Summary
Rheumatoid Arthritis (RA) is a multisystem disorder, which causes significant morbidity. An early diagnosis of RA is essential to prevent the development of irreversible bone and joint changes. The disease has characteristic clinical features, but an early evaluation of the quantum of disease may be difficult with plain radiography alone. Recent developments in the imaging of RA have contributed significantly to an early diagnosis of the disease. In this article, we review the role and current status of various imaging modalities including recent advances in the evaluation and follow-up of early RA.
MeSH Keywords: Arthritis, Rheumatoid; Diffusion Magnetic Resonance Imaging; Radiography
Background
Rheumatoid Arthritis (RA) is the commonest inflammatory arthritis that progressively involves the small joints of the hand and feet causing joint destruction and deformity with extensive morbidity [1]. It usually presents as a symmetrical polyarthritis, but may be asymmetrical in around 20% of patients [2]. The common clinical features of RA include painful swollen joints, morning stiffness, fatigue and myalgia. The disease primarily affects the synovium, causing its hypertrophy, and subsequently leading to cartilage and bone destruction.
Diagnosis of RA is relatively simple when all the characteristic features are seen, however, diagnosis is challenging as the classical clinical, imaging and serological features may not appear at the same time. Laboratory tests such as anti-cyclic citrullinated peptide antibody (ACPA or anti-CCP), C-reactive protein (CRP), Erythrocyte sedimentation rate (ESR) and Rheumatoid factor (RA Factor) may help in suggesting and confirming the diagnosis.
The ACR/EULAR criteria are often used to diagnose RA, though they were framed with the objective of classifying the newly presenting patients of undifferentiated inflammatory arthritis as RA. This was done primarily for the classification of RA for epidemiological studies and clinical trials [3]. The 1987 ACR criteria stated the presence of juxtaarticular erosions on wrist and hand radiographs as one of the seven criteria for the classification of RA. This does not help in making an early diagnosis, as erosions appear late on radiographs. According to the 2010 revised ACR/EULAR criteria, however, joint involvement, as evidenced by synovitis which may be confirmed on imaging, is included as one of the criteria and thus underlines the role of imaging in diagnosing RA.
There is a growing body of evidence that suggests the existence of a time period in the early course of the disease, called the “window of opportunity”, during which, if the appropriate treatment is started, there is a long-term improvement and a halt in progression of the disease. Thus, aggressive therapy may be started on the basis of strong clinical suspicion even before the classical hallmarks of disease have appeared. As an early diagnosis and treatment are essential for the long-term outcome in patients with early RA, confirmation or exclusion of the diagnosis must be made early after the onset of symptoms [4]
Imaging
Radiography
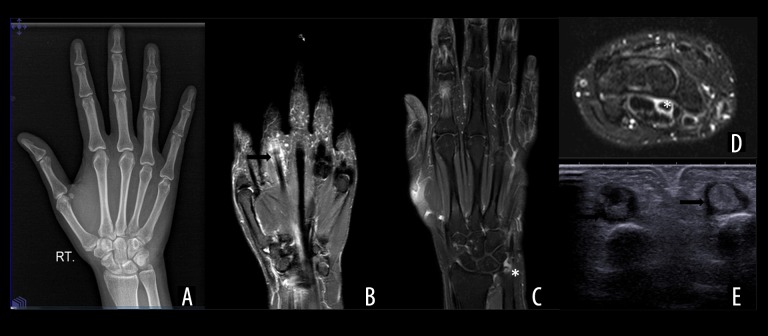
Analogue or digital radiography is the first-line and most commonly performed imaging modality for patients with RA. Its low cost and a wide availability makes it the modality of choice not only for diagnosis but also for follow-up. It demonstrates the bony changes of established RA such as narrowing of joint space and bony erosions. However, it has a low sensitivity in demonstrating soft tissue edema, synovial thickening and bone marrow changes in the early stages of the disease (Figure 1).
Figure 1.
(A) Normal AP radiograph of hand and wrist of a 23-year-old female patient presenting with difficult painful flexion of digits diagnosed as early Rheumatoid arthritis. (B, C) Fat-suppressed (FS) T2W and CE coronal MRI images of the same patient showing tenosynovitis (arrow)of the second flexor tendon with subtle marrow edema at the ulnar styloid (*). (D) Axial FST2W image showing similar high signal around the flexor tendon at the level of flexor retinaculum (*). (E) Axial sonographic image at the level of proximal phalanges showing hypoechoic cuffing around the flexor digitorum tendons of 2nd and 3rd digit (arrows).
The soft tissue thickening seen on the radiographs is due to a variable combination of synovial thickening, tenosynovitis and joint effusion. In small joints, it is seen as a focal bulge of periarticular soft tissue and an increase in soft tissue density. In larger joints, the displacement of the periarticular fat pads may be seen.
Joint space widening is the earliest radiographic abnormality and is transient in nature due to synovial thickening and joint effusion [5].
Osteopenia may be localized due to synovitis and resulting hyperemia, or diffuse in the later stages of the disease due to a painful disuse of the limb. It may be further compounded by steroid administration.
Erosions are the hallmark of RA and may not be seen at the time of initial presentation. Their incidence increases with the duration of the disease such that after 10 years of onset of symptoms erosions are seen in 90–95% of patients [6]. The appearance of erosions signals irreversibility of the joint changes. However, a few studies have shown that healing and repair of erosions may occur in patients undergoing treatment with Disease-Modifying AntiRheumatic Drugs (DMARDs) [7,8].
In advanced cases, there may be fusion at the involved joints. Bony fusion is relatively uncommon in joints other than the intercarpal joints [9]. Alignment deformities, marked joint destruction and stress fractures are other late changes of RA.
Ultrasonography (US)
Ultrasonography is widely available, relatively inexpensive and entails no exposure to ionizing radiation. US has gained popularity in not only detection of joint changes but also in follow-up of these changes in patients on various DMARDS.
US has come a long way since the time when the pathologies of RA were first described on gray scale and colour Doppler US in 1978 and 1994, respectively [10,11]. The availability of high resolution and high frequency linear transducers (up to 18 MHz) has made detection of synovial thickening, joint effusion and superficial erosions easy. Furthermore, the smaller footprint or hockey stick probes have simplified the sonographic evaluation of the small joints of the hand and feet. These technological advances are especially relevant in patients with RA as these patients require long and frequent follow-ups. However, US is a time-consuming and operator dependent modality.
On USG, synovial thickening is seen as a noncompressible hypoechoic soft tissue thickening of the synovial layer, which may appear as a hyperechoic layer in chronic disease, whereas synovial effusion appears as a compressible hypoechoic layer. The thickened synovium may demonstrate colour flow in the active inflammatory phase. Similar hypoechoic thickening in the tendon sheath is seen in the case of tenosynovitis. Erosions are depicted as discontinuity of the cortical bone visualized in two planes. Caution needs to be exercised in imaging of irregular bony surfaces and normal contouring of the bone, for which a thorough understanding of the bony anatomy is essential. (Figure 2A–2E).
Figure 2.
Ultrasound findings in different patients with newly diagnosed RA, hypoechoic synovial thickening at the metacarpophalangeal joint (*) (A), proliferative pannus (marked by calipers) with underlying bony erosion at the distal radius (arrow) (B), synovial hypertrophy around the scaphoid (C), wavy hypoechoic fluid in the flexor tendon sheath (arrow) (D), tenosynovitis involving all the flexor tendons at the wrist (E), intrasynovial and perisynovial increased vascularity (F).
Many investigators have studied the usefulness of US for the diagnosis and follow-up of RA. Gray scale US is more effective than conventional radiography in detection of bony erosions, which is a hallmark of the disease. It can also help in identifying bony surface irregularities and abnormalities of the tendons and synovium. Ultrasound has a high sensitivity of 79% and specificity of 97%, as compared to 32% and 98% of radiography in identifying changes of RA at the metatarsophalangeal joints [12]. High interobserver agreement has also been found in the identification of synovitis and bony erosions by different sonologists [13]. Large joints, such as the shoulder, are also amenable to US and are especially useful when radiographs are normal [14].
US also detects evidence of tenosynovitis by demonstrating fluid along the tendon sheaths, increased intratendinous vascularity with colour Doppler along with structural damage in the form of a partial or complete tear [15]. Even subclinical tenosynovitis has also been diagnosed in the ankle joint with an involvement of the tibialis posterior and peroneus longus tendons on ultrasound [16].
Doppler US evaluates the vascularization of the synovium, which correlates with disease activity (Figure 2F). The inflamed synovium demonstrates low resistance flow on Doppler US [17,18]. Chronic synovial hypertrophy results in an echogenic thickening without any increase in the Doppler signal. The sensitivity of the examination may be improved by the use of US contrast agents [19]. However, the diagnostic value of adding an ultrasound contrast is still unclear, especially considering its cost and an invasive nature.
Sonoelastography, which utilizes the principle of measuring tissue stiffness, has shown promise in the evaluation of tendon pathologies wherein a correlation has been found between the degree of tendon softening and tendinopathies involving the Achillis tendon and the rotator cuff tendons. Its role in the evaluation of synovial pathologies is still under evaluation [20–22].
There are many US scoring systems in place, which grade the degree of synovial pathology, erosions and other features of RA in varying number of joints. The number of the joints assessed range from four to 78 [23,24].
USG has a role in differentiating between inflammatory and non-inflammatory arthritis as well. A combination of structural and synovial assessments by US also aids in the differentiation between RA, osteoarthritis and normal joints [25].
Computed Tomography (CT)
CT is infrequently used for the evaluation of early RA primarily due to the use of ionizing radiation and also because of its limited soft tissue contrast, even though it has a high sensitivity in assessing the structural changes in the cortical bone. Its major role is in the assessment of cervical spine involvement in RA, especially in the atlanto-axial subluxations and fractures [26]. CT also demonstrates well bony ankylosis in the advanced stages. It is a valuable tool for the evaluation of the pulmonary parenchymal involvement in RA.
Magnetic Resonance Imaging (MRI)
MRI has proved to be the most sensitive of all the available modalities in making an early diagnosis and subsequent evaluation of RA. Its excellent soft tissue contrast, multiplanar capabilities and the use of gadolinium-based contrast allow for the differentiation of synovitis from joint effusion or tenosynovitis as well as for the diagnosis of bone marrow edema and erosions.
The MR scan in RA patients should be individualized based on the anatomic area, availability of coils, and strength of the magnet. In most cases, it should include a T1-weighted and fat-saturated, T2-weighted/STIR sequences with a contrast-enhanced T1W-fat-suppressed sequence. Isotropic 3D sequences are useful in small joints of the hand and feet. PD/fat-suppressed T1 sequences and cartilage sequences may also be performed. Diffusion-weighted images at a “b” value of 400 and 800 and ADC may additionally be acquired. Images should be acquired in minimum two planes depending on the area of interest. The slice thickness should not be more than 3 mm, with thinner slices preferable for small joints [27,28].
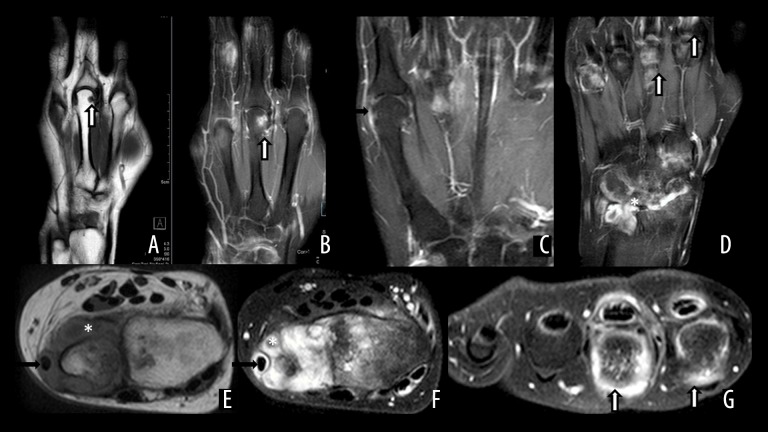
On MRI, synovitis is seen as thickened synovium with a bright signal on T2W images signifying edema, an increase in synovial volume and contrast-enhancement on post-gadolinium scans. CE MRI can differentiate between synovitis and joint effusion by demonstrating an early contrast enhancement of the inflamed synovium up to 5 minutes after contrast injection. In the late phase i.e., 10 minutes after contrast injection; gadolinium diffuses into the synovial fluid whence the differentiation of the synovium from joint effusion becomes difficult [29–31]. This differentiation may also be achieved by acquiring heavily T2W images in which the joint effusion appears brighter than the inflamed synovium. In some patients, however, gadolinium-based compounds cannot be used, either due to a history of contrast reaction or compromised renal function. In such patients, diffusion-weighted imaging may be helpful. (Figure 3). Synovitis is seen as a high signal in the synovium at a high b value of 800 [32]. In RA, bone marrow edema is frequently seen in the subchondral bone and is best demonstrated by MRI, in which it is seen as a bright signal in the fat-suppressed T2W/STIR images. This high signal is seen in contrast-enhanced T1W images as well. These areas of marrow edema are likely to be precursors of erosions [33,34] (Figure 4).
Figure 3.
MRI changes of early RA in a 29-year-old male patient presenting with isolated little finger pain, focal area of marrow edema and synovial thickening at the PIP joint of the fifth digit (arrow) on coronal fat-suppressed T2WI (A), show restricted diffusion on DWI (B) and ADC maps (C). There is synovial thickening (white arrows) on axial fat suppressed T2WI (D) and enhancement on axial CEMRI (E). Also a focal area of marrow edema in the proximal phalanx (black arrow) (D).
Figure 4.
MRI findings in different patients with RA. T1W and CE MRI (A, B), showing erosion at the third metacarpal head, with enhancement on post-contrast image (arrow). (C) Synovial thickening and enhancement at the fifth metacarpophalangeal joint (arrow). Advanced synovial thickening and enhancement at the intercarpal, distal radioulnar joints (*) with tenosynovititis (arrows) (D). Extensive proliferative pannus on T1W (E) and FS T2W (F) MRI around the distal ulna (*) with involvement of the extensor carpi ulnaris tendon (arrow). FST2W axial image showing inflammatory changes at the second and third metacarpal heads and the corresponding flexor tendons (arrow).
Erosions represent irreversible bone damage and MRI is more sensitive in detecting erosions in the hand and wrist in early RA than US and conventional radiography. The presence of erosions at baseline MRI has prognostic significance too, as they correlate with a poor long-term outcome [35,36]. It has also been observed that patients who do not have erosions at a baseline MRI demonstrate no sign of erosions at a 2-year follow-up [33].
Tenosynovitis is also an early feature of RA, with multiple patients presenting with an isolated tendon sheath involvement. MRI shows fluid distension and thickening of the tendon sheath with contrast enhancement. Tenosynovitis has to be differentiated from a normal tendon sheath fluid which is less than 1 mm in thickness or smaller than the diameter of the corresponding tendon [37,38]. Dorsal extensor tendons of the hand are more commonly involved than the volar flexor tendons in RA [8]. MRI also demonstrates the sequel of tenosynovitis i.e. a partial or complete rupture of tendons due to either weakening of the tendon sheath by invading synovium or due to friction resulting from movement of the tendons across the irregularly eroded bone surface [38,39].
Several studies have demonstrated the changes in MRI findings with treatment. These studies have shown a decrease in the relative early enhancement in response to intraarticular steroids, initiation of DMARDs and anti TNF alpha therapy [40–44].
Currently, MRI plays a role in improving diagnostic confidence, in predicting the progression of the disease to definitive RA rather than to undifferentiated inflammatory arthritis, in detecting evidence of persistent inflammation in the setting of clinical remission and in predicting treatment response. Since MRI is the gold standard for the detection of bone marrow edema, it is recommended to be used for independent prediction of subsequent bone damage (Table 1) [45].
Table 1.
Utility of various imaging modalities in Rheumatoid Arthritis (RA).
| RA features | Radiography | Grey scale ultrasound | Doppler (color/power) | Bone scan | CT | MRI |
|---|---|---|---|---|---|---|
| Early changes | ||||||
| Synovial thickening | − | ++ | +++ | − | + | +++ |
| Effusion | + | ++ | ++ | − | + | +++ |
| Synovial vascularity | − | − | +++ | − | − | +++ |
| Bone marrow oedema | − | − | − | + | − | +++ |
| Tenosynovitis | − | ++ | +++ | − | − | +++ |
| Joint space widening | + | − | − | − | +++ | + |
| Late changes | ||||||
| Osteopenia | ++ | − | − | − | ++ | − |
| Erosions | + | ++ | + | + | +++ | +++ |
| Bony ankyloses | ++ | + | − | − | +++ | + |
| Alignment deformity | +++ | − | − | − | +++ | + |
| Stress fractures | ++ | + | − | +++ | ++ | +++ |
RA – Rheumatoid Arthritis; CT – Computed Tomography; MRI – Magnetic Resonance Imaging; ‘−’ – not useful; ‘+’ – limited utility; ‘++’ – definitely useful; ‘+++’ – modality of choice.
Newer imaging modalities
Various biochemical techniques targeted towards identifying inflammatory changes are being studied, including Optical imaging techniques such as Thermography and Near Infrared imaging (NIR). These techniques are based on the detection of local increase in skin temperature secondary to an inflammatory process and transmission and/or scatter of light through an inflamed joint, respectively. Biochemical probes are also under development which are supposed to identify key molecular/enzymatic changes in the involved areas. Advances in PET and SPECT imaging are also being investigated for identifying changes in the biochemical milieu of the affected regions [46].
Conclusions
Even though radiography continues to be the mainstay for the diagnosis and follow-up of patients with RA, it has been proven by many investigators that US and MRI are more sensitive in detecting early and persistent changes of RA. A baseline radiograph is usually taken at the initiation of therapy to assess the severity of disease. Since RA patients require a regular follow-up, repeated radiographs expose them to unnecessary radiation without providing details of synovial and bony changes. Once diagnosed with RA, a patient may be followed up by ultrasound for persistent disease activity or deterioration. With the wide availability of US, the assessment of structural changes can be made with a greater sensitivity and specificity. In those cases where ultrasound findings are equivocal, a contrast MRI may be employed. Several studies have shown the value of MRI in not only an early assessment of the disease but also in predicting disease progression and treatment response. Currently, MRI plays an important role in an early diagnosis of RA, especially in radiographically normal joints and in follow-up of disease activity, treatment response, and in predicting treatment outcomes. Future research may shed light on the role and efficacy of the non-contrast MRI techniques such as diffusion-weighted MRI.
Footnotes
Conflict-of-interest statement
The authors declare no conflicting interests (including but not limited to commercial, personal, political, intellectual or religious interests).
Core tip: Various imaging modalities help the radiologist and the rheumatologist in making an early diagnosis of rheumatoid arthritis (RA). These modalities play an important role in identifying the severity and progression of the disease as well as in assessing the response to treatment. Latest advances in ultrasound, computed tomography and magnetic resonance imaging have further improved the specificity and sensitivity in identifying early changes of disease. In this review, we aim to elucidate the current role of imaging in making an early diagnosis of RA.
References
- 1.Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am. 2001;27(2):269–81. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 2.Helms CA. Arthritis. In: Helms CA, editor. Fundamentals of Skeletal Radiology. Philadelphia: WB Saunders; 1989. pp. 135–76. [Google Scholar]
- 3.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–81. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 4.Narvaez JA, Narvaez J, De Lama E, et al. MR imaging of early rheumatoid arthritis. Radiographics. 2010;30(1):143–63. doi: 10.1148/rg.301095089. [DOI] [PubMed] [Google Scholar]
- 5.Renner WR, Weinstein AS. Early changes of rheumatoid arthritis in the hand and wrist. Radiol Clin North Am. 1988;26:1185–93. [PubMed] [Google Scholar]
- 6.Renton P. Diseases of joints. In: Sutton D, editor. Textbook of Radiology and Imaging. 7th ed. Vol. 2. Churchill Livingstone; 2002. pp. 1201–45. [Google Scholar]
- 7.Sharp JT, Van Der Heijde D, Boers M, et al. Subcommittee on Healing of Erosions of the OMERACT Imaging Committee. Repair of erosions in rheumatoid arthritis does occur. Results from 2 studies by the OMERACT Subcommittee on Healing of Erosions. J Rheumatol. 2003;30(5):1102–7. [PubMed] [Google Scholar]
- 8.Ideguchi H, Ohno S, Hattori H, et al. Bone erosions in rheumatoid arthritis can be repaired through reduction in disease activity with conventional disease-modifying antirheumatic drugs. Arthritis Res Ther. 2006;8(3):R76. doi: 10.1186/ar1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenspan A. Inflammtory arthritides. In: Greenspan A, editor. Orthopaedic Radiology: A practical Approach. 3rd ed. Philadelphia: Lippincott Williams and Wilkins; 2000. pp. 453–78. [Google Scholar]
- 10.Cooperberg P, Tsang I, Truelove L, et al. Gray scale ultrasound in the evaluation of rheumatoid arthritis of the knee. Radiology. 1978;126:759–63. doi: 10.1148/126.3.759. [DOI] [PubMed] [Google Scholar]
- 11.Newman J, Adler R, Bude R, et al. Detection of soft-tissue hyperemia: Value of power Doppler sonography. Am J Roentgenol. 1994;163:385–89. doi: 10.2214/ajr.163.2.8037037. [DOI] [PubMed] [Google Scholar]
- 12.Szkudlarek M, Narvestad E, Klarlund M, et al. Ultrasonography of the metatarsophalangeal joints in rheumatoid arthritis: Comparison with magnetic resonance imaging, conventional radiography, and clinical examination. Arthritis Rheum. 2004;50:2103–12. doi: 10.1002/art.20333. [DOI] [PubMed] [Google Scholar]
- 13.Szkudlarek M, Court-Payen M, Jacobsen S, et al. Interobserver agreement in ultrasonography of the finger and toe joints in rheumatoid arthritis. Arthritis Rheum. 2003;48:955–62. doi: 10.1002/art.10877. [DOI] [PubMed] [Google Scholar]
- 14.Hermann K-G, Backhaus M, Schneider U, et al. Rheumatoid arthritis of the shoulder joint: Comparison of conventional radiography, ultrasound, and dynamic contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:3338–49. doi: 10.1002/art.11349. [DOI] [PubMed] [Google Scholar]
- 15.Filippucci E, Gabba A, Di Geso L, et al. Hand tendon involvement in rheumatoid arthritis: An ultrasound study. Semin Arthritis Rheum. 2012;41(6):752–60. doi: 10.1016/j.semarthrit.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez M, Pineda C, Salaffi F, et al. Is ankle involvement underestimated in rheumatoid arthritis? Results of a multicenter ultrasound study. Clin Rheumatol. 2016 doi: 10.1007/s10067-016-3226-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Qvistgaard E, Rogind H, Torp-Pedersen S, et al. Quantitative ultrasonography in rheumatoid arthritis: evaluation of inflammation by Doppler technique. Ann Rheum Dis. 2001;60:690–93. doi: 10.1136/ard.60.7.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terslev L, Torp-Pedersen S, Savnik A, et al. Doppler ultrasound and magnetic resonance imaging of synovial inflammation of the hand in rheumatoid arthritis: A comparative study. Arthritis Rheum. 2003;48:2434–41. doi: 10.1002/art.11245. [DOI] [PubMed] [Google Scholar]
- 19.Zordo T, Mlekusch SP, Feuchtner GM, et al. Value of contrast-enhanced ultrasound in rheumatoid arthritis. Eur J Radiol. 2007;64(2):222–30. doi: 10.1016/j.ejrad.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Lalitha P, Reddy M, Reddy KJ. Musculoskeletal applications of elastography: A pictorial essay of our initial experience. Korean J Radiol. 2011;12:365–75. doi: 10.3348/kjr.2011.12.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SU, Joo SY, Kim SK, et al. Real-time sonoelastography in the diagnosis of rotator cuff tendinopathy. J Shoulder Elbow Surg. 2016;25(5):723–29. doi: 10.1016/j.jse.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Ooi CC, Schneider ME, Malliaras P, et al. Diagnostic performance of axial-strain sonoelastography in confirming clinically diagnosed Achilles tendinopathy: Comparison with B-mode ultrasound and color Doppler imaging. Ultrasound Med Biol. 2015;41(1):15–25. doi: 10.1016/j.ultrasmedbio.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Ohrndorf S, Backhaus M. Supplement: Advances in sonographic scoring of rheumatoid arthritis. Ann Rheum Dis. 2013;72(Suppl 2):69–75. doi: 10.1136/annrheumdis-2012-202197. [DOI] [PubMed] [Google Scholar]
- 24.Backhaus M, Ohrndorf S, Kellner H, et al. Evaluation of a novel 7-joint ultrasound score in daily rheumatologic practise: A pilot project. Arthritis Rheum. 2009;61(9):1194–201. doi: 10.1002/art.24646. [DOI] [PubMed] [Google Scholar]
- 25.Kunkel GA, Cannon GW, Clegg DO. Combined structural and synovial assessment for improved ultrasound discrimination of rheumatoid, osteoarthritic, and normal joints: A pilot study. Open Rheumatol J. 2012;16:199–206. doi: 10.2174/1874312901206010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stach CM, Bauerle M, Englbrecht M, et al. Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high-resolution computed tomography. Arthritis Rheum. 2010;62:330–39. doi: 10.1002/art.27252. [DOI] [PubMed] [Google Scholar]
- 27.Boutry N, Hachulla E, Flipo RM, et al. MR imaging findings in hands in early rheumatoid arthritis: Comparison with those in systemic lupus erythematosus and primary Sjögren syndrome. Radiology. 2005;236(2):593–600. doi: 10.1148/radiol.2361040844. [DOI] [PubMed] [Google Scholar]
- 28.Szopińska S, Jurik AG, Eshed I, et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin Musculoskelet Radiol. 2015;19:396–411. doi: 10.1055/s-0035-1564696. [DOI] [PubMed] [Google Scholar]
- 29.Schoellnast H, Deutschmann HA, Hermann J, et al. Psoriatic arthritis and rheumatoid arthritis: findings in contrast-enhanced MRI. Am J Roentgenol. 2006;187(2):351–57. doi: 10.2214/AJR.04.1798. [DOI] [PubMed] [Google Scholar]
- 30.Farrant JM, O’Connor PJ, Grainger AJ. Advanced imaging in rheumatoid arthritis. Part: I. Synovitis. Skeletal Radiol. 2007;36(4):269–79. doi: 10.1007/s00256-006-0219-9. [DOI] [PubMed] [Google Scholar]
- 31.Østergaard M, Klarlund M. Importance of timing of post-contrast MRI in rheumatoid arthritis: What happens during the first 60 minutes after IV gadolinium-DTPA? Ann Rheum Dis. 2001;60(11):1050–54. doi: 10.1136/ard.60.11.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Liu X, Du X, Ye Z. Diffusion-weighted MR imaging for assessing synovitis of wrist and hand in patients with rheumatoid arthritis: A feasibility study. Magn Reson Imaging. 2014;32(4):350–53. doi: 10.1016/j.mri.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 33.McQueen FM, Stewart N, Crabbe J, et al. Magnetic resonance imaging of the wrist in early rheumatoid arthritis reveals progression of erosions despite clinical improvement. Ann Rheum Dis. 1999;58(3):156–63. doi: 10.1136/ard.58.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McQueen FM, Benton N, Perry D, et al. Bone edema scored on magnetic resonance imaging scans of the dominant carpus at presentation predicts radiographic joint damage of the hands and feet six years later in patients with rheumatoid arthritis. Arthritis Rheum. 2003;48(7):1814–27. doi: 10.1002/art.11162. [DOI] [PubMed] [Google Scholar]
- 35.Guermazi A, Taouli B, Lynch JA, Peterfy CG. Imaging of bone erosion in rheumatoid arthritis. Semin Musculoskelet Radiol. 2004;8(4):269–85. doi: 10.1055/s-2004-861575. [DOI] [PubMed] [Google Scholar]
- 36.Farrant JM, Grainger AJ, O’Connor PJ. Advanced imaging in rheumatoid arthritis. Part II. Erosions. Skeletal Radiol. 2007;36:381–89. doi: 10.1007/s00256-006-0220-3. [DOI] [PubMed] [Google Scholar]
- 37.Rubens DJ, Blebea JS, Totterman SM, Hooper MM. Rheumatoid arthritis: evaluation of wrist extensor tendons with clinical examination versus MR imaging – a preliminary report. Radiology. 1993;187(3):831–38. doi: 10.1148/radiology.187.3.8497640. [DOI] [PubMed] [Google Scholar]
- 38.Valeri G, Ferrara C, Ercolani P, et al. Tendon involvement in rheumatoid arthritis of the wrist: MRI findings. Skeletal Radiol. 2001;30(3):138–43. doi: 10.1007/s002560100322. [DOI] [PubMed] [Google Scholar]
- 39.McQueen F, Beckley V, Crabbe J, et al. Magnetic resonance imaging evidence of tendinopathy in early rheumatoid arthritis predicts tendon rupture at six years. Arthritis Rheum. 2005;52(3):744–51. doi: 10.1002/art.20947. [DOI] [PubMed] [Google Scholar]
- 40.Kalden-Nemeth D, Grebmeier J, Antoni C, et al. NMR monitoring of rheumatoid arthritis patients receiving anti-TNF-alpha monoclonal antibody therapy. Rheumatol Int. 1997;16:249–55. doi: 10.1007/BF01375657. [DOI] [PubMed] [Google Scholar]
- 41.Ostergaard M, Stoltenberg M, Henriksen O, Lorenzen I. Quantitative assessment of synovial inflammation by dynamic gadolinium-enhanced magnetic resonance imaging. A study of the effect of intra-articular methylprednisolone on the rate of early synovial enhancement. Br J Rheumatol. 1996;35:50–59. doi: 10.1093/rheumatology/35.1.50. [DOI] [PubMed] [Google Scholar]
- 42.Reece RJ, Kraan MC, Radjenovic A, et al. Comparative assessment of leflunomide and methotrexate for the treatment of rheumatoid arthritis, by dynamic enhanced magnetic resonance imaging. Arthritis Rheum. 2002;46:366–72. doi: 10.1002/art.10084. [DOI] [PubMed] [Google Scholar]
- 43.Lee J, Lee SK, Suh JS, et al. Magnetic resonance imaging of the wrist in defining remission of rheumatoid arthritis. J Rheumatol. 1997;24:1303–8. [PubMed] [Google Scholar]
- 44.Gardener JC, Aierhut ML, Gardner GC, et al. Vascularity in the RA wrist at 1–2 months following Initiation of anti-TNFalpha and methotrexate therapy. Rheumatology. 2003;47:S131. [Google Scholar]
- 45.Colebatch AN, Edwards CJ, Østergaard M, et al. EULAR recommendations for the use of imaging of the joints in the clinical management of rheumatoid arthritis. Ann Rheum Dis. 2013;72(6):804–14. doi: 10.1136/annrheumdis-2012-203158. [DOI] [PubMed] [Google Scholar]
- 46.Mountz JM, Alavi A, Mountz JD. Emerging optical and nuclear medicine imaging methods in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8:719–28. doi: 10.1038/nrrheum.2012.148. [DOI] [PubMed] [Google Scholar]