, although the patient's pain decreased. His medication was changed to 150 mg of etimicin sulfate administered intravenously by injection twice daily and 15 mg of dexamethasone orally once daily. The patient also began an alternating regimen of hourly drops of tobramycin during the day and topical ointment at night. After 18 days of therapy and hospitalization, the patient left the hospital but continued the oral and topical antibiotic regimen because his symptoms had not improved and there was a continuous discharge of purulent material from his injured eye. On 20 September 2002, he was admitted to another hospital and received treatment with cephradine, ceftriaxone, and penicillin for cellulitis and traumatic uveitis; however, there was no improvement in his condition after 2 months of this new therapy (Fig. 1). A cerebral computed tomography scan showed a protruding left eyeball with normal morphology and a normal interior. Also visible were patchy, dense shadows on the left ethmoid sinus (Fig. 2a) and left maxillary sinus (Fig. 2b). An incomplete osteoid structure of the orbital inside wall was found protruding into the left ethmoid sinus. Resection of the left ethmoid sinus and surgical drainage of the left orbit were performed. Many fistulas filled with creamy material ran through the orbit. The infected granuloma and purulent material from the orbit were used for direct smears and fungal cultures, and they were also examined histopathologically. Potassium hydroxide mounts revealed filamentous hyaline and septate hyphae, and Sabouraud dextrose agar (SDA) cultures grew maize-colored, powdery colonies (Fig. 3). The results of routine bacteriology cultures were negative. The isolated organism was subsequently identified as A. kalrae. All oral and topical antibacterial antibiotics were discontinued, and the patient began a regimen of topical nystatin and intravenous amphotericin B (AMB) beginning with a dose of 0.5 mg/kg of body weight/day, eventually increasing to 50 mg/day (0.6 mg/kg/day) for 20 days. The AMB treatment was discontinued and replaced with itraconazole (ITCZ) at a dose of 400 mg/day because of a severe alimentary tract reaction. After 24 days of antifungal therapy, the discharge of pus stopped, and fungal cultures of fluid washed from the injured eye on two separate occasions were negative. The patient left the hospital and continued to take ITCZ orally (200 mg/day). After 1 week, his eye began to discharge pus again. Two new fistulas appeared at the middle part of upper eyelid, where there was a discharge of purulent material. KOH mounts showed fungal elements, and cultures were positive for the same isolate identified previously. The patient was admitted to another hospital on 4 January 2003. A computed tomography scan was performed and confirmed that the fungal infection was extensive inside and outside the orbit, involving the maxillary and ethmoid sinuses. The patient was placed on a regimen of oral ITCZ (200 mg/day) and atomized allitridum. The openings of the sinus tracts were washed with 0.2% fluconazole (FLCZ) every day, and 0.2% FLCZ was applied topically to the injured eye. The maxillary, ethmoid, and paranasal sinuses and the skin fistula were surgically removed. The eyeball, lacrimal sac, and infected orbit granuloma were also surgically removed. Several attempts to culture fungi at different times from fluid washed from the patient's eye were negative. Eventually, the patient healed and was released from the hospital in March 2003. We have observed him for 6 months postrelease, and he has not relapsed.
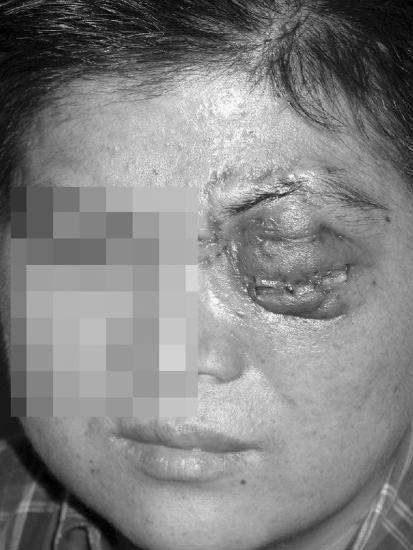
FIG. 1.
Patient with pus discharging from left eye showing severe edema.
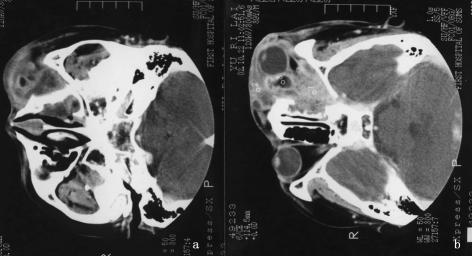
FIG. 2.
Cerebral computed tomography scan showing a protruding left eyeball with normal morphology and a normal interior. Also visible are patchy dense shadows on the left ethmoid sinus (a) and left maxillary sinus (b). An incomplete osteoid structure of the orbital inside wall was found protruding into the left ethmoid sinus.
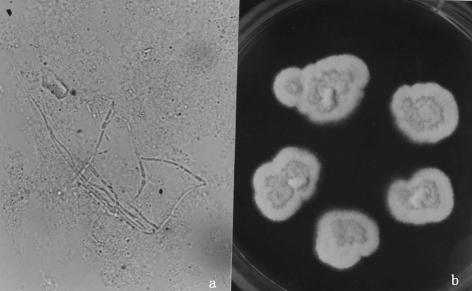
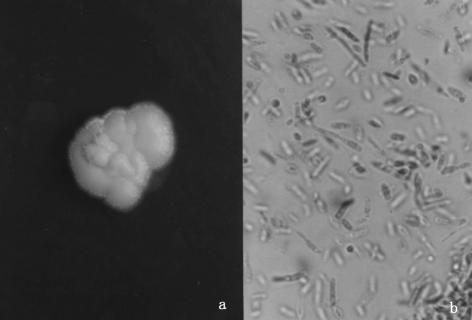
FIG. 3.
(a) KOH mounts of the infective granuloma and purulent material from the orbit revealed filamentous hyaline, septate hyphae. Magnification, ×400. (b) SDA cultures at 25°C for 7 days revealed a powdery colony with a maize color.
Microbiology. (i) Morphologic studies.
In direct examination, potassium hydroxide mounts of the infected granuloma and purulent material from the orbit revealed filamentous hyaline and septate hyphae (Fig. 3a).
For the fungal cultures, the infected granuloma and purulent material from the orbit were inoculated onto SDA and incubated at 25°C. After 2 days of incubation, small colonies appeared, and good growth was observed after 7 days of incubation. The initial colony was cream colored and glabrous. It gradually became maize or yellow in color and velvety, and it ultimately formed a powdery, yellowish colony after 7 days. The texture of the colony was soft and frangible (Fig. 3b). Slide cultures were grown on SDA and potato dextrose agar (PDA) at 25 and 37°C, respectively. The isolate produced unbranched or irregularly branched hyphae, with dendritic or tree-like conidiophores; chains of single-celled, rectangular arthroconidia not separated by disjunction cells; and occasional hyaline, sessile, subglobose conidia (Fig. 4). The isolates were inoculated onto brain heart infusion agar (BHIA) and PDA and incubated at 25 and 37°C. A typical velvety colony appeared after 7 days of culture on PDA at 25°C, a typical cream-like colony appeared after 5 days of incubation on BHIA, and then a fine velvety colony formed after 5 days of incubation at 37°C. Lactophenol cotton blue (LPCB)-stained mounts of cells grown on BHIA, incubated at 37°C, showed mainly oval to elongated yeast cells (2.5 to 4 μm in diameter) and short hyphae (Fig. 5). The growth characteristics described above indicate that the isolated fungus was dimorphic. Finally, the isolate was capable of growth at 42°C.
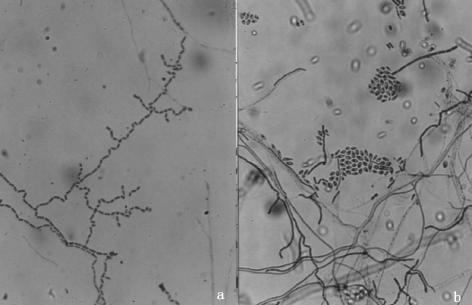
FIG. 4.
(a) Chains of one-celled, rectangular dendritic or tree-like arthroconidia (2 to 4 μm in diameter). Magnification, ×400. (b) Subglobose conidia along the sides and accumulating in slimy heads (LPCB stain).
FIG. 5.
(a) Typical cream-like colony with fine velvety appearance after incubation at 37°C on BHIA for 5 days. (b) LPCB-stained mount showing mainly oval to elongate yeast cells (2.5 to 4 μm in diameter) and short hyphae. Magnification, ×400.
Several biochemical tests were done. For the cycloheximide tolerance test, the isolate was incubated on SDA containing 0.05% cycloheximide at 30°C. Good growth was seen after 3 days of incubation. For the urea hydrolysis test, freshly subcultured isolates incubated on PDA for 3 days were inoculated onto Christensen's urea agar and incubated at 37°C. Candida albicans ATCC 90028 and Cryptococcus neoformans were used as the negative and positive controls, respectively. The results were positive after 4 h of incubation. The isolate was also inoculated into wells of an ID 32 plate (Biomerieux, Marcy l'Etoile, France). Results were obtained after 72 h of incubation. The profile code of 3607300637 did not correspond to any taxon scored in the manufacturer's database. For the ubiquinone system analysis, the method reported by Fukushima et al. (8) was adopted. The major ubiquinone of this isolate, like that of the type strain UAMH 3616 (University of Alberta Microfungus Collection and Herbarium, Alberta, Canada), was identified as dihydroubiquinone-10.
We also performed DNA sequence analysis. To aid in isolate identification, the sequences of two regions (D1 and D2) and of the internal transcribed spacer (ITS) of the ribosomal DNA from this isolate and UAMH 3616 were analyzed. The sequences of regions D1 and D2 (604 bp) and the ITS (508 bp) from both our isolate and the A. kalrae UAMH 3616 type strain were identical, with 100% homology. Judging from the sequence data, our isolate was confirmed to be A. kalrae and is preserved at the Research Center for Pathogenic Fungi and Microbial Toxicoses, Chiba University, Chiba, Japan, under collection number IFM 52423.
(ii) Histopathological and susceptibility studies.
In the histopathological examination, hematoxylin and eosin stains of purulent material from the left eye orbit revealed mainly masses of granuloma tissue and a pyogenic response. A few positively stained oval to elongated fungal spores were seen inside the abscesses by use of periodic acid-Schiff stain.
For in vitro antifungal susceptibility testing, MICs were determined using a frozen plate kit (Eiken Chemical Co., Tokyo, Japan). This kit is based on almost the same principle as that of the NCCLS system. The MIC was interpreted to be the lowest concentration of antifungal agent which allowed no visible growth of the tested fungus (30°C, 72 h). The MICs of AMB, 5-flucytosine, FLCZ, ITCZ, and miconazole were determined to be 2, >64, 16, 1, and 0.5 μg/ml, respectively.
A. kalrae is a member of the Hyphomycetes Cochet ex Sigler and Carmichael (4). It was previously named Oidiodendron kalrai in 1976 (14, 18). The teleomorph of the type species, A. kalrae, was considered to be Eremomyces langeronii by Malloch and Sigler (11). The anamorphic (asexual) phase of Arthrographis consists of five species: A. kalrae, A. cuboidea, A. lignicola, A. pinicola, and A. alba (14, 15, 16). At first, A. alba appeared to be an atypical A. kalrae strain, but molecular studies confirmed that it was a distinct new species (9). A. cuboidea, A. lignicola, and A. pinicola have been isolated from wood as contaminates. A. kalrae can commonly be isolated from soil and compost but has only rarely been reported to be an opportunistic pathogen of humans and animals (19). A. kalrae has been described as a pathogen in infections of the dorsal hand (causing eumycotic mycetoma) (3), the lung (in bronchial alveolar lavage fluid) (17), and a corneal ulcer, as well as a cause of keratitis (13) and invasive sinusitis and meningitis (2). This report deals with the first Chinese case of panophthalmitis and invasive fungal maxillary and ethmoid sinusitis caused by A. kalrae and is only the second report in Asia. The first report described a case in India in 1961 (19). The isolate we are reporting was identified as A. kalrae by colony and microscopic morphologies, by biochemical assays, and by DNA sequence analysis. Chin-Hong et al. (2) reported invasive fungal sinusitis and meningitis caused by A. kalrae in an AIDS patient from the United States, but our patient was a strong, healthy man before his eye was injured. It was confirmed that he did not have human immunodeficiency virus. The infection could have come from contaminated steel strips that may have injured his eye. However, a fungal cause was not suspected at first, and corticosteroids were given frequently. This case should remind ophthalmologists to consider the possibility of infections being fungal when antibacterial agents have no effect and the patient's condition worsens.
Nucleotide sequence accession numbers.
The sequence data for our A. kalrae isolate were deposited in the DNA Data Bank of Japan with accession numbers AB116536 (for the ITS) and AB116544 (for regions D1 and D2).
Acknowledgments
Glenn S. Bulmer, Kevin C. Hazen, James Masuoka, David Singleton, and Joann Eliason gave great assistance in the English composition and revision.
This study was performed as part of the “Frontier Studies and International Networking of Genetic Resources in Pathogenic Fungi and Actinomycetes (FN-GRPF)” program through special coordination funds for promoting science and technology from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
REFERENCES
- 1.Behrens-Braumann, W. 1999. Mycosis of the eye and its adnexa. Dev. Ophthalmol. 32:i-ix, 1-201. [PubMed]
- 2.Chin-Hong, P. V., D. A. Sutton, M. Roemer, M. A. Jacobson, and J. A. Aberg. 2001. Invasive fungal sinusitis and meningitis due to Arthrographis kalrae in a patient with AIDS. J. Clin. Microbiol. 39:804-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Degavre, B., J. M. Joujoux, M. Dandurand, and B. Guillot. 1997. First report of mycetoma caused by Arthrographis kalrae: successful treatment with itraconazole. J. Am. Acad. Dermatol. 37:318-320. [PubMed] [Google Scholar]
- 4.De Hoog, G. S., J. Guarro, J. Gené, and M. J. Figueras. 2000. Hyphomycetes. Genus: Arthrographis, p. 440-441. In G. S. De Hoog, J. Guarro, J. Gené, and M. J. Figueras (ed.), Atlas of clinical fungi, 2nd ed. Centraalbureau voor Schimmelcultures, Utrecht, The Netherlands.
- 5.Eckburg, P. B., A. R. Zolopa, and J. G. Montoya. 1999. Invasive fungal sinusitis due to Scedosporium apiospermum in a patient with AIDS. Clin. Infect. Dis. 29:212-213. [DOI] [PubMed] [Google Scholar]
- 6.Fenelon, L., and S. Kennedy. 1996. Fungal disease in ophthalmology, p. 227-233. In C. C. Kibbler, D. W. R. Mackenzie., and F. C. Odds (ed.), Principles and practice of clinical mycology. John Wiley & Sons Ltd., West Surrey, England.
- 7.Foster, C. S. 1992. Fungal keratitis. Infect. Dis. Clin. North Am. 6:851-857. [PubMed] [Google Scholar]
- 8.Fukushima, K., K. Takizawa, K. Okada, Y. Maebayashi, K. Nishimura, and M. Miyaji. 1993. Suitability of sterilization methods for ubiquinone analysis of pathogenic fungi. Trans. Mycol. Soc. Jpn. 34:473-480. [Google Scholar]
- 9.Gené, J., J. M. Guillamón, K. Ulfig, and J. Guarro. 1996. Studies on keratinophilic fungi. X. Arthrographis alba sp. nov. Can. J. Microbiol. 42:1185-1189. [Google Scholar]
- 10.Guarro, J., A. L. Höfling-Lima, J. Gené, D. De Freitas, P. Godoy, M. L. Zorat-Yu, L. Zoror, and O. Fischman. 2002. Corneal ulcer caused by the new fungal species Sarcopodium oculorum. J. Clin. Microbiol. 40:3071-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malloch, D., and L. Sigler. 1988. The Eremomycetaceae (Ascomycotina). Can. J. Bot. 66:1929-1932. [Google Scholar]
- 12.Meyer, R. D., C. R. Caultier, J. T. Yamashita, R. Babapour, H. E. Pitchon, and P. R. Wolfe. 1994. Fungal sinusitis in patients with AIDS: report of 4 cases and review of the literature. Medicine 73:69-78. [DOI] [PubMed] [Google Scholar]
- 13.Perlman, E. M., and L. Binns. 1997. Intense photophobia caused by Arthrographis kalrae in a contact lens-wearing patient. Am. J. Ophthalmol. 123:547-549. [DOI] [PubMed] [Google Scholar]
- 14.Sigler, L., and J. W. Carmichael. 1976. Taxonomy of Malbranchea and some other hyphomycetes with arthroconidia. Mycotaxon 4:349-488. [Google Scholar]
- 15.Sigler, L., and J. W. Carmichael. 1983. Redisposition of some fungi referred to Oidium microspermum and a review of Arthrographis. Mycotaxon 18:495-507. [Google Scholar]
- 16.Sigler, L., Y. Yamaoka, and Y. Hiratsuka. 1990. Taxonomy and chemistry of a new fungus from bark beetle infected Pinus contorta var. latifolia. Part 1. Arthrographis pinicola sp. nov. Can. J. Microbiol. 36:77-82. [Google Scholar]
- 17.Sigler, L., and M. J. Kennedy. 1999. Aspergillus, Fusarium, and other opportunistic moniliaceous fungi, p. 1212-1241. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. ASM Press, Washington, D.C.
- 18.Tewari, R. P., and C. R. MacPherson. 1971. A new dimorphic fungus, Oidiodendron kalrai: morphological and biochemical characteristics. Mycologia 63:604-611. [PubMed] [Google Scholar]
- 19.Tewari, R. P., and C. R. Macpherson. 1968. Pathogenicity and neurological effects of Oidiodendron kalrai for mice. J. Bacteriol. 95:1130-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]