Abstract
The aim of this study was to perform a follow-up evaluation of the Streptococcus mutans and Streptococcus sobrinus colonization profile of children's oral cavities, which included the pattern of vertical transmission from mother to child, genotypic diversity, and stability of the strains. The subjects were 16 mother-child pairs, who were monitored for 20 months. Samples of saliva, tongue dorsum, alveolar ridge mucosa, and dental plaque from the children were collected bimonthly. Saliva samples from the mothers were also collected. After isolation and identification, the arbitrarily primed PCR method was performed for the genotypic characterization of S. mutans (968 isolates) and S. sobrinus (111 isolates). At the time the strains were acquired, the children harbored one to four distinct genotypes of S. mutans and only one genotype of S. sobrinus. Although S. mutans prevalence and genotypic diversity were greater than those of S. sobrinus, the presence of matching genotypes of S. mutans and S. sobrinus was similar (in 81.25 and 83.33% of mother-child pairs, respectively), suggesting vertical transmission for both species. This longitudinal study showed an increase in genotypic diversity of S. mutans in the oral cavity during the follow-up period: most of the initially acquired genotypes persisted, normally those genotypes transmitted by the mother, and some were lost during follow-up; new strains were also acquired. In conclusion, S. mutans and S. sobrinus genotypes acquired from maternal or alternative sources may show effective persistence in the oral cavity and/or transitory detection in the children's mouths, reflecting the continuous development of oral microbiota in children.
Dental caries is a transmissible infectious disease that still represents a significant public health problem in many countries (27, 30).
Streptococcus mutans and Streptococcus sobrinus are the main microorganisms associated with caries in humans (12, 25). Studies using phenotyping and/or genotyping methods suggest that the mother is the major primary source of infection for children who carry S. mutans and/or S. sobrinus strains (1, 7, 8, 18, 21, 35). However, detection of genotypes that are not found in children's mothers or other family members indicates that S. mutans and/or S. sobrinus may also be acquired from other sources (7, 8, 18, 28).
It has been observed that children harbor one to five distinct genotypes of mutans streptococci (MS) at different ages (1, 4, 8, 11, 18, 19, 20, 28). Genotypes of MS have a fairly high degree of consistency in children aged 3 to 8 years, indicating persistence of some strains (2, 7). The conservation of strains in a mother-child pair over a 3-year interval has also been demonstrated (4). Nevertheless, little is known about the stability of the genotypes detected at the time of initial acquisition.
Information on the stability of colonization with this microorganism in children could help elucidate the natural history of the development of caries. Thus, this paper presents a follow-up evaluation from the profiles done at the the time of initial acquisition of the S. mutans and S. sobrinus colonization in the healthy infant oral cavity, which included the pattern of vertical transmission from mother to child, genotypic diversity, and stability of the strains.
MATERIALS AND METHODS
Study population.
The Institutional Ethical Committee in Research at the Piracicaba School of Dentistry, State University of Campinas, previously approved the study protocol. The study was carried out with MS strains isolated from mother-child pairs. Sixteen mother-infant pairs were selected for this study, based on following previously described criteria (36): (i) absence of erupted teeth in the child during the first collection visit, (ii) absence of more than four missing posterior teeth and no more than one missing tooth per quadrant, (iii) absence of any chronic diseases or daily intake of medicines, and (iv) completion of the 20-month follow-up period. The average age of the edentulous children during the first sample was 5.9 ± 1.5 months. The mothers' age averaged 28.6 ± 5.3 years. The mothers' average decayed, missing, and filled teeth (DMF-T) score was 10.4 ± 6.3. The children were a nursery school population of 6 boys and 10 girls from a low economic level, who stayed at the nursery school five days a week, 10 h per day. The drinking water in Piracicaba (São Paulo, Brazil) has been fluoridated since 1971 (0.7 ppm F).
Sampling.
For every infant, at least 1 h after the last food intake, samples from different sites (i.e., saliva in the sublingual area, tongue dorsum, the mucosa of maxillary and mandibular dental ridges, and dental plaque, when teeth were present) were obtained by swabbing each site for 10 s. The samples were obtained bimonthly for an average of 20 months. Saliva from each mother was collected (6) once, at least 1 month after persistent colonization by MS in their children. Persistent colonization was defined as the presence of two consecutive positive cultures for MS from any sample site; following initial detection, a second confirmatory sample was obtained, usually 2 weeks after the primary positive sample.
Microbiological processing.
The samples were dispersed from swabs and diluted in saline solution (0.9% NaCl). Aliquots of each dilution were inoculated, in duplicate, in plates containing mitis salivarius bacitracin agar (10). The plates were incubated at 37°C for 48 h in an atmosphere of 10% CO2. Thereafter, a stereoscopic microscope was used to verify the presence of CFU resembling MS.
If present, up to 30 typical morphotype colonies (9) of culture plates of samples from children and 15 colonies of culture plates of samples from mothers were collected, seeded in brain heart infusion broth, and incubated for 24 h (10% CO2; 37°C). An aliquot of 1.5 ml of bacterial culture was submitted to bacterial identification, and other aliquots were stored in glycerol stock (−70°C). The bacterial identification of MS was performed by PCR.
Extraction of chromosomal DNA.
DNA from a total of 1,181 suspect MS isolates was extracted with a simpler DNA preparation in which the cells from a brain heart infusion broth were washed and boiled for 10 min with TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]), the debris was pelleted, and the supernatant was diluted for identification by PCR and genotyping by arbitrarily primed PCR (AP-PCR), modified from a previously described method (13).
PCR identification.
The DNA samples from MS isolates were identified by PCR with previously described primers (29): S. mutans primer pair GTFB-F and GTFB-R (517 bp) and S. sobrinus primer pair GTFI-F and GTFI-R (712 bp). The PCR was processed in 25 μl of a reaction mixture containing 1× reaction buffer Taq polymerase, 1.5 mM MgCl2, 0.1 mM deoxynucleoside triphosphate, 0.2 μM each primer, 1.5 U of Taq DNA polymerase, and 2.5 μl of DNA sample. All PCR reagents were obtained from Invitrogen-Life Technology, São Paulo, Brazil. Besides the samples, positive and negative controls were used in each experiment: purified genomic DNA from S. mutans (CCT 3440) and S. sobrinus (ATCC 27607) were used as positive controls, and distilled water was used as a negative control. PCR amplification was performed with a GeneAmp PCR System 2400 (Perkin-Elmer-Applied Biosystems) under thermal conditions described previously (29). The PCR products were separated by electrophoresis in 1.5% agarose gels in Tris-borate-EDTA running buffer (pH 8.0). The DNA was stained with 0.5 μg of ethidium bromide/ml and visualized under UV illumination.
AP-PCR typing.
DNA from a total of 1,079 MS isolates (968 S. mutans isolates and 111 S. sobrinus isolates) was used for genotyping. The AP-PCR fingerprinting was performed with two primers, OPA-02 (5′-TGCCGAGCTG-3′) and OPA-13 (5′-CAGCACCCAC-3′) (22). The AP-PCR was processed as previously described (31). Amplification products were analyzed electrophoretically with a 1.5% agarose gel with Tris-borate-EDTA running buffer (pH 8.0). A 100-bp DNA ladder was included in each gel. Ethidium bromide-stained gel images were captured with the LISCAP digital imaging system (Image Master VDS; Amersham Pharmacia Biotech). Molecular sizes for each band were computed and analyzed with SigmaGel gel analysis software (Jandel Scientific Corporation). NTSYS-pc software, version 1.7 (Applied Biostatistics, Inc.) was used to generate similarity dendrograms (Dice coefficient, >95%; unweighted pair group method with arithmetic mean) was used to show the S. mutans and S. sobrinus genotypes.
Data analysis.
The chi-square test and Fisher's exact test were used to compare the proportions among various category groups. Spearman correlation analysis was used to verify the association between numbers of erupted teeth and number of genotypes detected. The levels of MS in CFU/ml of mother's saliva were converted into logarithms (log10) for statistical analysis. The Student t test was used to compare the numbers of CFU/ml between mothers in matching and nonmatching groups (the matching group was defined as a subset of mother-child pairs in which the same genotype was detected in the mother and in her child; the nonmatching group was defined as a subset of mother-child pairs in which the same genotype was not shared by the mother and child). Pearson correlation analysis was used to correlate the amount of MS in saliva of the mothers with their DMF-T scores. The limit for rejection of the null hypothesis was set at 5%.
RESULTS
A total of 1,181 strains recovered from the oral cavities of 16 mother-child pairs were submitted to identification by PCR method. A total of 102 (8.64%) strains remained unclassified, probably other species of the MS group. The isolation frequency of MS revealed distinct individual differences between the species characterized (Table 1). S. mutans was detected in all individuals enrolled in the study. However, only six mothers harbored both S. mutans and S. sobrinus in their saliva, and S. sobrinus was not detected in four children in any of the samples tested. Of the 951 isolates from all children's samples, 773 (90.2%) strains were identified as S. mutans and 84 (9.8%) strains were identified as S. sobrinus. Comparable values were found in the mothers' saliva, with the isolation frequency of S. mutans (87.8%) higher than that of S. sobrinus (12.2%).
TABLE 1.
The isolated and identified strains from 16 mother-child pairs
| Subject | No. of strains isolated
|
Total | |
|---|---|---|---|
| S. mutans | S. sobrinus | ||
| Mother | 195 | 27 | 222 |
| Child | 773 | 84 | 857 |
| Total | 968 | 111 | 1,079 |
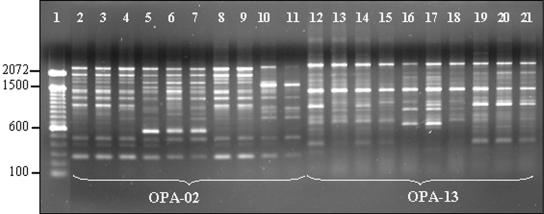
The AP-PCR fingerprinting profile analysis with primers OPA-02 and OPA-13 (Fig. 1) showed the distinct genotypes of S. mutans and S. sobrinus detected in each mother-child pair (Tables 2 and 3).
FIG. 1.
AP-PCR fingerprinting profiles of strains isolated from mother-child pair 8. Lane 1, 100-bp DNA ladder; lanes 2 to 11, primer OPA-02; lanes 12 to 21, primer OPA-13. The data presented in lanes 2 to 11 and the data presented in lanes 12 to 21 are for the same respective strains. The analysis of molecular sizes for each band from both primers provides five distinct S. mutans genotypes: A (lanes 2 and 13), B (lanes 3 and 13, 4 and 14, and 8 and 18), C (lanes 5 and 15, 6 and 16, 7 and 17), D (lanes 9 and 19), and E (lanes 10 and 20, and 11 and 21).
TABLE 2.
Distribution and number of S. mutans genotypes isolated from oral cavities of 16 mother-child pairs at time of S. mutans initial acquisition and during follow-up
| Group (%)a | Pair no. (gender)b |
S. mutans genotype(s) in childreng
|
S. mutans genotype(s) in mothersg | |
|---|---|---|---|---|
| Initial acquisition, ageh | Followupi | |||
| Matching (81.25) | 2 (F)c | 3 (A,B,C), 15.4 | 2 (A*, D) | 1 (A) |
| 3 (F) | 3 (A, B, C), 16.8 | 1 (B*, D) | 1 (A) | |
| 4 (F) | 1 (A), 17.6 | 1 (A*) | 4 (A, B, C, D) | |
| 5 (F) | 2 (A, B), 14.2 | 2 (A*, B*) | 2 (A, B) | |
| 7 (M)d | 1 (C), 17.8 | 2 (A, D, E) | 2 (A, B) | |
| 8 (F)ef | 2 (A, B), 12.8 | 5 (A*, B*, C, D, E) | 3 (A, B, C) | |
| 10 (M)d | 1 (C), 11.6 | 2 (A, D) | 2 (A, B) | |
| 12 (M) | 1 (A), 16.6 | 1 (E) | 4 (A, B, C, D) | |
| 13 (F) | 1 (A), 14.5 | 1 (A*) | 2 (A, B) | |
| 17 (F) | 1 (A), 16.6 | — | 1 (A) | |
| 20 (F) | 2 (A, C), 13.4 | 2 (A*, C) | 2 (A, B) | |
| 23 (F)f | 2 (A, B), 16.1 | 4 (C, D, E, F) | 2 (A, B) | |
| 24 (M) | 2 (A, C), 18 | — | 1 (A) | |
| Nonmatching (18.75) | 18 (M) | 4 (F, G, H, J), 14.6 | 2 (I, L) | 5 (A, B, C, D, E) |
| 28 (F) | 1 (C), 18.4 | 5 (D, E, F, G, H) | 2 (A, B) | |
| 32 (M) | 3 (C, D, E), 12.6 | 2 (D*, E*) | 2 (A, B) | |
| Total of distinct genotypes detected | 30 | 22 | 36 | |
| Total of genotypes transmitted | 13 | 3 | 16 | |
Fisher's exact test showed statistical difference among the number of children who acquired or did not acquire S. mutans genotypes from their mothers (P = 0.0011).
Number of pairs = 16. Gender, gender of child; M, male; F, female.
The child in pair 2 is the only child who showed incipient caries on the buccal surfaces of the upper anterior incisors. No other child showed caries lesions during this study.
The children from pairs 7 and 10 did not acquire genotypes from their mothers at the time of initial acquisition. However, these children acquired S. mutans from their mothers in later samplings.
The child in pair 8 acquired one more genotype from his mother in later samplings, in addition to genotypes acquired the at the time of initial acquisition.
The children from pairs 8 and 23 acquired two genotypes from their mothers at the time of initial acquisition.
Letters in parentheses indicate genotypes detected in the subjects. Genotypes indicated by the same letters are identical only within that mother-child pair and bear no relation to types indicated by the same letter in any other mother-child pair.
Age is indicated in months.
Asterisks indicate S. mutans genotypes considered to be stable genotypes. [—], S. mutans was not isolated in posterior samplings.
TABLE 3.
Distribution and number of S. sobrinus genotypes isolated from oral cavities of 16 mother-child pairs at time of S. sobrinus initial acquisition and during follow-up
| Group (%)-a | Pair no. (gender)b |
S. sobrinus genotype(s) in childrenc
|
S. sobrinus genotype(s) in mothersc | |
|---|---|---|---|---|
| Initial acquisition, aged | Followupe | |||
| Matching (83.33) | 4 (F) | 1 (A), 23.5 | 1 (A)* | 1 (A) |
| 7 (M) | 1 (A), 21.6 | 2 (B; C) | 1 (A) | |
| 17 (F) | 1 (A), 21.2 | — | 1 (A) | |
| 20 (F) | 1 (A), 13.4 | 1 (B) | 1 (A) | |
| 28 (F) | 1 (A), 18.4 | — | 1 (A) | |
| No matching (16.67) | 2 (F) | 1 (D), 21.5 | 1 (E) | 3 (A, B, C) |
| Children of mothers not colonized by S. sobrinus | 3 (F) | 1 (A), 16.8 | 1 (A)* | — |
| 5 (F) | — | — | — | |
| 8 (F) | 1 (A), 12.8 | — | — | |
| 10 (M) | 1 (A), 15.1 | 1 (A)* | — | |
| 12 (M) | 1 (A), 16.6 | — | — | |
| 13 (F) | — | — | — | |
| 18 (M) | 1 (A), 14.6 | — | — | |
| 23 (F) | 1 (A), 16.1 | — | — | |
| 24 (M) | — | — | — | |
| 32 (M) | — | — | — | |
| Total of distinct genotypes detected | 12 | 4 | 8 | |
| Total of genotypes transmitted | 5 | 0 | 5 | |
Fisher's exact test did not show statistical difference among the number of children who acquired and did not acquire S. sobrinus genotypes from theirs mothers (P = 0.0801).
Number of pairs = 16. Gender, gender of child; M, male; F, female.
Letters in parentheses indicate genotypes detected in the subjects. Genotypes indicated by the same letters are identical only within that mother-child pair and bear no relation to types indicated by the same letter in any other mother-child pair.
Age is indicated in months.
Asterisks indicate three children (pairs 3, 4, and 10) in whom the same S. sobrinus genotype (A) was detected twice, with no stability. —, S. sobrinus was not isolated in posterior samplings; this bacterium was not detected in the mothers either.
S. mutans and S. sobrinus acquisition.
At the time of the first acquisition of S. mutans and S. sobrinus, the average age of the children tested was 15.4 ± 2.12 months for S. mutans and 17.63 ± 3.56 months for S. sobrinus. The times when each child acquired S. mutans and S. sobrinus are shown in Tables 2 and 3. S. sobrinus was detected in 12 of 16 children, and 7 of 12 children acquired both S. sobrinus and S. mutans at same time, while for 5 of 12 children, S. sobrinus was detected later than S. mutans.
S. mutans and S. sobrinus transmission.
The mother-child pairs were separated into one of two subgroups, matching or nonmatching.
The S. mutans genotypes detected in the mother-child pairs (Table 2) showed S. mutans vertical transmission occurrence in the matching subgroup corresponding to 81.25% (13 of 16) of the mother-child pairs. At the time of initial acquisition, or during the 20-month period, one to three S. mutans genotypes were transmitted from the mothers to their children.
The occurrence of S. sobrinus vertical transmission in the matching subgroup is shown in Table 3. From the time of initial acquisition and throughout the follow-up period, only one S. sobrinus genotype was transmitted from the mothers to their children in five of six mother-child pairs (83.33%) in which S. sobrinus were detected in the mother's oral cavity. An additional six children were infected by S. sobrinus, but this bacterium was not detected in their mothers.
There was no statistical difference (Student t test; P = 0.0694) between the mothers' saliva levels of MS (CFU/ml), in either matching or nonmatching groups (Table 4). The amount of MS in saliva samples of the mothers did not correlate significantly with their DMF-T scores (r = −0.03; P = 0.90; Pearson correlation analysis) (Table 4).
TABLE 4.
Mothers' DMF-T Score and MS salivary levels
| Mother | DMF-T score | MS (CFU/ml of saliva) |
|---|---|---|
| 2 | 8 | 4.59 × 105 |
| 3 | 6 | 2.11 × 106 |
| 4 | 14 | 2.38 × 106 |
| 5 | 4 | 3.78 × 105 |
| 7 | 6 | 8.34 × 106 |
| 8 | 1 | 7.77 × 105 |
| 10 | 24 | 3.02 × 106 |
| 12 | 16 | 1.06 × 107 |
| 13 | 11 | 8.51 × 105 |
| 17 | 20 | 1.60 × 104 |
| 18 | 3 | 7.08 × 104 |
| 20 | 13 | 4.50 × 105 |
| 23 | 12 | 6.03 × 105 |
| 24 | 5 | 1.29 × 106 |
| 28 | 13 | 3.65 × 105 |
| 32 | 10 | 1.42 × 105 |
Vertical transmission of both S. mutans and S. sobrinus occurred in four mother-child pairs (pairs 4, 7, 17, and 20). Additionally, in two mother-child pairs (pairs 18 and 32) neither S. mutans nor S. sobrinus was transmitted. In pair 28, only S. sobrinus was shared.
Considering that some genotypes detected in children are not found in their mothers, we decided to compare the S. mutans and/or S. sobrinus genotypes detected in children who were in the same classroom in nursery school: children in pairs 4 and 5; in pairs 2, 3 and 7; in pairs 12 and 13; and in pairs 24, 28, and 32. No horizontal transmission was observed, since no matching of S. mutans and/or S. sobrinus genotypes was observed among children attending the same nursery (data not shown).
S. mutans and S. sobrinus genotypic diversity.
At the time of acquisition, the children harbored one to four distinct genotypes of S. mutans; among these children, only one (the child in pair 18) harbored four distinct genotypes, which were not acquired from the mother. The larger number of children (seven) acquired only one S. mutans genotype initially, five children acquired two genotypes, and three children acquired three genotypes (Table 2). Among the 773 S. mutans strains isolated from the children's oral cavities, 52 distinct genotypes were detected: 30 genotypes at the time of initial acquisition and 22 during the follow-up period. Among the 195 S. mutans strains isolated from the mothers' oral cavities, 36 distinct genotypes were found, 16 of which were transmitted to the mothers' children.
Table 3 shows that only one S. sobrinus genotype was detected at the time of initial acquisition. Among the 84 S. sobrinus strains isolated from the children's oral cavities (12 children), 16 distinct genotypes were detected: 12 genotypes were detected at the time of initial acquisition in 12 children, and 4 distinct genotypes were detected during the follow-up period in 3 children. During the follow-up period, only one child (9) was infected by three distinct genotypes, and two children (from pairs 2 and 20) harbored two distinct genotypes. Among the 27 S. sobrinus strains isolated from six mothers' oral cavities, 8 distinct genotypes were found.
Genotypic diversity in S. mutans and S. sobrinus in the four sampling sites (saliva, tongue dorsum, alveolar ridge mucosa, and dental plaque) from children's oral cavities and the number of strains isolated in each site are shown in Table 5. Dental plaque was an important site, with the larger number of S. mutans (35 of 52) and S. sobrinus (10 of 16) genotypes and strains isolated.
TABLE 5.
S. mutans and S. sobrinus genotypic diversity in sampling sites from children's oral cavities and number of strains isolated in each site
| Sitea | No. of genotypes detected (%)b
|
No. of strains analyzed (%)c
|
||
|---|---|---|---|---|
| S. mutansd | S. sobrinuse | S. mutansf | S. sobrinusg | |
| S | 29 (55.77) | 6 (37.5) | 194 (25.09) | 11 (13.10) |
| T | 25 (48.07) | 8 (50) | 165 (21.34) | 17 (20.24) |
| G | 24 (46.15) | 5 (31.25) | 142 (18.37) | 6 (7.14) |
| D | 35 (67.30) | 10 (62.5) | 272 (35.19) | 50 (59.52) |
Abbreviations: S, saliva, T, dorsum of tongue; G, alveolar ridge mucosa; D, dental plaque.
In all, 52 distinct S. mutans gentoypes and 16 distinct S. sobrinus genotypes were detected in children's oral cavities.
The total number of S. mutans and S. sobrinus strains analyzed from children's oral cavities was 773 and 84, respectively.
Fisher's exact test showed a statistical difference among the numbers of S. mutans gentoypes from dental plaque and alveolar ridge mucosa (P = 0.0478). Comparison among genotypes from other sites (S [times] D, T [times] D, S [times] T, S [times] G, and T [times] G) did not show a significant statistical difference (P = 0.05).
Fisher's exact test did not show a statistical difference among the genotypes from each site (P = 0.005).
The chi-square test showed significant statistical difference among the numbers of strains isolated from dental plaque and the other three sites (D [times] S, D [times] T, and D [times] G) (P = 0.0000). Also, significant statistical difference occurs among the numbers of strains isolated from saliva and alveolar ridge mucosa (P = 0.0017). Comparison among the other sites (S [times] T and T [times] G) using the same statistical test did not show a significant statistical difference (P = 0.05).
The chi-square test showed significant statistical difference among the numbers of strains isolated from dental plaque and the other three sites (D × S, D × T, and D × G) (P = 0.0000). Also, significant statistical difference occurs among the numbers of strains isolated from saliva and alveolar ridge mucosa (P = 0.0017). Comparison among the other sites (S × T, S × G, and T × G) using the same statistical test did not show a significant statistical difference (P = 0.05).
There was no association between the number of erupted teeth and number of genotypes detected (Spearman correlation analysis; P > 0.05) (data not shown).
S. mutans and S. sobrinus genotypic stability.
The genotypes considered stable genotypes were the ones detected until the final sampling: first, they were detected at the time of initial acquisition and then in the subsequent confirmatory sample collection. The genotypes detected during the follow-up period, from initial colonization to the time when the child was 26 months old, but not until the final sampling, and the genotypes detected at the time of initial acquisition, which were not detected during the follow-up period, were classified as transitory genotypes.
Eight children showed S. mutans genotypic stability. From the 30 distinct S. mutans genotypes detected at the time of initial acquisition and in the subsequent confirmatory sample collections, 19 (63.33%) were transitory and 11 (36.67%) were stable. Interestingly, eight (72.73%) of the stable genotypes were those transmitted by mothers to their children. An effective stability of S. sobrinus genotypes was not detected in any children infected by this bacterium.
DISCUSSION
The identification of the source of MS transmission is essential to the development of strategies for the prevention of dental caries. The presence of matching genotypes of S. mutans and S. sobrinus during the follow-up period was similar in 81.25% (13 of 16) and 83.33% (5 of 6) mother-child pairs, respectively, suggesting vertical transmission. This rate was higher than previously reported in several studies, with ranges between 24 and 71% (1, 4, 8, 18, 20, 21, 24). On the other hand, these results are very similar to a longitudinal study of intrafamilial MS ribotypes that showed at least one common S. mutans ribotype among strains recovered from 14 mother-child pairs; among those pairs, 12 pairs (85%) shared the same ribotype; and among eight maternal strains of S. sobrinus ribotyped, six strains (75%) were detected in the child (14).
Furthermore, it is important to emphasize that the children spent about 10 h per day in nursery schools and that the rates of S. mutans and S. sobrinus maternal transmission differ from those found in Chinese children (45%) (24) and American children (71%) (21) attending day care nurseries. This contradiction is probably because the source of transmission is very variable, depending on behavior: the frequency of salivary contact between mother and child and a mother's salivary MS level mutans streptococci (16) and cultural and environmental conditions of the population studied (8, 18, 24). Furthermore, variability in transmission can be associated with children's individual susceptibilities, including the period defined as a window of infectivity (5), which was reported to be earlier in Brazilian children (27); the number of erupted teeth (3, 5); the emergence of molars (5); the presence of enamel hypoplasia (23); sucrose consumption (27); the action of unspecific factors of the salivary and mucosal immune systems (21); and immunological conditions in children (32).
Data about S. sobrinus colonization, diversity, and transmission are rare. Kozai et al. (18) found that S. sobrinus transmission occurred in 50% of Chinese families; Köhler et al. (14) detected 75% genotypic similarity between mother and child. In our study, S. sobrinus was present in 75% of children; however, only 37.5% of the mothers were colonized by S. sobrinus, and vertical transmission occurred in 83.33% of mother-child pairs positive for this species. In contrast, in another study with Swedish families (8), only one mother and four fathers among 11 families harbored S. sobrinus, and this species was not transmitted to the children. However, it is interesting that six children harbored this species, at least transitorily; in addition, their mothers were negative for S. sobrinus.
In our study, a total of 52 distinct genotypes were detected in the children and only 16 of them were transmitted by mothers (13 of 16 mothers); thus, there are two questions. (i) Where did the other genotypes detected in the children come from? (ii) Why were only 16 of the 36 genotypes detected in the mothers transmitted to their children? We can try to answer these questions as follows. (i) The other genotypes were acquired from alternative transmission sources. The first report of S. mutans-matching genotypes in children from unrelated families and attending the same nursery that showed evidence for horizontal transmission reported that one genotype existed between two Brazilian children (28). Also, a study of Japanese children attending a day nursery showed that transmission occurred between the children; it was found that 6 of 39 children shared the same strain type of MS (34). We also compared the S. mutans and/or S. sobrinus genotypes detected in children who were in the same classroom in the nursery school, but we did not find similar genotypes shared by children attending the same institution. Therefore, more studies with a large population sample may be necessary to investigate horizontal transmission in this kind of institution. (ii) Different clonal types of MS detected within the oral cavity of one subject can have different phenotypic properties; some strains express virulence-associated characteristics that promote their colonization and survival (11).
At the time of acquisition, the children harbored one to four distinct genotypes of S. mutans and only one genotype of S. sobrinus; these results are in agreement with previous genotypic diversity studies that showed children harboring one to five distinct genotypes (1, 4, 8, 11, 18, 19, 20, 28). The genotype diversity represented by strains from the mothers and the fact that only some of these strains (around 45%) can be successfully transmitted to their children's oral cavities suggest a distinct capacity of colonization and/or infectivity by these different genotypes. Also, the high genotypic diversity present in S. mutans and S. sobrinus strains can result in oral cavity colonization by strains with distinct virulence (15, 26), as well as distinct sources (28).
Among the four sites sampled (saliva, tongue dorsum, alveolar ridge mucosa, and dental plaque), the dental plaque was an important site where the majority of S. mutans and S. sobrinus genotypes and strains were isolated. However, this site was not representative of all genotypes detected in the children's oral cavities. Tanner et al. (33) found that species detection from tooth and tongue samples was highly associated, with most species detected more frequently from tongue samples than from tooth samples in children younger than 18 months of age. This agrees with our finding that several genotypes were also present in the tongue, suggesting that the tongue is a potential microbial reservoir. In addition, there was no association between numbers of erupted teeth and the number of S. mutans or S. sobrinus genotypes detected, in agreement with data from Mattos-Graner et al. (28).
Once established, bacterial species tend to persist in children's oral cavities (5, 17). It has been shown that genotypes of MS have a fairly high degree of consistency in children aged 3 to 8 years, indicating persistence of the strains (2, 7); the conservation of strains in a mother-child pair over a 3-year interval has also been demonstrated (4). Likewise, the stability of MS over a 16-year period has recently been demonstrated (14). Nevertheless, the turnover rate at the genotypic level seems to be high in children; the extended interval between the times of the first and second samplings in those studies may be sufficient to allow reinfection of the child's oral cavity by the same genotype or ribotype, not reflecting the persistence throughout the observation period of of the same genotype or ribotype that was initially acquired.
This longitudinal epidemiological study with bimonthly sampling provides data about the persistence or lack of persistence of S. mutans and/or S. sobrinus genotypes from the time of their initial acquisition. We detected S. mutans genotypic stability in samples from half of the children; on the other hand, we were unable to detect stability for S. sobrinus genotypes. Again, it is important to emphasize that the genotypes with no stability (transitory genotypes) might not have been detected, due to a bias imposed by the detectable limits of the culture method used in this study.
A tendency toward effective stability of genotypes transmitted by mothers also occurred in this research. This fact can tentatively be explained by data presented in the literature that suggest facility in genotypes and/or strains acquired from the mothers, because the passively acquired immunoglobulins from the mothers influence the infant's selection of which bacteria are allowed to persist. Which bacteria are eliminated is a problem that remains to be solved (21, 32).
This longitudinal study showed an increase in the genotypic diversity of S. mutans and S. sobrinus genotypes in the oral cavities of children. Some genotypes persisted or were lost during follow-up; new strains were also acquired. In conclusion, S. mutans and S. sobrinus genotypes acquired from maternal or alternative sources may show effective persistence in the oral cavity and/or transitory detection in the children's mouth, reflecting the continuous development of the oral microbiota in children.
Acknowledgments
The following were sources of support for this work: CAPES and FAPESP (grant 99/12819-5, 00/08350-0).
REFERENCES
- 1.Alaluusua, S., J. Mättö, L. Grönroos, S. Innitä, H. Torkko, S. Asikainen, H. Jousimies-Somer, and M. Saarela. 1996. Oral colonization by more than one clonal type of mutans streptococcus in children with nursing-bottle dental caries. Arch. Oral Biol. 41:167-173. [DOI] [PubMed] [Google Scholar]
- 2.Alaluusua, S., J. S. Alaluusua, J. Karjalainen, M. Saarela, T. Holttinen, M. Kallio, P. Hölttä, H. Torkko, P. Relander, and S. Asikainen. 1994. The demonstration by ribotyping of the stability of oral Streptococcus mutans infection over 5 to 7 years in children. Arch. Oral Biol. 39:467-471. [DOI] [PubMed] [Google Scholar]
- 3.Berkowitz, R. J., H. V. Jordan, and G. White. 1975. The early establishment of Streptococcus mutans in the mouths of infants. Arch. Oral Biol. 20:171-174. [DOI] [PubMed] [Google Scholar]
- 4.Caufield, P. W., and T. M. Walker. 1989. Genetic diversity within Streptococcus mutans evident from chromosomal DNA restriction fragment polymorphisms. J. Clin. Microbiol. 27:274-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caufield, P. W., G. R. Cutter, and A. P. Dasanayake. 1993. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J. Dent. Res. 72:37-45. [DOI] [PubMed] [Google Scholar]
- 6.Dasanayake, A. P., P. W. Caufield, G. R. Cutter, J. M. Roseman, and B. Köhler. 1995. Differences in the detection and enumeration of mutans streptococci due to differences in methods. Arch. Oral Biol. 40:345-351. [DOI] [PubMed] [Google Scholar]
- 7.Emanuelsson, I. R., and E. Thorqvist. 2000. Genotypes of mutans streptococci tend to persist in their host for several years. Caries Res. 34:133-139. [DOI] [PubMed] [Google Scholar]
- 8.Emanuelsson, I. R., Y. Li, and D. Bratthall. 1998. Genotyping shows differents strains of mutans streptococci between father and child and within parental pairs in Swedish families. Oral Microbiol. Immunol. 13:271-277. [DOI] [PubMed] [Google Scholar]
- 9.Emilson, C. G. 1983. Prevalence of Streptococcus mutans with different colonial morphologies in human plaque and saliva. Scand. J. Dent. Res. 91:26-32. [DOI] [PubMed] [Google Scholar]
- 10.Gold, O. C., H. V. Jordan, and J. van Houte. 1973. A selective medium for S. mutans. Arch. Oral Biol. 18:1356-1364. [DOI] [PubMed] [Google Scholar]
- 11.Grönroos, L., M. Saarela, J. Mättö, U. Tanner-Salo, A. Vuorela, and S. Alaluusua. 1998. Mutacin production by Streptococcus mutans may promote transmission of bacteria mother to child. Infect. Immun. 66:2595-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamada, S., and H. D. Slade. 1980. Biology, immunology and cariogenicity of Streptococcus mutans. Microbiol. Rev. 44:331-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein, M. I., and R. B. Gonçalves. 2003. Detection of Tannerella forsythensis (Bacteroides forsythus) and Porphyromonas gingivalis by polymerase chain reaction in subjects with different periodontal status. J. Periodontol. 74:798-802. [DOI] [PubMed] [Google Scholar]
- 14.Köhler, B., A. B. Lundberg, D. Birkhed, and P. N. Papapanou. 2003. Longitudinal study of intrafamilial mutans streptococci ribotypes. Eur. J. Oral Sci. 111:383-389. [DOI] [PubMed] [Google Scholar]
- 15.Köhler, B., and B. Krasse. 1990. Human strains of mutans streptococci show different cariogenic potential in the hamster model. Oral Microbiol. Immunol. 5:177-180. [DOI] [PubMed] [Google Scholar]
- 16.Köhler, B., and I. Andréen. 1984. Influence of caries-preventive measures in mothers on cariogenic bacteria and caries experience in their children. Arch. Oral Biol. 39:907-911. [DOI] [PubMed] [Google Scholar]
- 17.Könönen, E., A. Kanervo, A. Takala, S. Asikainen, and H. Jousimies-Somer. 1999. Establishment of oral anaerobes during the first year of life. J. Dent. Res. 78:1634-1639. [DOI] [PubMed] [Google Scholar]
- 18.Kozai, K., R. Nakayama, U. Tedjosasongko, S. Kuwahara, J. Suzuki, M. Okada, and N. Nagasaka. 1999. Intrafamilial distribution of mutans streptococci in Japanese families and possibility of father-to-child transmission. Microbiol. Immunol. 43:99-106. [DOI] [PubMed] [Google Scholar]
- 19.Kreulen, C. M., H. J. de Soet, R. Hogeveen, and J. S. J. Veerkamp. 1997. Streptococcus mutans in children using nursing bottles. ASDC J. Dent. Child. 64:107-111. [PubMed] [Google Scholar]
- 20.Kulkarni, G. V., K. H. Chan, and J. Sandham. 1989. An investigation into the use of restriction endonuclease analysis for the study of transmission of mutans streptococci. J. Dent. Res. 68:1155-1161. [DOI] [PubMed] [Google Scholar]
- 21.Li, Y., and P. W. Caufield. 1995. The fidelity of initial acquisition of mutans streptococci by infants from their mothers. J. Dent. Res. 74:681-685. [DOI] [PubMed] [Google Scholar]
- 22.Li, Y., and P. W. Caufield. 1998. Arbitrarily primed chain reaction fingerprinting for the genotypic identification of mutans streptococci from humans. Oral Microbiol. Immunol. 13:17-22. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y., J. M. Navia, and P. W. Caufield. 1994. Colonization by mutans streptococci in the mouth of 3- and 4-year-old Chinese children with or without enamel hiplopasia. Arch. Oral Biol. 39:1057-1062. [DOI] [PubMed] [Google Scholar]
- 24.Li, Y., W. Wang, and P. W. Caufield. 2000. The fidelity of mutans streptococci transmission and caries status correlates with breast-feeding experience among Chinese families. Caries Res. 34:123-132. [DOI] [PubMed] [Google Scholar]
- 25.Loesche, W. L. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattos-Graner, R. O., D. J. Smith, W. F. King, and M. P. Mayer. 2000. Water-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12- to 30-month-old children. J. Dent. Res. 79:1371-1377. [DOI] [PubMed] [Google Scholar]
- 27.Mattos-Graner, R. O., F. Zelante, R. C. Line, and M. P. Mayer. 1998. Association between caries prevalence and clinical, microbiological and dietary variables in 1.0 to 2.5-year-old Brazilian children. Caries Res. 32:319-323. [DOI] [PubMed] [Google Scholar]
- 28.Mattos-Graner, R. O., Y. Li, P. W. Caufield, M. Duncan, and D. J. Smith. 2001. Genotypic diversity of mutans streptococci in Brazilian nursery children suggests horizontal transmission. J. Clin. Microbiol. 39:2313-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oho, T., Y. Yamashita, Y. Shimazaki, M. Kushiyama, and T. Koga. 2000. Simple and rapid detection of Streptococcus mutans and Streptococcus sobrinus in human saliva by polymerase chain reaction. Oral Microbiol. Immunol. 15:258-262. [DOI] [PubMed] [Google Scholar]
- 30.Ramos-Gomez, F. J., J. A. Weintraub, S. A. Gansky, C. I. Hoover, and J. D. Featherstone. 2002. Bacterial, behavioral, and environmental factors associated with early childhood caries. J. Clin. Pediatr. Dent. 26:165-173. [DOI] [PubMed] [Google Scholar]
- 31.Saarela, M., J. Hannula, J. Mättö, S. Asikainen, and S. Alaluusua. 1996. Typing of mutans streptococci by arbitrarily primed polymerase chain reaction. Arch. Oral Biol. 41:821-826. [DOI] [PubMed] [Google Scholar]
- 32.Smith, D. J., W. F. King, H. Akita, and M. A. Taubman. 1998. Association of salivary immunoglobulin A antibody and initial mutans streptococcal infection. Oral Microbiol. Immunol. 13:278-285. [DOI] [PubMed] [Google Scholar]
- 33.Tanner, A. C. R., P. M. Milgrom, R. Kent, Jr., S. A. Mokeem, R. C. Page, C. A. Riedt, P. Weinstein, and J. Bruss. 2002. The microbiota of young children from tooth and tongue samples. J. Dent. Res. 80:2060-2065. [DOI] [PubMed] [Google Scholar]
- 34.Tedjosasongko, U., and K. Kozai. 2002. Initial acquisition and transmission of mutans streptococci in children at day nursery. ASDC J. Dent. Child. 69:284-288, 234-235. [PubMed] [Google Scholar]
- 35.van Houte, J., G. Gibbs, and C. Butera. 1982. Oral flora of children with “nursing bottle caries.” J. Dent. Res. 61:382-385. [DOI] [PubMed] [Google Scholar]
- 36.van Loveren, C., J. F. Buijs, and J. M. ten Cate. 2000. Similarity of bacteriocin activity profiles of mutans streptococci within the family when the children acquire the strains after the age of 5. Caries Res. 34:481-485. [DOI] [PubMed] [Google Scholar]