Abstract
Background
Sacrococcygeal teratoma (SCT) is a relatively uncommon tumor. Recurrence with poor survival and anorectal dysfunction are the 2 leading problems for patients. Here, we would review the clinic features of patients with SCTs in our hospital to identify risk factors of recurrent SCTs and to analyze anorectal functional sequelae.
Material/Methods
A retrospective review of all patients with SCTs in our center between 2007 and 2013 was performed. We analyzed the recorded data on each patient and performed follow-up through phone calls.
Results
Our study included 105 inpatients (78 girls and 27 boys); 104 cases underwent surgical resection, and 62.5% cases had a mature histopathology. The proportion of malignant teratomas rose with increasing age. Fifteen children developed recurrent SCTs with a median of 11.5 months, and most of them had an elevation of AFP levels. Four recurrent children experienced a second tumor relapse. We observed a statistically significant difference in survival rate through Kaplan-Meier method between relapsed (66.7%) and non-relapsed (94.4%) patients. In univariate analysis, incomplete primary resection and malignant histology were proven to increase recurrence risks. Nearly half of patients had at least 1 of the parameters reflecting abnormal bowel function (e.g., involuntary bowel movements, fecal incontinence, and constipation). For those recurrent SCTs patients, difficulty defecating was a major problem.
Conclusions
Tumor recurrence affected the prognosis of children with SCT. In our research, incomplete resection and malignant histology were considered risk factors. Constipation was the main problem in anorectal functional sequelae for children who had recurrence.
MeSH Keywords: Pediatrics, Prognosis, Recurrence, Risk Factors, Sacrococcygeal Region, Teratoma
Background
Sacrococcygeal teratoma (SCT) is thought to arise from Hensen’s node, which is usually located on the coccyx [1]. With an incidence of approximately 1: 35 000 to 1: 40 000 in live births, SCT is a rare tumor; however, it is still the most common germ cell tumor (GCT) of infancy and early childhood [2].
For decades, diagnosis and treatment of SCTs have made progress and become fairly standardized, and good prognosis is usually achieved. Nevertheless, increasing numbers of recurrent cases with poor survival rates have become a major challenge [3]. Incomplete resection, tumor spillage, and residual coccyx have been identified as the main risk factors for recurrence [3,4]. Further research may lead to better insight into therapeutics and prognosis of this tumor.
Anorectal dysfunction is a leading problem after surgical therapy for SCT patients. It has been reported that these sequelae, with a high incidence (over 35%), frequently continue into adulthood and can severely affect quality of life [5]. However, few studies have evaluated anorectal functional sequelae in patients who experience recurrence.
The present study was performed to determine: (a) the clinical features of patients with SCT relapse treated in a single center over the past 7 years; (b) risk factors associated with the recurrence of SCTs; and (c) how these patients, especially those who have recurrence, experience anorectal functional sequelae.
Material and Methods
Patients
A retrospective review of all patients with SCTs in our center between January 2007 and December 2013 was performed. We analyzed the recorded data of each patient concerning demographics, age at diagnosis, signs and symptoms, tumor markers, therapeutic methods, Altman classification (type I–IV), histology (mature, immature, malignant), complete resection of coccyx (yes, no), complete resection of tumor (yes, no), and primary tumor size (150 cm3 or less, 151–500 cm3, more than 500 cm3). For those patients with recurrent SCTs, more data were collected, for example: age and interval of recurrence, signs and symptoms of relapse, histopathology of recurred tumor, and chemotherapy/radiotherapy course. Patients with a presacral teratoma as part of the Currarino triad were excluded.
We determined post-surgical survival through phone calls, and to evaluate anorectal functional sequelae, some parents completed a brief oral questionnaire in the follow-up. The scale used in the questionnaire was based on Krichenbeck’s classification assessing 3 aspects: involuntary bowel movements, fecal incontinence, and constipation [6].
Statistical analysis
Categorical variables were compared with the χ2 test and Fisher’s exact test. Overall survival (OS) was calculated by the Kaplan-Meier method. Statistical analysis was performed using SPSS statistical software for Windows, version 18.0 (SPSS, Chicago, IL). Unless stated otherwise, data are expressed as mean ±SD. Proportions are presented with 95% confidence intervals. A p-value of less than 0.05 was considered statistically significant.
Results
Demographics
We treated 105 inpatients with a diagnosis of SCT in our hospital during the study period. There were 78 girls and 27 boys, with a male-to-female ratio of 1: 2.89. The diagnosis of SCT was made within the first month in 29 cases (antenatally suspected in 6, gestational ages 22–36 weeks), between 1 month and 1 year in 35, and at a later age in 41 patients. Their mean gestational age was 36.5±1.2 weeks, with a mean birth weight of 3240±390 g.
The most prominent clinical sign was a sacrococcygeal mass; other signs included constipation, urinary retention, skin burst, and ulceration in the sacrococcygeal region. According to Altman’s classification, 46.7% of patients were type I (Table 1). Except for 1 preterm patient who died before surgery because of intratumoral hemorrhage and sepsis, the remaining 104 patients underwent surgical resection and were analyzed further.
Table 1.
Altman’s classification at diagnosis and histology after surgery.
| Altman’s classification | Histological classification | ||
|---|---|---|---|
| Type I | 49 (46.7%) | Mature | 65 (62.5%) |
| Type II | 26 (24.8%) | Immature | 11 (10.6%) |
| Type III | 13 (12.4%) | Malignant: Yolk Sac Tumor | 26 (25%) |
| Type IV | 15 (14.3%) | Germinoma | 2 (1.9%) |
| Unkown | 2 (1.9%) | ||
The median age at time of first surgery was 10.2 months (range 1 day to 12 years). Intraoperatively, a sacral approach was performed in all cases, combined with a laparotomy in 7. Laparoscopic surgery was performed on 5 children for pelvic exploration and clipping of the median sacral artery, in 97 children the coccyx was removed completely, in 4 it was just partially resected, and in 3 this information was unknown.
We discharged 103 patients, and 1 patient died due to circulatory failure and severe pneumonia shortly after surgery. Tumor histology is listed in Table 1, and the proportion of malignant teratomas increased with increasing age (P<0.05) (Table 2). All patients with malignant and Grade III immature teratomas underwent adjuvant chemotherapy, and the commonly used regimens included PVB, PEB, C-PEB, JEB, and JEB/VAC. Some patients with malignancy received chemotherapy combined with radiotherapy.
Table 2.
Age and histology after resection of primary SCTs.
| Age at diagnosis | Number of patients | ||
|---|---|---|---|
| Mature | Immature | Malignant | |
| ≤1 month | 19 | 8 | 1 |
| 1 month to 1 year | 25 | 2 | 8 |
| >1 year | 21 | 1 | 19 |
Eighty-eight cases were followed-up until December 2015, with a median duration of 51.2 months. The OS of all patients was 90.4% (94/104). According to histologic type, the overall OS of mature, immature, and malignant teratoma were 96.9%, 81.8%, and 78.6%, respectively (Figure 1).
Figure 1.
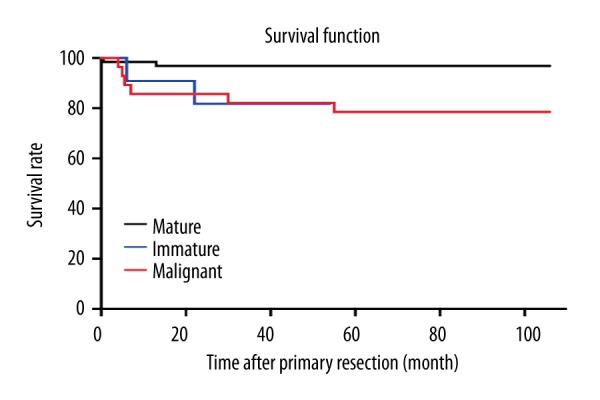
Outcomes of different histology of sacrococcygeal teratoma.
Recurrent sacrococcygeal teratoma
Fifteen children (14.4%) developed recurrent SCT, with a median duration of 11.5 months (range 5–84 months) postoperatively. Seven recurrences were found as a palpable sacrococcygeal or sacroanterior masses by physical examination during routine follow-up. Three children were suspected to have a recurrent mass owing to chronic sinus discharge; 3 cases did not have abnormal signs or symptoms, but serum AFP levels were found elevated; and in the remaining 2, gradual constipation drew the attention of their parents, and ultrasonic examination confirmed tumor recurrence. The AFP levels of 12 patients were raised (range 76.18–58632 ng/ml) after tumor relapse, and histology revealed 11 of them had malignant relapse after reoperation. In 14 of them, the recurrence was confined locally, and in 1 was combined with metastatic lesion in the liver, and all of their coccyges had been previously removed. All 15 recurrent cases received a second operation and tumors were removed completely.
It should be noted that 4 of the children with recurrence experienced a second tumor relapse, with a median of 11 months (range 7–80 months) after second surgeries. Three cases of second relapse were found to have high AFP (range 276.4–35350 ng/ml), and digital rectal examination subsequently revealed sacroanterior masses. Only 1 female patient had coccydynia with normal AFP level. All of these second recurrent lesions were local without metastasis, and third operations were performed for them with en bloc resection.
Chemotherapy with or without radiotherapy was performed on those malignant recurrent patients. In 2 of the children who had 2 recurrences, we performed identification of genomic markers of drug chemosensitivity to improve regimens for individualized treatment.
The histology of the primary tumor was compared with that of the recurrent and second recurrent tumor (Table 3). In 4 tumors, there was a shift towards malignancy. In 1 child who had chemotherapy after first surgery, the initial malignant tumor had shifted to mature at recurrence. Five children died after detection of tumor recurrence. We observed a statistically significant difference in OS among patients who relapsed (66.7%) and those who did not (94.4%) (Figure 2).
Table 3.
Histology of SCTs at original operation and recurrent operation.
| Histology before recurrence | Number of patients | Histology after first recurrence (Histology after second recurrence) | ||
|---|---|---|---|---|
| Mature | Immature | Malignant | ||
| Mature | 65 | 3 (1) | 0 | 3 |
| Immature | 11 | 0 | 0 | 1 (1) |
| Malignant | 28 | 1 | 0 | 7 (2) |
Figure 2.
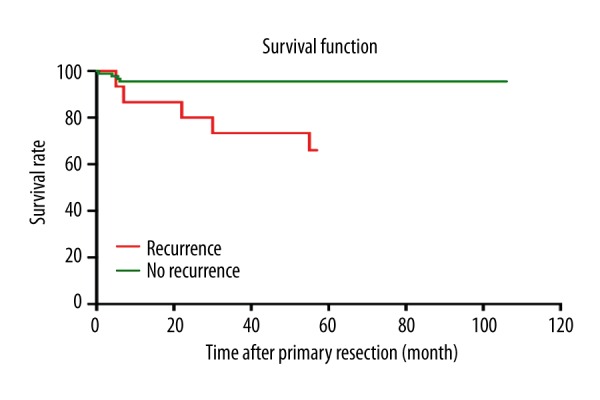
Overall survival rate according to SCTs relapse or not.
Risk factors for recurrence
The tumor recurrence rate was 10.8% in mature teratoma, 9.1% in immature teratoma, and 28.6% in malignant teratoma. Residual neoplasm in the resection margins was found in 17 patients, leading to recurrence in 6 (35.3%). The recurrence rate of patients in whom the tumor was completely resected was 10.3% (9/87). We found that the recurrence rate gradually rose with increasing tumor volume.
In univariate analysis, incomplete resection during the primary surgery and malignant histology were proven to be significant risk factors for recurrence. Other factors, including Altman’s classification, size of the tumor, immature histology, age at diagnosis, and whether to remove the coccyx, were not risk factors for SCT recurrence (Table 4).
Table 4.
Analysis of risk factors for recurrence of SCTs.
| Recurrence rate | Univariate analysis | ||
|---|---|---|---|
| Odds ratio (95% CI) | P | ||
| Complete resection | |||
| Yes | 9/87 | 1 | |
| No | 6/17 | 4.727 (1.409, 15.862) | 0.007 |
| Altman classification | |||
| I | 4/49 | 1 | |
| II | 4/26 | 2.045 (0.467, 8.956) | 0.335 |
| III | 3/13 | 3.375 (0.651, 17.509) | 0.131 |
| IV | 3/15 | 2.812 (0.553, 14.308) | 0.199 |
| Histology | |||
| Mature | 7/65 | 1 | |
| Immature | 1/11 | 0.829 (0.092, 7.479) | 0.867 |
| Malignant | 8/28 | 3.314 (1.066, 10.307) | 0.032 |
| Tumor volume (cm3) | |||
| ≤150 | 8/68 | 1 | |
| 151 to 500 | 4/23 | 1.579 (0.428, 5.831) | 0.491 |
| >500 | 3/13 | 2.250 (0.509, 9.946) | 0.275 |
| Age at surgery | |||
| ≤1 month | 5/28 | 1 | |
| 1 month to 1 year | 4/35 | 0.594 (0.143, 2.458) | 0.469 |
| ≥1 year | 6/41 | 0.789 (0.215, 2.888) | 0.72 |
| Coccyx completely removed | |||
| Yes | 15/97 | 1 | |
| No | 0/4 | 0 | 0.999 |
CI – confidence interval.
Anorectal functional sequelae of SCTs
Taking into account that most normal children over 3 years of age are well toilet trained, only 71 questionnaires were included in our study for assessing anorectal functional sequelae. Table 5 provides a schematic overview of the results of the above 71 SCTs patients and 15 recurrent SCTs patients, using Krichenbeck’s classification.
Table 5.
Schematic overview of the anorectal functional sequelae of patients SCTs.
| Ratio for 71 primary SCTs | Ratio for 15 recurrent SCTs | ||
|---|---|---|---|
| Voluntary bowel movements Feeling of urge capacity to verbalize hold the bowel movement |
No | 5 (7%) | 2 (13.3%) |
| Yes | 66 (93%) | 13 (86.7%) | |
| Soiling | No | 60 (84.5%) | 13 (86.7%) |
| Grade 1 Occasionally (once or twice per week) |
Yes | 2 (2.8%) | 0 (0) |
| Grade 2 Every day, no social problem |
Yes | 6 (8.5%) | 1 (6.7%) |
| Grade 3 Constant, social problem |
Yes | 3 (4.2%) | 1 (6.7%) |
| Constipation | No | 48 (67.6%) | 8 (53.3%) |
| Grade 1 Manageable by changes in diet |
Yes | 15 (21.2%) | 2 (13.3%) |
| Grade 2 Requires laxative |
Yes | 5 (7%) | 3 (20%) |
| Grade 3 Resistant to laxatives and diet |
Yes | 3 (4.2%) | 2 (13.3%) |
Of the 71 patients, 49.3% had at least 1 of the parameters reflecting bowel function as abnormal. Five patients had involuntary bowel movements and 3 of them also had fecal incontinence (Grade 3), and these patients were considered to have total fecal incontinence. Constipation was reported in 23 patients and all of them had normal voluntary bowel movements.
We also described the anorectal functional sequelae of those recurrent patients. In the 15 recurrent cases, 13 (86.7%) patients had normal voluntary bowel movements. Two patients suffered from fecal incontinence in their underwear every day with or without social problems, including 1 who suffered both of these 2 sequelae, and he was considered as having total fecal incontinence. It is worth noting that the proportion of patients with constipation increased, and nearly half of recurrent children had problems defecating, with various degrees of severity.
Discussion
This study describes our 7-year experience in management of SCTs from a single center in China. Based on a large series of over 100 patients, in some respects (e.g., morbidity sex ratio, proportions according to Altman’s classification, clinical manifestations, surgical methods, and chemotherapy regimens) our findings are consistent with the literature and may be useful in meta-analyses.
With improved imaging techniques, more SCTs are diagnosed antenatally. It has been reported that over 40% of patients with SCTs are detected at 22–36 weeks of gestation in some Western countries [7]. However, in our study only 5.7% of patients were diagnosed prenatally; suggesting that much improvement is still needed in prenatal examination technologies and methods.
Tumor recurrence is considered to be an important factor affecting prognosis of SCTs. Previous studies showed a mean recurrence rate of 12.5% (range 2–35%) [3,8]. From a histological perspective, mature teratoma has a mean recurrence rate of 10%, and immature and malignant teratoma have means of 33% and 18%, respectively [9]. The tumor recurrence rate of our study was similar to that reported in the literature. However, in our study, malignant tumors showed a higher recurrence rate than other pathologic types, in contrast to previous reports. We reviewed the therapeutic process used in our cases, and found 7 of the 11 immature SCTs were Grade III tumors, and all of Grade III cases were received chemotherapy, confirming that chemotherapy plays important roles in the treatment of immature or malignant SCTs.
Many reports suggested the necessity for 3-year follow-up for SCTs, as most recurrent cases occurred within 3 years [4,10,11]. In our study, 4 cases recurred with a mean duration of 58.5 months after primary operations, and there were 4 recurrent cases who had second relapses. We recommend at least 5 years of multidisciplinary follow-up, including regular physical examination, monitoring of AFP lever, and imaging surveillance. For those patients with recurrence, it is essential to establish special files for making detailed records and extending the follow-up process.
SCT has 5-year OS over 90% after early surgery, but for recurrent cases, survival is about 50% [4,12]. In our series, OS for no-recurrence patients significantly differed from that of the patients with recurrence, which further confirms the negative influences of tumor relapse on prognosis. Therefore, it is important to pay more attention to risk factors of tumor recurrence.
As previously mentioned, many factors are thought to contribute to tumor recurrence. In our series, the most important risk factor was incomplete resection after primary surgery. In addition, malignant histology of the primary tumor was also a positive predictor of recurrence. However, incomplete removal of the coccyx was not thought to be correlated with the tumor relapse, which was usually thought to be a definite positive factor [13,14]. We thought it might be due to the mature pathological type in these cases. Notably, there were also relapses after radical excisions, and some reports cited small remnants left in situ or minute yolk sac components missed in the pathological examination [15]. We agree with these views, and we suggest careful assessment of microscopic involvement of the resection margin. Once the foci of YST are identified, there will be a need to appropriately expand the excision range, and chemotherapy after the operation is warranted. In addition, the tumors should be thoroughly sampled for histology to detect small foci of YST, because it is essential to prevent histological underscoring of those benign tumors that recur as YST.
As SCTs are close to the anal-rectal region, anorectal functional sequelae are common after operations. It is necessary to pay more attention and to make more accurate evaluation for these sequelae. We designed an objective and simple questionnaire, and parameters were defined in the Krickenbeck conference (2005) [6].
In previous studies, fecal incontinence was reported to affect 13% to 27% of the patients in all age groups, and constipation was reported to affect a wide range of 8% to 39% [5,16]. The percentage of patients in our study who developed functional sequelae is comparable to that in previous reports. Some controversies remain about the risk factors for functional sequelae. Previously, Malone et al. [17] found an association between the incidence of functional sequelae and tumors of Altman type IV. However, other studies did not support this view [18,19]. We also found Altman classification was not a risk factor (data not shown).
Few studies have described the anorectal functional sequelae in recurrent patients, and we investigated this aspect. Compared with non-recurrent SCTs, the proportion of patients with fecal incontinence was similar in recurrent ones. However, both incidence and severity of constipation were increased in patients with recurrence. The cause for this phenomenon remains unclear. It might be related to surgical injuries to the pelvic neuroplexus. Persistent tumor compression could cause problems with defecation, which might aggravate dilation of the rectum, and worsen constipation. Recently, as benefits of laparoscopy have been realized, Szyllo et al. [20] has reported this technology was used in resection of recurred presacral teratoma. Laparoscopy facilitated accurate visualization of pelvic tumors, and careful sharp dissection of the tissue near the rectum could minimize nerve injury, thus reducing anorectal functional sequelae.
Conclusions
In conclusion, although diagnostic technology, surgical procedures, and chemotherapy regimens are improving, SCT recurrence is still a major problem causing significantly decreased survival rates. Complete resection with careful microscopic detection of tumor margins is warranted. Regardless of whether the histopathology result is mature, immature, or malignant, we suggest performing long and close follow-up for these young patients, especially for those with recurrence. Some reports suggested that anorectal functional sequelae of SCT might be improving over time [21]. Nevertheless, for these pediatric patients, quality of life could be seriously affected. For those with recurrence, constipation is a major problem that needs more attention from clinicians.
Acknowledgements
Zhixiang Wu assisted with statistical analysis, Kai Chen helped with data collection, and Yihan Zheng gave us writing assistance.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Source of support: Departmental sources
References
- 1.Frazier AL, Weldon C, Amatruda J. Fetal and neonatal germ cell tumors. Semin Fetal Neonatal Med. 2012;17(4):222–30. doi: 10.1016/j.siny.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Rescorla FJ, Sawin RS, Coran AG, et al. Long-term outcome for infants and children with sacrococcygeal teratoma: A report from the Childrens Cancer Group. J Pediatr Surg. 1998;33(2):171–76. doi: 10.1016/s0022-3468(98)90426-2. [DOI] [PubMed] [Google Scholar]
- 3.Derikx JP, De Backer A, van de Schoot L, et al. Factors associated with recurrence and metastasis in sacrococcygeal teratoma. Br J Surg. 2006;93(12):1543–48. doi: 10.1002/bjs.5379. [DOI] [PubMed] [Google Scholar]
- 4.Yao W, Li K, Zheng S, et al. Analysis of recurrence risks for sacrococcygeal teratoma in children. J Pediatr Surg. 2014;49(12):1839–42. doi: 10.1016/j.jpedsurg.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 5.Derikx JP, De Backer A, van de Schoot L, et al. Long-term functional sequelae of sacrococcygeal teratoma: A national study in The Netherlands. J Pediatr Surg. 2007;42(6):1122–26. doi: 10.1016/j.jpedsurg.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 6.Holschneider A, Hutson J, Peña A, et al. Preliminary report on the International Conference for the Development of Standards for the Treatment of Anorectal Malformations. J Pediatr Surg. 2005;40(10):1521–26. doi: 10.1016/j.jpedsurg.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Ho KO, Soundappan SV, Walker K, Badawi N. Sacrococcygeal teratoma: The 13-year experience of a tertiary paediatric centre. J Paediatr Child Health. 2011;47(5):287–91. doi: 10.1111/j.1440-1754.2010.01957.x. [DOI] [PubMed] [Google Scholar]
- 8.De Backer A, Madern GC, Hakvoort-Cammel FG, et al. Study of the factors associated with recurrence in children with sacrococcygeal teratoma. J Pediatr Surg. 2006;41(1):173–81. doi: 10.1016/j.jpedsurg.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 9.Schneider DT, Wessalowski R, Calaminus G, et al. Treatment of recurrent malignant sacrococcygeal germ cell tumors: Analysis of 22 patients registered in the German protocols MAKEI 83/86, 89, and 96. J Clin Oncol. 2001;19(7):1951–60. doi: 10.1200/JCO.2001.19.7.1951. [DOI] [PubMed] [Google Scholar]
- 10.Niramis R, Anuntkosol M, Buranakitjaroen V, et al. Long-term outcomes of sacrococcygeal germ cell tumors in infancy and childhood. Surg Res Pract. 2015;2015:398549. doi: 10.1155/2015/398549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha S, Kumar Sarin Y, P Deshpande V. Neonatal sacrococcygeal teratoma: Our experience with 10 cases. J Neonatal Surg. 2013;2(1):4. [PMC free article] [PubMed] [Google Scholar]
- 12.Huddart SN, Mann JR, Robinson K, et al. Sacrococcygeal teratomas: The UK Children’s Cancer Study Group’s experience. I. Neonatal Pediatr Surg Int. 2003;19(1–2):47–51. doi: 10.1007/s00383-002-0884-2. [DOI] [PubMed] [Google Scholar]
- 13.Mann JR, Gray ES, Thornton C, et al. Mature and immature extracranial teratomas in children: The UK Children’s Cancer Study Group Experience. J Clin Oncol. 2008;26(21):3590–97. doi: 10.1200/JCO.2008.16.0622. [DOI] [PubMed] [Google Scholar]
- 14.Barakat MI, Abdelaal SM, Saleh AM. Sacrococcygeal teratoma in infants and children. Acta Neurochir. 2011;153(9):1781–86. doi: 10.1007/s00701-011-1048-8. [DOI] [PubMed] [Google Scholar]
- 15.Yoneda A, Usui N, Taguchi T, et al. Impact of the histological type on the prognosis of patients with prenatally diagnosed sacrococcygeal teratomas: The results of a nationwide Japanese survey. Pediatr Surg Int. 2013;29(11):1119–25. doi: 10.1007/s00383-013-3384-7. [DOI] [PubMed] [Google Scholar]
- 16.Shalaby MS, Walker G, O’Toole S, et al. The long-term outcome of patients diagnosed with sacrococcygeal teratoma in childhood. A study of a national cohort. Arch Dis Child. 2014;99(11):1009–13. doi: 10.1136/archdischild-2014-306414. [DOI] [PubMed] [Google Scholar]
- 17.Malone PS, Spitz L, Kiely EM, et al. The functional sequelae of sacrococcygeal teratoma. J Pediatr Surg. 1990;25(6):679–80. doi: 10.1016/0022-3468(90)90362-d. [DOI] [PubMed] [Google Scholar]
- 18.Havránek P, Hedlund H, Rubenson A, et al. Sacrococcygeal teratoma in Sweden between 1978 and 1989: Long-term functional results. J Pediatr Surg. 1992;27(7):916–18. doi: 10.1016/0022-3468(92)90398-q. [DOI] [PubMed] [Google Scholar]
- 19.Rintala R, Lahdenne P, Lindahl H, et al. Anorectal function in adults operated for a benign sacrococcygeal teratoma. J Pediatr Surg. 1993;28(9):1165–67. doi: 10.1016/0022-3468(93)90156-f. [DOI] [PubMed] [Google Scholar]
- 20.Szyllo K, Lesnik N. Sacrococcygeal teratoma – case report and review of the literature. Am J Case Rep. 2013;14:1–5. doi: 10.12659/AJCR.883727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kremer ME, Dirix M, Koeneman MM, et al. Quality of life in adulthood after resection of a sacrococcygeal teratoma in childhood: A Dutch multicentre study. Arch Dis Child Fetal Neonatal Ed. 2015;100(3):F229–32. doi: 10.1136/archdischild-2014-307589. [DOI] [PubMed] [Google Scholar]


