Abstract
Objective
To assess role of some inflammatory mediators in patients with primary and recurrent lumbar disc herniation. Expression of IL-6, transforming growth factor (TGF)-1, insulin-like growth factor (IGF)-1, and Bcl-2-associated X protein (BAX) have been shown to be more intense in the primary group than the recurrent goup, but this mediators may be important aspects prognostic.
Methods
19 patients underwent primary and revision operations between June 1, 2009 and June 1, 2014, and they were included in this study. The 19 patients’ intervertebral disc specimens obtained from the primary procedures and reoperations were evaluated. Expression of IL-6, TGF-1, IGF-1, and BAX were examined immunohistochemically in the 38 biopsy tissues obtained from the primary and recurrent herniated intervertebral discs during the operation.
Results
For IL-6 expression in the intervertebral disc specimens, there was no difference between the groups. The immunohistochemical study showed that the intervertebral disc specimens in the primary group were stained intensely by TGF-1 compared with the recurrent group. Expression of IGF-1 in the primary group was found moderate. In contrast, in the recurrent group of patients was mild expression of IGF-1. The primary group intervertebral disc specimens were stained moderately by BAX compared with the recurrent group.
Conclusion
The results of our prognostic evaluation of patients in the recurrent group who were operated due to disc herniation suggest that mediators may be important parameters.
Keywords: Inflammatory mediators, Disc herniation, Immunohistochemical
INTRODUCTION
Lumbar disc herniation (LDH) is the most frequent disorder of the lumbar spine, and it is treated surgically at times5). However, in 15–30% of cases, discectomy-related complications occur, such as hemorrhage, soft-tissue infection, nerve root injury, dural tear, recurrent or residual disc herniation, epidural scar formation, discitis, arachnoiditis, pseudomeningocele, facet joint fracture (iatrogenic or stress related), spinal stenosis, and epidural hematoma3,28). The total failure rate after discectomy is 3–20%6,17,28). Additionally, the recurrence rate at the same level regardless of ipsilateral or contralateral herniation has been reported to be 5–11%.6,7,18,28). Reherniation has been suggested to be the most common cause of lumbar disc reoperation1,17). The risk of adverse outcomes such as obesity, diabetes, vibration works, drivers, and psychological factors, which increase the incidence of recurrence, should be considered before surgery11). Elevated levels of molecular mediators of inflammation have been reported in pathologic disc tissue, with their quantities increasing with the extent of degeneration16,35). The aim of the present study was to assess role of inflammatory mediators such as IL-6, transforming growth factor (TGF)-1, insulin-like growth factor (IGF)-1, and Bcl-2-associated X protein (BAX) in patients with primary and recurrent lumbar disc herniation.
MATERIALS AND METHODS
The procedure adopted herein was approved by the Ethics Committee of Abant Izzet Baysal University. Among 500 patients treated surgically for LDH in our department, 19 patients underwent primary and revision operations between June 1, 2009 and June 1, 2014, and they were included in this study. The 19 patients’ intervertebral disc specimens obtained from the primary procedures and reoperations were evaluated. Ten of the patients were men, and nine were women. Informed consent for specimen collection was obtained from all patients. The levels involved were L4–5 (12 patients), L5–S1 (6 patients), and L3–4 (1 patients). Disc herniation was assessed by computed tomography (CT)/magnetic resonance imaging (MRI) and confirmed using operative findings. Disc materials were collected by both microsquestrectomy and discectomy from disc space through annulus. Expression of IL-6, TGF-1, IGF-1, and BAX were examined immunohistochemically in the 38 biopsy tissues obtained from the primary and recurrent herniated intervertebral discs during the operation and embedded in paraffin.
Immunohistochemistry
The tissues were fixed in Bouin’s solution and embedded in paraffin. Sections of 4-μm thickness were cut, dewaxed in xylene, and incubated for 20 min in 0.3% H2O2 to block endogenous peroxidase activity. Then, the sections were processed following the standard avidin-biotin peroxidase complex procedure. Nonspecific binding was blocked with 3% normal goat serum in a phosphate buffered saline (PBS) solution pH7.4 for 30 min at room temperature. The slides were then incubated overnight with primary antibodies at 4°C. The following antibodies were used: polyclonal mouse anti-IGF-1 (GeneTex Inc., Irvine, CA, USA, 1:100), polyclonal rabbit anti-IL-6 (GeneTex, 1:00), polyclonal rabbit anti-TGF-1 (GeneTex, 1:25), and monoclonal rabbit Bax (GeneTex, 1:250). Rabbit and mouse immunoglobulins of the same dilutions as primary antibodies were used as controls. Peroxidase activity was revealed by incubation in 0.05% 3,3′diaminobenzidine tetrahydrochloride in PBS containing 0.03% peroxide for 5 min at room temperature; the slides were then washed, dehydrated, and mounted with Entellan (Merck KGaA, Darmstadt, Germany). The experiments were reproduced in triplicate, and the slides were examined under a Nikon E600 light microscope by two experienced observers blinded to each other and to the harvested tissues. The immunostaining intensity was evaluated by considering the reactive cells or fibers in 20 random fields per slide (lens: 20). The immunuhistochemical expressions were evaluated in three categories: mild (value=1), moderate (value=2), and intense (value=3).
Statistical analysis
SPSS 11.0 software (SPSS Inc., Chicago, IL, USA) for Windows was used for statistical analyses. Data are expressed as mean SD and analyzed for statistical significance using one-way analysis of variance (ANOVA), followed by the post-hoc Tukey test. Differences between the mean values were considered significant when p<0.05.
RESULTS
We included 19 patients to the study. The mean age of the patients was 39.5 years (6.4 SD). The results of IL-6, TGF-1, IGF-1, and BAX expressions examined immunohistochemically using the biopsy tissues taken from the intervertebral discs of patients of primary and recurrent lumbar disc herniation are summarized in Table 1.
Table 1.
Expression of IL-6, TGF-1, IGF-1, and BAX examined immunohistochemically using biopsy tissues taken from primary and recurrent disc hernia patients.
| Groups | IL-6 | TGF | IGF-1 | BAX |
|---|---|---|---|---|
| Primary (n=19) | 1.9±0.6 | 2.6±0.5* | 1.9±0.7† | 1.5 ± 0.5‡ |
| Recurrent (n=19) | 1.4±0.6 | 1.8±0.7 | 1.4±0.6 | 1.1 ± 0.3 |
Values expressed as meanstandard deviation.
p<0.003 compared with recurrent group.
p<0.05 compared with recurrent group.
p<0.05 compared with recurrent group.
IL-6 : interleukin 6, TGF-1 : transforming growth factor-1, IGF-1 : insulin-like growth factor-1, BAX : Bcl-2-associated X protein
Clinical data of the patients before and after surgery summarized in Table 2.
Table 2.
Clinical data of the patients before and after surgery
| Before surgery (n) | After surgery (n) | |
|---|---|---|
| Back pain | 19 | 0 |
| Leg pain | 19 | 0 |
| Leg numbness | 19 | 1 |
| motor lost | 3 | 1 |
| Reflex loss | 8 | 2 |
| Sexual dysfunction | 1 | 1 |
| Urinary incontinence | 1 | 1 |
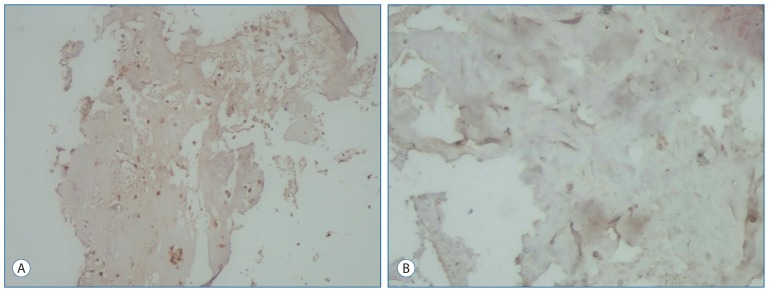
IL-6 expression in the intervertebral disc specimens of the primary group was stained moderately (Fig. 1A). IL-6 expression in the intervertebral disc specimens was mild in the recurrent group when compared with that in primary group. However, there was no difference between the groups (Fig. 1B).
Fig. 1.
Immunohistochemical staining of intervertebral disc for IL-6 expression. A : IL-6 expression in chondrocytes of the intervertebral disc was moderate in the primary group (Immun-Peroxidase, original magnification 100). B : In the recurrent group, in chondrocytes of the intervertebral disc were stained mild by IL-6 (Immuno-Peroxidase, original magnification 100).
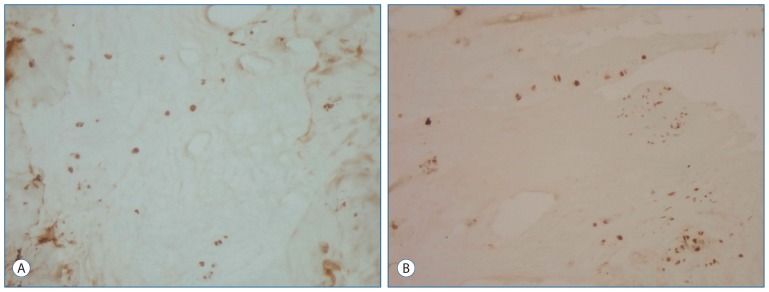
The immunohistochemical study showed that the intervertebral disc specimens in the primary group were stained intensely by TGF-1 compared with the recurrent group (p<0.003) (Fig. 2A, B).
Fig. 2.
Immunohistochemical staining of the intervertebral disc for TGF. A : The chondrocytes of the intervertebral disc in primary group were stained intensely by TGF (Immun-Peroxidase, original magnification 100). B : In contrast, the chondrocytes of the intervertebral disc in the recurrent group were stained moderate by TGF (Immuno-Peroxidase, original magnification 100). TGF : transforming growth factor.
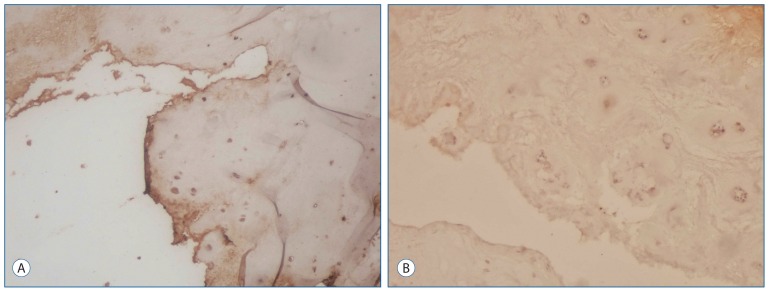
Fig. 3A shows moderate expression of IGF-1 in the primary group. In contrast, Fig. 3B shows mild IGF-1 expression in the intervertebral disc tissue from the recurrent group of patients (p<0.05).
Fig. 3.
Immunohistochemical staining of the intervertebral disc for IGF-1 expression. A : An IGF-1 expression was moderate in the primary group (Immuno-Peroxidase, original magnification 100). B : In the recurrent group, the chondrocytes in the intervertebral disc were stained mild by IGF-1 (Immun-Peroxidase, original magnification 100). IGF-1 : insulin-like growth factor-1.
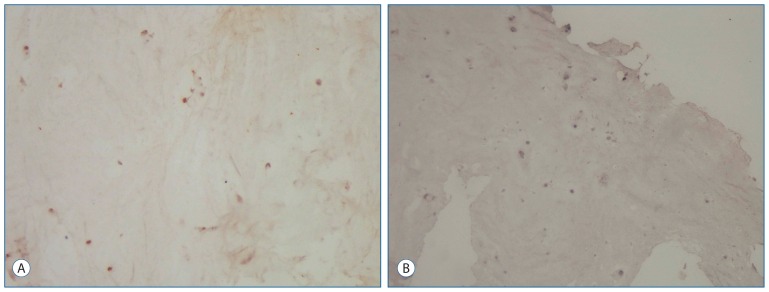
The primary group intervertebral disc specimens were stained moderately by BAX compared with the recurrent group (p<0.05) (Fig. 4A, B).
Fig. 4.
Immunohistochemical staining of the intervertebral disc for BAX. A : The chondrocytes of the intervertebral disc in primary group were stained moderately by BAX (Immun-Peroxidase, original magnification 100). B : In contrast, the chondrocytes of the intervertebral disc in the recurrent group were stained mild by BAX (Immuno-Peroxidase, original magnification 100). BAX : Bcl-2-associated X protein.
DISCUSSION
Despite the development of modern and microsurgery techniques and the reporting of successful results in lumbar disc surgery, more than 10% of patients suffer from long-term recurrent pain, which increases healthcare costs2). The precise mechanism of recurrent lumbar disc herniation remains unclear36). The causes and characteristics of reoperations after different primary surgeries have been not specified in the population-based data.
In recent clinical studies, elevated levels of proinflammatory cytokines (IFN-c, IL-1b, IL-2, IL-6, and TNF-a) correlated with elevated pain scores in various types of neurological and rheumatologic disorders20,31,33). While IL-6 production occurs in various cell types, Takada et al.29) showed that macrophages are the major cells in disc herniation–associated pathology. IL-6 helps mediate the acutephase response to injury, promote monocyte differentiation into macrophages, and activate maturation of both B- and T-lineage lymphocytes. Burke et al.4) found significantly higher levels of IL-6 and IL-8 in discs from low back-pain patients compared those in to discs from sciatica patients, independent of the presence of an intact anulus fibrosus or nuclear extrusions. TGF-β1 is known to be involved the repair process of connective tissue and to induce the formation of inflammatory and granulation tissues15). The role of TGF-β 1 in repair is directed toward several cell types such as fibroblasts, monocytes, and endothelial cells15). TGF-β 1 improves the synthesis of collagens and fibronectin, and the arrangement of the extracellular matrix25). Moreover, TGF-β 1 is a chemoattractant for fibroblasts and monocytes and may hence induce the movement of cells to repair-needing areas24). Additionally, the use of TGF-β on hernia disc cells causes an anabolic response, characterized by increased proteoglycan synthesis and decreased tissue resorption via decreased MMP-2 secretion8,22). In addition, a transient proliferative effect was observed on cultivated disc cells8).
IGF-1 increases proteoglycan synthesis in the nucleus pulposus and stimulates the growth of bone and cartilage; then, the increased IGF-1 expression works toward repairing the extruded disc material30,27). IGF-1 is produced predominantly in the liver in response to the action of growth hormone and is transferred into the serum, which is a key anabolic factor in the growth and differentiation of normal cartilage tissue23,26). In a previous study, local release of IGF-1 was detected in the intervertebral disc: the expression of IGF-1 mRNA and IGF-1-receptor in disc tissue was greater in cells of the nucleus pulposus of fetal bovine intervertebral discs than in those of the adult disc21). Jennische et al.10) suggest that the production of IGF-1 during tissue repair in adult herniated intervertebral discs might represent an attempt to repair the matrix.
BAX (Bcl-2-associated X protein) is required for the release of cytochrome c, a critical factor in the initiation of cell death pathways derived from the mitochondrion14,32,34). It has been suggested that the downregulation of key apoptosis-regulating proteins such as Bcl-2 promotes mitochondrial membrane permeability, which subsequently leads to activation of the intrinsic caspase-dependent pathway via caspase-9 and, finally, caspase-3/7-mediated apoptosis9,12,19). In addition, studies have demonstrated that the down regulation of collagen type II is closely related to a substantial reduction in the level of Bcl-2 protein13).
We shall discuss the reasons why inflammatory cytokines in second intervention were not as high as in those first surgical procedure. The initial pathological process of disc hernia is associated with greater burden of inflammation. Symptoms of the patients with hernia recurrence was mostly a consequence of anatomical changes (e.g. discontinuation of posterior ligament structure) rather than inflammation.
In this study, for the first time, some mediators in the same patient group with primary and recurrent hernia were investigated. Tissue levels of mediators were found to be decreased in the relapse. Decrease in TGF-1, IGF, and Bax levels were statistically significant. The presence of higher levels of mediators in the primary group shows that there is greater activity in these patients. Additionally, the mediators investigated in the present study are considered to be effective at lower activity levels in cases of recurrence in correlation with an inflammatory process than in patients in the primary group. The results of our prognostic evaluation of patients in the recurrent group who were operated due to disc herniation suggest that mediators may be important parameters.
References
- 1.Aizawa T, Ozawa H, Kusakabe T, Nakamura T, Sekiguchi A, Takahashi A, et al. Reoperation for recurrent lumbar disc herniation: a study over a 20-year period in a Japanese population. J Orthop Sci. 2012;17:107–113. doi: 10.1007/s00776-011-0184-6. [DOI] [PubMed] [Google Scholar]
- 2.Ambrossi GLG, McGirt MJ, Sciubba DM, Witham TF, Wolinsky JP, Gokaslan ZL, et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 2009;65:574–578. doi: 10.1227/01.NEU.0000350224.36213.F9. discussion 578. [DOI] [PubMed] [Google Scholar]
- 3.Babar S, Saifuddin A. MRI of the post-discectomy lumbar spine. Clin Radiol. 2002;57:969–981. doi: 10.1053/crad.2002.1071. [DOI] [PubMed] [Google Scholar]
- 4.Burke JG, Watson RWG, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 5.Cheng JW, Wang HW, Zheng WJ, Li CQ, Wang J, Zhang ZF, et al. Reoperation after lumbar disc surgery in two hundred and seven patients. Int Orthop. 2013;37:1511–1517. doi: 10.1007/s00264-013-1925-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fandino J, Botana C, Viladrich A, Gomezbueno J. Reoperation after lumbar disc surgery - results in 130 cases. Acta Neurochir (Wien) 1993;122:102–104. doi: 10.1007/BF01446994. [DOI] [PubMed] [Google Scholar]
- 7.Gibson JNA, Waddell G. Surgical interventions for lumbar disc prolapse - updated cochrane review. Spine. 2007;32:1735–1747. doi: 10.1097/BRS.0b013e3180bc2431. [DOI] [PubMed] [Google Scholar]
- 8.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta 1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 9.Heyde CE, Tschoeke SK, Hellmuth M, Hostmann A, Ertel W, Oberholzer A. Trauma induces apoptosis in human thoracolumbar intervertebral discs. BMC Clin Pathol. 2006;6:5. doi: 10.1186/1472-6890-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jennische E, Skottner A, Hansson HA. Dynamic changes in insulin-like growth factor I immunoreactivity correlate to repair events in rat ear after freeze-thaw injury. Exp Mol Pathol. 1987;47:193–201. doi: 10.1016/0014-4800(87)90074-8. [DOI] [PubMed] [Google Scholar]
- 11.Kara B, Tulum Z, Acar U. Functional results and the risk factors of reoperations after lumbar disc surgery. Eur Spine J. 2005;14:43–48. doi: 10.1007/s00586-004-0695-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiechle FL, Zhang XB. Apoptosis: biochemical aspects and clinical implications. Clin Chim Acta. 2002;326:27–45. doi: 10.1016/s0009-8981(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 13.Kinkel MD, Horton WE., Jr Coordinate down-regulation of cartilage matrix gene expression in Bcl-2 deficient chondrocytes is associated with decreased SOX9 expression and decreased mRNA stability. J Cell Biochem. 2003;88:941–953. doi: 10.1002/jcb.10442. [DOI] [PubMed] [Google Scholar]
- 14.Korsmeyer SJ, Wei MC, Saito M, Weller S, Oh KJ, Schlesinger PH. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7:1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 15.Laiho M, Keski-Oja J. Growth factors in the regulation of pericellular proteolysis: a review. Cancer Res. 1989;49:2533–2553. [PubMed] [Google Scholar]
- 16.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto M, Watanabe K, Hosogane N, Tsuji T, Ishii K, Nakamura M, et al. Recurrence of Lumbar Disc Herniation after Microendoscopic Discectomy. J Neurol Surg A Cent Eur Neurosurg. 2013;74:222–227. doi: 10.1055/s-0032-1320031. [DOI] [PubMed] [Google Scholar]
- 18.Morgan-Hough CV, Jones PW, Eisenstein SM. Primary and revision lumbar discectomy - a 16-year review from one centre. J Bone Joint Surg Br. 2003;85:871–874. [PubMed] [Google Scholar]
- 19.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 20.Ohtori S, Suzuki M, Koshi T, Takaso M, Yamashita M, Inoue G, et al. Proinflammatory cytokines in the cerebrospinal fluid of patients with lumbar radiculopathy. Eur Spine J. 2011;20:942–946. doi: 10.1007/s00586-010-1595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, et al. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 22.Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by transforming growth factor-beta(1)and insulin like growth factor-I. Cell Biol Int. 2001;25:679–689. doi: 10.1006/cbir.2000.0718. [DOI] [PubMed] [Google Scholar]
- 23.Phillip M, Maor G, Assa S, Silbergeld A, Segev Y. Testosterone stimulates growth of tibial epiphyseal growth plate and insulin-like growth factor-1 receptor abundance in hypophysectomized and castrated rats. Endocrine. 2001;16:1–6. doi: 10.1385/ENDO:16:1:01. [DOI] [PubMed] [Google Scholar]
- 24.Postlethwaite AE, Keskioja J, Moses HL, Kang AH. Stimulation of the chemotactic migration of human fibroblasts by transforming growth factor beta. J Exp Med. 1987;165:251–256. doi: 10.1084/jem.165.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci U S A. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwander JC, Hauri C, Zapf J, Froesch ER. Synthesis and secretion of insulin-like growth factor and its binding protein by the perfused rat liver: dependence on growth hormone status. Endocrinology. 1983;113:297–305. doi: 10.1210/endo-113-1-297. [DOI] [PubMed] [Google Scholar]
- 27.Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11:145–151. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suk KS, Lee HM, Moon SH, Kim NH. Recurrent lumbar disc herniation: results of operative management. Spine (Phila Pa 1976) 2001;26:672–676. doi: 10.1097/00007632-200103150-00024. [DOI] [PubMed] [Google Scholar]
- 29.Takada T, Nishida K, Doita M, Miyamoto H, Kurosaka M. Interleukin-6 production is upregulated by interaction between disc tissue and macrophages. Spine (Phila Pa 1976) 2004;29:1089–1092. doi: 10.1097/00007632-200405150-00007. discussion 1093. [DOI] [PubMed] [Google Scholar]
- 30.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral-disk by growth-factors. Spine (Phila Pa 1976) 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Uceyler N, Kafke W, Riediger N, He L, Necula G, Toyka KV, et al. Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology. 2010;74:1806–1813. doi: 10.1212/WNL.0b013e3181e0f7b3. [DOI] [PubMed] [Google Scholar]
- 32.von Ahsen O, Renken C, Perkins G, Kluck RM, Bossy-Wetzel E, Newmeyer DD. Preservation of mitochondrial structure and function after Bid- or Bax-mediated cytochrome c release. J Cell Biol. 2000;150:1027–1036. doi: 10.1083/jcb.150.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang HL, Ahrens C, Rief W, Gantz S, Schiltenwolf M, Richter W. Influence of depression symptoms on serum tumor necrosis factor-alpha of patients with chronic low back pain. Arthritis Res Ther. 2010;12:R186. doi: 10.1186/ar3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weiler C, Nerlich AG, Bachmeier BE, Boos N. Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine (Phila Pa 1976) 2005;30:44–53. doi: 10.1097/01.brs.0000149186.63457.20. [DOI] [PubMed] [Google Scholar]
- 36.Wera GD, Marcus RE, Ghanayem AJ, Bohlman HH. Failure within one year following subtotal lumbar discectomy. J Bone Joint Surg Am. 2008;90:10–15. doi: 10.2106/JBJS.F.01569. [DOI] [PubMed] [Google Scholar]