Abstract
Background
Dendritic cells (DCs) could be used as potential cellular adjuvant for the production of specific tumor vaccines.
Objectives
Our study was aimed to evaluate the safety and efficacy of autologous pulsed DC vaccine in advanced hepatocellular carcinoma (HCC) patients in comparison with supportive treatment.
Methods
Thirty patients with advanced HCC not suitable for radical or loco-regional therapies were enrolled. Patients were divided into 2 groups, group I consisted of 15 patients received I.D vaccination with mature autologous DCs pulsed ex vivo with a liver tumor cell line lysate. Group II (control group, no. 15) received supportive treatment. One hundred and 4 ml of venous blood were obtained from each patient to generate DCs. DCs were identified by CD80, CD83, CD86 and HLA-DR expressions using flow cytometry. Follow up at 3, and 6 months post injection by clinical, radiological and laboratory assessment was done.
Results
Improvement in overall survival was observed. Partial radiological response was obtained in 2 patients (13.3 %), stable course in 9 patients (60 %) and 4 patients (26.7 %) showed progressive disease (died at 4 months post-injection). Both CD8+ T cells and serum interferon gamma were elevated after DCs injection.
Conclusion
Autologous DC vaccination in advanced HCC patients is safe and well tolerate.
Keywords: DCs, HCC, Adjuvant immunotherapy
Introduction
Hepatocellular carcinoma (HCC), which accounts for 80–90 % of primary liver cancer, is characterized by a very poor prognosis and is associated with high mortality (Parkin et al. 2001). Anticipant chronic viral hepatitis B and C infections are the most important risk factors, responsible for 80 % of HCC worldwide. Advanced HCC is an aggressive illness, a significant proportion of patients are not suitable for conventional therapeutic modalities for HCC such as resection, transplantation, radiofrequency or trans-arterial chemoembolization (Schwartz et al. 2007). This conveys the thinking for implication of alternative treatment options like tumor immunotherapy.
Dendritic cells (DCs) vaccination is one of the new modalities of immunotherapy in cancer that posses less frequency of complications, minimal invasive procedures and very good tolerability by most of the patients. DCs are potent APCs that play a pivotal role in antitumor host responses. Therapeutic cancer vaccines target the cellular arm of the immune system to initiate a cytotoxic T-lymphocyte (CTL) response against tumor-associated antigens (Lilah and Morris 2007). They can process and present antigens to T cells and hence generation of tumor-specific CD8+ T cells, which recognize peptides derived from intracellular proteins, and presented on MHC class I complexes then effective antigen-specific T cell immunity will be induced. DCs could be pulsed with tumor-specific antigens (TSA) that able to stimulate antitumor immune responses. Given these properties, DCs have attracted considerable attention as potential cellular adjuvant for the production of specific tumor vaccines (Lee 2010). The use of DC vaccination have been tried with different protocols in various advanced cancers such as melanoma, renal cell cancer, prostate cancer, colon cancer and others (Fields et al. 1998; Nestle et al. 1998; Thurner et al. 1999). Our study was aimed to evaluate the safety and efficacy of autologous pulsed DC vaccine in advanced HCC patients in comparison with supportive treatment.
Subjects and methods
Subjects
Thirty patients with advanced HCC not amenable to curative resection, transplantation, local ablation or chemoembolization were eligible for inclusion in this study. Diagnosis of HCC was confirmed by a known predisposing chronic liver disease (anticipant liver cirrhosis due to hepatitis C or hepatitis B virus infection with Child grade was B or C and Model of End—Stage Liver Disease (MELD) score >8) and elevated AFP, and characteristic imaging. The thirty patients were divided randomly into 2 groups. Group I included 15 patients who received autologous DC vaccine. They were 9 males and 6 females; their age ranged from 49 to 79 years with mean value 62.66 ± 7.37 years. Group II, control group, included 15 ages- and sex-matched HCC patients who maintained on their ordinary liver supportive treatment including albumin, fresh plasma and vitamin K. They were 11 males and 4 females; their age ranged from 45 to 72 years with mean value 59.8 ± 9.57 years. Patients were selected from Kasr El-Aini outpatient clinics during a period from 2009 to 2010. All patients were subjected to the following: detailed history taken, complete clinical examination with special emphasis on abdominal examination and Laboratory investigation included complete blood count (CBC), liver function tests, prothrombin concentration (PC), alpha fetoprotein (AFP) assay, kidney function tests and radiological investigation including abdominal ultrasonography (US) with Doppler and duplex study of portal system and triphasic computed tomography scan (CT). Written informed consent was obtained from the vaccinated group before enrollment into the study.
Methods
Sampling
One hundred and 4 ml of venous blood were obtained from each patient by a sterile vein-puncture and divided as follows: 10 ml were put into each of 10 sterile tubes containing preservative-free heparin for MNC separations and cell culture. Two ml were placed in a sterile vacutainer containing EDTA as an anticoagulant for performing CBC and flowcytometric detection of CD83 and CD8. Finally, 2 ml were placed in sterile plain vacutainer for performing interferon gamma (IFN-γ) by enzyme-linked immunosorbent assay (ELISA) technique.
Separation of mononuclear cells
The mononuclear cell (MNC) layer was separated under aseptic conditions using a Ficoll-Hypaque Density Gradient (density 1.077, Sigma, St. Louis, MO) by centrifugation at 1,800 rpm for 20 min then immunophenotyped by flowcytometric detection of CD14 for enumeration of proportion of monocyte in MNCs, CD14 revealed ~20 % expression.
Dendritic cells generation in GMP condition according to the method described by Iwashita et al. (2003)
Preparation of liquid culture system
In brief, the mononuclear cells were adjusted at 1 × 106 cell/ml then suspended in liquid culture media that contained 45 ml EMEM, 10 % inactivated Fetal Calf Serum, 500 μl Penicillin/Streptomycin and 500 μl amphotericin B (all from Sigma). MNCs were differentiated into DCs by adding the growth factors GM-CSF (100 μ/ml) and IL-4 (20 ng/ml) (R&D System, Minneapolis, MN) to the suspension then incubated at 37 °C under 5 % CO2 for 6 days; the medium was changed every 2–3 days.
Pulsing of DCs with tumor lysates
Tumor cell lysate was prepared according to a previously published method (Zhang et al. 2005). Immature DCs were recovered on day 6 and pulsed with tumor lysate prepared from the HepG2 cell line for 3 h. Briefly, HepG2 cell line was propagated, undergoing repeated 4 cycles of freezing and thawing, passed through 0.22-μm filters, and finally, protein content was adjusted to be 120 μg/ml, and aliquots were stored at −80 °C.
Morphological identification of DCs
On day 6, the cultured MNCs were evaluated for morphological changes using cytospin preparation stained with Giemsa. Cells having a large size, copious gray cytoplasm and long cytoplasmic processes were identified as DCs (Fig. 1). Viability of generated DCs was assessed by trypan blue dye exclusion test (Merck, Darmstadt, Germany). Eighty percent viable DCs were obtained.
Fig. 1.
Light microscopy of DCs. a Generation of inactivated DCs; MNCs were separated using a Ficoll-Hypaque density gradient; then, MNCs were differentiated into DCs by suspending them in liquid culture medium containing EMEM, 10 % FCS, penicillin (100 U/ml), streptomycin (10 mg/ml), amphotericin B and gentamycin and adding the growth factors rhIL-4 (20 ng/ml) and rhGM-CSF (100 U/ml) to the suspension pulsed with HepG2 cell line lysate in sterile tissue culture tubes that were incubated at 37 °C in 5 % CO2 for 7 days. The medium was changed every 2–3 days. b Generation of activated DCs was done on day 6 by adding 10 ng/ml TNF α; the cultured MNCs were evaluated for morphological changes using cytospin preparation stained with Giemsa. Cells having a large size, copious gray cytoplasm and long cytoplasmic processes were identified as mature DCs
Maturation step
On day 6, the DCs were activated by adding Tumor necrosis factor α (TNF-α; 10 ng/ml; R&D System).
Flowcytometric identification of DCs
To insure that the DCs were mature, they were immunophenotyped by flowcytometry using monoclonal antibodies against HLA-DR, CD80, CD83 and CD86. DCs (1 × 106 cells/100 μl) were suspended in phosphate-buffered saline (PBS, Sigma) containing 1 % BSA and were stained with fluorochrome-conjugated mAbs (anti-mouse mAanti-HLA-DR, mAanti CD80, mAanti CD83 and mAanti CD86) (BD Biosciences, San Diego, CA) for 20 min on ice. Flowcytometric analysis was performed using FACSCaliber (BD Biosciences) equipped with CellQuest Software (BD Biosciences). Ten thousand cells were passed in front of the laser for each sample. Each sample was analyzed in duplicate. A cutoff value at 20 % was set to categorize samples as positive. Markers of DC maturation (HLA-DR, CD80, CD83 and CD86) were ~90 % expressions.
Injection of DCs
Once the mature DCs were obtained on day 7 (~90 % purity and 80 % viability), they were trypsinized, and about 20 × 106 DCs were suspended in 1 ml of sterile saline in an insulin syringe. Then, the patient was given five intradermal (ID) injections on each of their forearms. Each patient remained under observation in an inpatient for approximately 18 h for monitoring of any side effects. All patients tolerated the DC injections well with no serious adverse events reported. The most common adverse events were injection site rash, mild fever and headache. There were no changes in vital signs. Laboratory parameters including liver function tests, kidney function tests and full blood count were unchanged.
Follow-up by monitoring immunological response
(a) Flowcytometric assessment of the percentage of CD8+ T cells before and 1 month after injection of the DCs was done. The DC suspension (1 × 106 cells/100 μl) was mixed with 10 μl fluorochrome-conjugated anti-CD8 (anti-mouse mAanti-CD8) (BD Biosciences). The mixture was incubated in the dark at 4 °C for 30 min followed by washing with PBS containing 2 % BSA. A non-reactive mAb of the same isotype and conjugated with the same fluorochrome was used as a negative control. Flowcytometric analysis was performed using FACSCaliber (BD Biosciences) equipped with CellQuest Software. Ten thousand cells were passed in front of the laser for each sample. Each sample was analyzed in duplicate. A cutoff value at 20 % was set to categorize samples as positive. (b) Measurement of interferon gamma (IFN-γ) was done before and 1 month after DCs injection using colorimetric enzyme-linked immunosorbent assay (ELISA) for quantification of human IFN-γ in serum (BD Biosciences Pharmingen, NJ, USA). In brief, blood sample was withdrawn from the patients of group I before and 1 month after DCs injection. Samples and standards are incubated in wells coated with antibodies recognizing human IFN-γ; then, assay was performed according to the manufacturer’s guidance.
Statistical analysis of data
Quantitative values are expressed as the mean ± SD and were compared using a Student’s t test. Qualitative data were compared using a χ2 test. P < 0.05 was considered to be significant while P < 0.01 was considered to be highly significant. SPSS version 15 was used.
Declaration of ethics
This study was approved by the review board of Kasr Al-Aini hospital, and written informed consent was obtained from all patients according to Helsinki guidelines of research ethics.
Results
Demographic, baseline clinical and laboratory data of both groups, vaccinated patients (group I) and control group (group II), are listed in Table 1.
Table 1.
Comparison between the 2 groups regarding demographic, baseline clinical and laboratory data
| Item | Group (I) n = 15 | Group (II) n = 15 | P value | Significance |
|---|---|---|---|---|
| Age | ||||
| Range | 49–79 | 45–72 | 0.77 | NS |
| Mean ± SD | 62.66 ± 7.37 | 59.80 ± 9.57 | ||
| Sex | ||||
| Males (No./%) | 9/60 % | 11/73.3 % | 0.669 | NS |
| Females (No./%) | 6/40 % | 4/26.7 % | ||
| Clinical data | ||||
| Jaundice | ||||
| Present | 11/73.3 % | 6/40 % | 0.139 | NS |
| Absent | 4/26.7 % | 9/60 % | ||
| Ascites | ||||
| Present | 15/100 % | 15/100 % | 0.08 | NS |
| Moderate-sever | 14/93.3 % | 9/60 % | ||
| Mild | 1/6.7 % | 6/40 % | ||
| Absent | 0/0 % | 0/0 % | ||
| LL edema | ||||
| Present | 15/100 % | 11/73.3 % | 0.09 | NS |
| Absent | 0/0 % | 4/26.7 % | ||
| Encephalopathy | ||||
| Present | 8/53.3 % | 9/60 % | 0.9 | NS |
| Absent | 7/46.7 % | 6/40 % | ||
| Child grade | ||||
| A | 0/0 % | 0/0 % | 0.9 | NS |
| B | 15/100 % | 14/93.3 % | ||
| C | 0/0 % | 1/6.7 % | ||
| Laboratory data (all data were expressed as mean ± SD apart from GGT and AFP were expressed as median and range) | ||||
| AST(10–42 U/l) | 76.93 ± 43.15 | 77.80 ± 34.97 | >0.05 | NS |
| ALT (10–42 U/l) | 50.6 ± 29.82 | 61.33 ± 35.56 | >0.05 | NS |
| Alk. Phosph (40–120 U/l) | 209.69 ± 76.51 | 328.63 ± 82.94 | <0.05 | S |
| GGT (0–65 U/l) | 120 (3.10–480.00) | 51 (33.00–480.00) | >0.05 | NS |
| Albumin (3.5–5 gm/dl) | 2.79 ± 0.43 | 2.90 ± 0.58 | >0.05 | NS |
| Bilirubin (0.1–1.0 gm/dl) | 2.19 ± 1.1–0 | 2.54 ± 1.32 | >0.05 | NS |
| PC % (80–120 %) | 69.1 ± 12.68 | 60.53 ± 18.47 | >0.05 | NS |
| AFP (0–10 ng/dl) | 20 (2.00–1,000.00) | 18 (4.10–902.00) | 0.3 | NS |
| MELD score | 12.25 ± 1.84 | 12.2 ± 2.70 | >0.05 | NS |
| Urea (10–50 mg/dl) | 54.45 ± 25.68 | 41.41 ± 9.97 | >0.05 | NS |
| Creatinine (0.4–1.0 mg/dl) | 0.84 ± 0.18 | 1.10 ± 0.34 | <0.05 | S |
| Hb % (12–16 gm/dl) | 11.69 ± 1.35 | 11.76 ± 1.17 | >0.05 | NS |
| RBCs (4–6 × 106/ml) | 3.79 ± 0.54 | 4.07 ± 0.608 | >0.05 | NS |
| WBCs (4–11 × 103/ml) | 4.73 ± 1.69 | 5.60 ± 4.85 | >0.05 | NS |
| Platelets (150–450 × 103/ml) | 87.40 ± 47.63 | 111.41 ± 65.32 | >0.05 | NS |
S significant, NS non significant, HS highly significant
There was a highly statistical significant difference between expression of CD80, CD83, CD86 and HLA-DR before and after culture with higher expression at the end of the culture (P < 0.002). This indicated successful culturing of MNC and its differentiation into DCs (Table 2).
Table 2.
Comparison between the CD80, CD83, CD86 and HLA-DR % before and after DC culture
| Item | Group I (No. 15) | P value | Significance | |
|---|---|---|---|---|
| Before culture | After culture | |||
|
CD 80 % Mean ± SD |
5.50 ± 1.51 | 90.30 ± 5.85 | <0.001 | HS |
|
CD 83 % Mean ± SD |
3.80 ± 0.91 | 95.00 ± 6.05 | <0.001 | HS |
|
CD 86 % Mean ± SD |
4.02 ± 0.98 | 92.65 ± 7.22 | <0.001 | HS |
|
HLA-DR Mean ± SD |
8.82 ± 2.86 | 89.95 ± 4.85 | <0.001 | HS |
There was no statistically significant difference between both groups regarding demographic (age and sex) and baseline clinical data including jaundice, ascites, LL edema, encephalopathy and Child Pugh grade, P > 0.05. Regarding the baseline laboratory data, there was a statistically significant difference between the two groups regarding alkaline phosphatase and serum creatinine, P < 0.05. Meanwhile, there was no statistically significant difference regarding other laboratory data. Regarding the radiological parameters, as shown by US, 9 cases had one large lesion (60 %), 5 cases had multiple lesions (>3) (33.3 %) and one case had 2 lesions (6.7 %) compared to 5 patients with 1 lesion, 1 patient with 2 lesions and 9 patients with multiple lesions. In group I, there was a vascular invasion (portal vein thrombosis) in 6 patients (40 %) in comparison with 7 patients (46.7 %) in group II.
On monitoring of vaccinated patients serologically 1 month after DCs injection, there was a highly statistical significant difference between mean value of CD8+ T cells % before and after DCs injection with higher expression after injection (P < 0.001); however, IFN-γ did not elicit an equivalent result (Table 3).
Table 3.
Comparison between mean value of both CD8 % and interferon gamma level before and after injection of DC vaccine
| Item | Group I (No. 15) | P value | Significance | |
|---|---|---|---|---|
| Before injection | After injection | |||
|
CD 8+ % Mean ± SD |
13.10 ± 3.48 | 40.60 ± 8.90 | <0.001 | HS |
|
Interferon gamma level Mean ± SD |
24.98 ± 7.45 | 29.05 ± 8.28 | 0.168 | NS |
Three months after DC injection, group I was re-evaluated, clinically and through laboratory testing and radiological examination. Regarding clinical parameters, 10 patients had clinically evident jaundice (66.7 %), in comparison with 11 patients (73.3) before starting treatment. In group II, all patients had clinically evident jaundice. In group I, 14 patients had moderate ascites (93.3 %) and one patient who had mild ascites (6.7 %) compared to 13 patients who had severe ascites (86.6 %) and 2 patients who had mild ascites (13.3) in group II. All patients had LL edema in both groups. In group I, 9 patients (60 %) had encephalopathy compared to 12 patients (80 %) in group II. In group I, 2 patients progressed to Child’s grade C, (13.3 %) and 13 patients were stationary grade B (83.7 %) while 3 patients in group II progressed to Child’s grade C (20 %). Regarding laboratory data, there was a significant difference between the 2 groups as regards albumin, which was 2.7 ± 0.36 in group I in comparison with 2.3 ± 0.38 in group II, bilirubin which was 2.4 ± 0.98 in group I in comparison with 3.9 ± 2.0 in group II, alkaline phosphatase which was 120.9 ± 64.4 in group I compared to 192.6 ± 48 in group II (P < 0.05). also there was a highly statistical difference regarding creatinine which was 1.05 ± 0.38 in group I in comparison with 2.04 ± 0.77 in group II (P < 0.001) and AFP, the median was 30 in group I in comparison with 95 in group II (P = 0.002) (Table 4). Regarding radiological parameters, 9 patients with 1 lesion, 3 patients with 2 lesions and 3 patients with multiple lesions, in group I compared to 2 patients with 1 lesion, 2 patients with 2 lesions and 11 patients with multiple lesions in group II. Vascular invasion (PV thrombosis) was evident in 6 patients (40 %) in group I compared to 10 patients (66.7 %) in group II (Table 5).
Table 4.
Comparison between the 2 groups regarding clinical and laboratory data after 3 months of DC vaccination
| Item | Group (I) n = 15 | Group (II) n = 15 | P value | Significance |
|---|---|---|---|---|
| Clinical data | ||||
| Jaundice | ||||
| Present | 10/66.7 % | 15/100 % | 0.042 | S |
| Absent | 5/33.3 % | 0/0 % | ||
| Ascites | ||||
| Present | 15/100 % | 15/100 % | 1.0 | NS |
| Moderate-sever | 14/93.3 % | 13/86.7 % | ||
| Mild | 1/6.6 % | 2/13.3 % | ||
| Absent | 0/0 % | 0/0 % | ||
| LL edema | ||||
| Present | 15/100 % | 15/100 % | 1.0 | NS |
| Absent | 0/0 % | 0/0 % | ||
| Encephalopathy | ||||
| Present | 9/60 % | 12/80 % | 0.427 | NS |
| Absent | 6/40 % | 3/20 % | ||
| Child grade | ||||
| A | 0/0 % | 0/0 % | 1.0 | NS |
| B | 13/86.7 % | 12/80 % | ||
| C | 2/13.3 % | 3/20 % | ||
| Laboratory data (all data were expressed as mean ± SD apart from GGT and AFP were expressed as median and range) | ||||
| AST(10–42 U/l) | 68.57 ± 30.78 | 72.33 ± 30.48 | >0.05 | NS |
| ALT (10–42U/l) | 44.92 ± 21.66 | 53.6 ± 22.87 | >0.05 | NS |
| Alk. Phosph (40–120 U/l) | 120.91 ± 64.43 | 192.6 ± 48.91 | <0.05 | S |
| GGT (0–65U/l) | 116 (43.00–450.00) | 180 (111.00–500.00) | >0.05 | NS |
| Albumin (3.5–5 gm/dl) | 2.71 ± 0.36 | 2.35 ± 0.38 | <0.05 | S |
| Bilirubin (0.1–1.0 gm/dl) | 2.43 ± 0.98 | 3.96 ± 2.00 | <0.05 | S |
| PC % (80–120 %) | 59.42 ± 11.87 | 56.85 ± 16.96 | >0.05 | NS |
| AFP (0–10 ng/dl) | 30 (4.60–1,000.00) | 95 (5.10–3,150.00) | <0.001 | HS |
| Urea (10–50 mg/dl) | 54.45 ± 25.68 | 41.41 ± 9.97 | >0.05 | NS |
| Creatinine (0.4–1.0 mg/dl) | 1.05 ± 0.38 | 2.04 ± 0.77 | <0.001 | HS |
| Hb % (12–16 gm/dl) | 11.35 ± 1.08 | 12 ± 2.66 | >0.05 | NS |
| RBCs (4–6 × 106/ml) | 3.60 ± 0.40 | 4.01 ± 0.76 | >0.05 | NS |
| WBCs (4–11 × 103/ml) | 4.8 ± 1.79 | 4.43 ± 1.59 | >0.05 | NS |
| Platelets (150–450 × 103/ml) | 94.78 ± 61.27 | 115 ± 94.71 | >0.05 | NS |
S significant, NS non significant, HS highly significant
Table 5.
Comparison between 2 groups regarding radiological findings
| Items | Group I | Group II | ||||
|---|---|---|---|---|---|---|
| Baseline | After 3 months | After 6 months | Baseline | After 3 months | After 6 months | |
| Radiological parameters (No. %) | ||||||
| One lesion | 9; 60 % | 9; 60 % | 9; 60 % | 5; 33.3 % | 2; 13.3 % | 1; 6.7 % |
| Two lesions | 1; 6.7 % | 3; 20 % | 4; 26.7 % | 1; 6.7 % | 2; 13.3 % | 1; 6.7 % |
| Multiple lesions (>3) | 5; 33.3 % | 3; 20 % | 2; 13.3 % | 9; 60 % | 11; 73.3 % | 13; 86.6 % |
| Vascular invasion (No. %) | 6; 40 % | 6; 40 % | 8; 53.3 % | 7; 46.7 % | 10; 66.7 % | 15; 100 % |
After 6 months, regarding clinical parameters, all patients in group I and II had clinically evident jaundice, moderate-severe ascites and LL edema (100 %). In group I, 6 patients (66.6 %) had encephalopathy, compared to 14 patients who developed encephalopathy (83.3 %) in group II. In group I, 9 patients progressed to Child’s grade C (53.3 %) and 6 patients were stationary grade B (46.7 %) while all patients in group II progressed to child’s grade C (100 %). Regarding laboratory data, there was a significant difference in laboratory parameters between the 2 groups regarding albumin, which was 2.63 ± 0.26 in group I in comparison with 2.06 ± 0.22 in group II (P < 0.001), bilirubin, which was 3.12 ± 1.23 in group I in comparison with 4.31 ± 0.98 in group II (P < 0.05), alkaline phosphatase which was 129.25 ± 51.3 in group I compared to 192.6 ± 48 in group II (P < 0.05), creatinine which was 1.1 ± 0.5 in group I in comparison with 2.98 ± 0.84 in group II with highly significant difference between the 2 groups (P < 0.001), PC which was 57.44 ± 9.72 in group I in comparison with 41.66 ± 7.5 in group II with highly significant difference between the 2 groups and AFP, the median, which was 21.5 in group I in comparison with 120 in group II with significant difference between the 2 groups (P < 0.001) (Table 6). Regarding radiological parameters, 9 patients with 1 lesion, 4 patients with 2 lesions and 2 patients with multiple lesions in group I compared to 1 patient with 1 lesion, 1 patient with 2 lesions and 13 patients with multiple lesions in group II. Regarding the tumor response detected radiologically, CT revealed partial response in 2 patients (13.3 %), stable course in 9 patients (60 %) and progressive disease in 4 patients (26.7 %) and were not complete the follow-up period (died at 4 months post-injection) in group I compared to group II; there were stable course in 2 patients (13.3 %) and progressive course in 13 patients (86.6 %). Vascular invasion was detected in 8 patients (53.3 %) in group I compared to 15 patients (100 %) in group II (Table 5 and Fig. 2).
Table 6.
Comparison between the 2 groups regarding clinical and laboratory data after 6 months of DC vaccination
| Item | Group (I) n = 15 | Group (II) n = 15 | P value | Significance |
|---|---|---|---|---|
| Clinical data | ||||
| Jaundice | ||||
| Present | 15/100 % | 15/100 % | 1.0 | NS |
| Absent | 0/0 % | 0/0 % | ||
| Ascites | ||||
| Present | 15/100 % | 15/100 % | 1.0 | NS |
| Moderate-sever | 9/53.3 % | 15/100 % | ||
| Mild | 6/46.7 % | 0/0 % | ||
| Absent | 0/0 % | 0/0 % | ||
| LL edema | ||||
| Present | 15/100 % | 15/100 % | 1.0 | NS |
| Absent | 0/0 % | 0/0 % | ||
| Encephalopathy | ||||
| Present | 6/66.7 % | 14/83.3 % | 0.60 | NS |
| Absent | 9/33.3 % | 1/16.7 % | ||
| Child grade | ||||
| A | 0/0 % | 0/0 % | 0.103 | NS |
| B | 6/46.7 % | 0/0 % | ||
| C | 9/53.3 % | 15/100 % | ||
| Laboratory data (all data were expressed as mean ± SD apart from GGT and AFP were expressed as median and range) | ||||
| AST(10–42U/L) | 64 ± 27.32 | 53.5 ± 16.41 | >0.05 | NS |
| ALT (10–42U/L) | 41 ± 22.54 | 30.83 ± 7.83 | >0.05 | NS |
| Alk. Phosph (40–120 U/L) | 129.25 ± 51.30 | 192.6 ± 48.91 | <0.05 | S |
| GGT (0–65U/L) | 125 (28.00–390.00) | 130 (111.00–480.00) | >0.05 | NS |
| Albumin (3.5–5 gm/dl | 2.63 ± 0.26 | 2.06 ± 0.22 | <0.001 | HS |
| Bilirubin (0.1–1.0 gm/dl | 3.12 ± 1.23 | 4.31 ± 0.98 | <0.05 | S |
| PC % (80–120 %) | 57.44 ± 9.72 | 41.66 ± 7.52 | <0.001 | HS |
| AFP (0–10 ng/dl) | 21.5 (6.20–1,000.00) | 120 (12.00–6,036.00) | <0.001 | HS |
| Urea (10–50 mg/dl) | 54.45 ± 25.68 | 41.41 ± 9.97 | >0.05 | NS |
| Creatinine (0.4–1.0 mg/dl) | 1.1 ± 0.50 | 2.98 ± 0.84 | <0.001 | HS |
| Hb % (12–16 gm/dl) | 11.35 ± 1.58 | 12.00 ± 2.66 | >0.05 | NS |
| RBCs (4–6 × 106/ml) | 3.66 ± 0.43 | 2.90 ± 0.58 | >0.05 | NS |
| WBCs (4–11 × 103/ml) | 5.78 ± 2.31 | 2.54 ± 1.32 | >0.05 | NS |
| Platelets (150–450 × 103/ml) | 94.78 ± 61.27 | 115 ± 94.71 | >0.05 | NS |
S Significant, NS non significant, HS highly significant
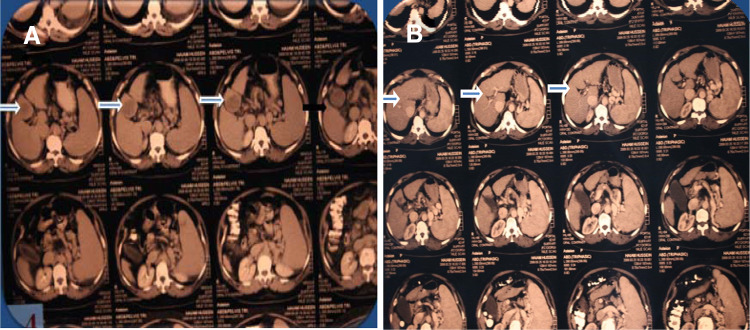
Fig. 2.
a CT scan before DC vaccine showed solitary lesion of HCC in one patient, b CT after DC vaccine showed disappearance of the lesion after 6 months
The median survival time in group I patients receiving DC vaccine was 7 months, (with a mean value of 9.8 ± 7.8 months) compared to 4 months in group II (with a mean value of 5.2 ± 2.6 months) (P = 0.008).
Discussion
Improvements in cancer treatment have been renewed in the last decade. The efficacy of anti-tumor vaccines including DC vaccination has been proven in a variety of animal models and clinical trials (Zhou et al. 2011; Nakamoto and Kaneko 2010). DCs from HCC patients can be transduced using an AFP expressing adenovirus in order to stimulate AFP-specific immune responses (Gonzalez-Carmona et al. 2006). In addition, DCs loaded with RNA cells from HepG2 tumor cells were also able to generate anti-HCC T cells (Zhang et al. 2005). Loading of DCs with Hsp70-peptide complexes derived from human HCC cells resulted in maturation of DCs which in turn stimulated proliferation of autologous HCC-specific cytotoxic T lymphocytes (CTLs) (Wang et al. 2005). Intratumoral injection of DCs in HCC patients relies on the ability of DCs to capture antigens from the tumor cells and transport them to draining lymph nodes, where the tumor antigen is presented to T cells (Kumagi et al. 2005; Chi et al. 2005). In addition, trials using DCs pulsed with autologous HCC tumors or tumor cell lines have just been initiated (Palmer et al. 2005).
Our work aimed to investigate a new modality “DC vaccine” in treatment of advanced HCC in Egypt where increasing numbers of HCC patients are evident, with a minimal treatment options. In the present work, 30 patients with advanced HCC were included: 15 received DC vaccine and 15 were followed while on their usual supportive therapy (control). The identification of dominant TAAs in HCC remains an open challenge due to their ability to stimulate therapeutic antitumor immune responses. In our study, pulsing of DCs with tumor cell lysate prepared from HepG2 cell line was done. This makes it a possible candidate as an antigenic target for anti-HCC immunotherapy. Butterfield et al. (2006) confirmed this possibility by the detection of AFP-specific CD8+ cell responses in HCC patients undergoing AFP-peptides vaccination. These results are promising because they further demonstrate the possibility to break tolerance to a self-protein-like AFP. However, many tumor-specific T cell responses are probably restricted to individual patients because of the known genetic heterogeneity of HCC.
Our study showed generation of DCs from all patients with a highly statistical significant difference of CD83 % before and after culture (P = 0.008). This is consistent with the study done by Lechmann et al. (2003) who said that up-regulation of co-stimulatory molecules such as CD80, CD86 and CD40 and expression of CD83 occur upon DC maturation. This also agrees with Katharina et al. (2003) who obtained 61 % positivity of CD83 after differentiation of immature DCs into mature DCs. Most patients developed lymphadenopathy after vaccination. This may be due to migration of the DCs to the lymph nodes which caused proliferation of lymphocytes leading to their enlargement. This finding is supported by Mullins et al. (2003) who suggested that SC or ID vaccination leads to DC migration to lymph nodes. Morse et al. (1999) reported that DCs injected intravenously initially localized in the lungs and then redistributed to the liver, spleen, bone marrow, but apparently not to the lymph nodes, while DCs injected intradermally were migrated to regional lymph nodes. De Vries et al. (2003) also said that most human studies have proven that SC or intradermally administered mature DCs are found indeed in draining lymph nodes.
By the end of the follow-up period (6 months), there was a significant improvement in liver functions in the group received DC vaccine including serum bilirubin, serum albumin and PC. These findings may be explained by tumor regression or stabilization of disease course that prevents further deterioration in hepatic functions. Other clinical parameters as ascites, LL edema and jaundice showed no significant difference between the two groups which may be explained by a low number of cases and advanced disease stage. Also, there was a highly statistical significant difference between the 2 groups regarding serum creatinine level which also reflect the delayed development of hepatorenal syndrome, one of the most important complications of liver cell failure and cause of death among HCC patients. Chi et al. (2005) studied 14 patients who received 8 sessions of radiotherapy followed by 1–2 doses of immature DCs intratumorally. Toxicity was clinically insignificant. Three patients had a reduced serum AFP level, and 8 of 10 patients had AFP-specific immunologic improvements. Furthermore, our results showed that there was partial radiological response in 2 patients (13.4 %), stationary course in 4 patients (26.6 %) and progressive disease in 9 patients (60 %) in group I treated by DC vaccination, compared to stationary course in 2 patients (13.3 %) and progressive disease in 13 patients (86.6 %) in group II (control). Our results showed some difference from the work done by Lee et al. (2005) who showed that among the 31 patients who received DC vaccine, 4 patients (12.9 %) exhibited partial response, 17 patients (54.8 %) had stable disease and 10 patients (32.3 %) had progressive disease.
In our study, flowcytometric assessment of CD8+ T cells and measurement of IFN-γ secretion by ELISA was tested in sera of patients of group I before and 1 month after treatment with pulsed DCs. There was a highly statistical significant difference between the mean value of CD8+ T cells before and after DCs injection (13.10 ± 3.48 vs. 40.60 ± 8.90, P < 0.01). Regarding IFN-γ level, although there was not a statistical significant difference before and 1 month after DCs injection, the mean value of IFN-γ released by CTLs after vaccination (29.05 ± 8.28 pg/ml) was higher than that released by CTLs before vaccination (23.98 ± 7.45 pg/ml), P > 0.05. This may indicate that DC vaccination helped in boosting the immunity of advanced HCC patients. Palmer et al. (2009) also reported that DC vaccine have induced antigen-specific CTL and Th1 responses in healthy volunteers and in patients with a variety of advanced cancers. In match with our work, Zhang et al. (2005) showed a similar results but on an in vitro model, and they reported that CTLs co-cultured with HepG2 cell line released higher level of IFN-γ than that released by CTLs co-cultured with SMMC7721 and K562 cell lines.
Improvement in survival is one of the main outcomes after treatment with DC vaccination in our patients; a median survival time of 7 months (mean 9.8 ± 7.8 months) was observed compared to 4 months in control group (mean 5.2 ± 2.6 months); this result was compatible with findings of Palmer et al. (2009) who showed that the median survival time was 168 days (6-month and 1-year survival rate was 33 and 11 %, respectively).
The tolerability of DC vaccine was excellent with minimal side effects as low-grade fever and mild bone aches, which lasts only for few days which agree with Palmer et al. (2009) who said that DC vaccine is safe when administered intravenously, with no significant toxicity, and has minimal side effects; also, they reported that despite loading with multiple antigens from a whole cell lysate, no evidence of autoimmunity could be noticed. No significant difference between all hematological parameters was also an indication for safety of DC vaccine.
Finally, we can conclude that DC vaccination showed partial improvement in encephalopathy, liver functions, serum creatinine, survival and radiological outcome in group I of patients. DC vaccine was well tolerated with minimal side effects. However, optimization of the source of DC, the loading/pulsing of DC and even the dose and route of vaccination all are perquisites before we can expect better immunological maneuver to become an adjuvant therapy with other treatment modalities of HCC, for example radiofrequency. DC vaccine could be used as a palliative treatment option in cases of advanced HCC in a larger number of patients where other treatment options are not applicable. Also, it can be used as adjuvant therapy to other treatment modalities as TACE and radiofrequency ablation in patients with better general condition and earlier stages of HCC.
Acknowledgments
This study was supported in part by grants from Kasr Al-Aini Hospital. We also thank El-Ansary private laboratories and our patients for their willing participation in our research.
Conflict of interest
The authors declare no conflicts of interest.
Abbreviations
- AFP
Alfa feto protein
- BM
Bone marrow
- CTL
Cytotoxic T-lymphocyte
- DC
Dendritic cells
- HCC
Hepatocellular carcinoma
- ID
Intradermal
- IFN-γ
Iterferon gamma
- TACE
Transarterial chemoembolization
- TSA
Tumor-specific antigens
Footnotes
ClinicalTrials.gov number: REC 49-2010.
References
- Butterfield LH, Ribas A, Dissette VB et al (2006) A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res 12:2817–2825 [DOI] [PubMed] [Google Scholar]
- Chi KH, Liu SJ, Li CP et al (2005) Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother 28:129–135 [DOI] [PubMed] [Google Scholar]
- De Vries IJ, Krooshoop DJ, Scharenborg NM et al (2003) Effective migration of antigen-pulsed dendritic cells to lymph nodes in melanoma patients is determined by their maturation state. Cancer Res 63:12–17 [PubMed] [Google Scholar]
- Fields RC, Shimizu K, Mule JJ (1998) Murine dendritic cells pulsed with whole tumor lysates mediate potent antitumor immune responses in vitro and in vivo. Proc Natl Acad Sci USA 95:9482–9487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Carmona MA, Marten A, Hoffmann P et al (2006) Patient-derived dendritic cells transduced with an a-fetoprotein-encoding adenovirus and co-cultured with autologous cytokine-induced lymphocytes induce a specific and strong immune response against hepatocellular carcinoma cells. Liver Int 26:369–379 [DOI] [PubMed] [Google Scholar]
- Iwashita Y, Tahara K, Goto S et al (2003) A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother 52:155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katharina T, Thomas MC, Helena H et al (2003) Disparate functions of immature and mature human myeloid dendritic cells: implications for dendritic cell-based vaccines. J Leukoc Biol 74:69–80 [DOI] [PubMed] [Google Scholar]
- Kumagi T, Akbar SM, Horiike N et al (2005) Administration of dendritic cells in cancer nodules in hepatocellular carcinoma. Oncol Rep 14:969–973 [PubMed] [Google Scholar]
- Lechmann M, Berchtold S, Hauber J et al (2003) CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol 23:273–275 [DOI] [PubMed] [Google Scholar]
- Lee DH (2010) Dendritic cells-based vaccine and immune monitoring for hepatocellular carcinoma. Korean J Physiol Pharmacol 14:11–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Wang HC, Hung CF et al (2005) Vaccination of advanced hepatocellular carcinoma patients with tumor lysate-pulsed dendritic cells: a clinical trial. J Immunother 28:496–504 [DOI] [PubMed] [Google Scholar]
- Lilah F, Morris MD, Antoni Ribas MD (2007) Therapeutic cancer vaccines. Surg Oncol Clin N Am 16(4):819–831 [DOI] [PubMed] [Google Scholar]
- Morse MA, Coleman RE, Akabani G, Niehaus N, Coleman D, Lyerly HK (1999) Migration of human dendritic cells after injection in patients with metastatic malignancies. Cancer Res 59:56–58 [PubMed] [Google Scholar]
- Mullins DW, Sheasley SL, Ream RM et al (2003) Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med 198:1023–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto Y, Kaneko S (2010) Dendritic cell-based immunotherapy for hepatocellular carcinoma. Gan To Kagaku Ryoho 37(3):413–416 [PubMed] [Google Scholar]
- Nestle FO, Alijagic S, Gilliet M et al (1998) Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4:328–332 [DOI] [PubMed] [Google Scholar]
- Palmer DH, Hussain SA, Johnson PJ (2005) Gene- and immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther 5:507–523 [DOI] [PubMed] [Google Scholar]
- Palmer DH, Midgley RS, Mirza N et al (2009) A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology 49:124–132 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J et al (2001) Estimating the world cancer burden: globocan 2000. Int J Cancer 94:153–156 [DOI] [PubMed] [Google Scholar]
- Schwartz M, Roayaie S, Konstadoulakis M (2007) Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol 4:424–432 [DOI] [PubMed] [Google Scholar]
- Thurner B, Haendle I, Roder C et al (1999) Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med 190:1669–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XH, Qin Y, Hu MH et al (2005) Dendritic cells pulsed with hsp70-peptide complexes derived from human hepatocellular carcinoma induce specific anti-tumor immune responses. World J Gastroenterol 11:5614–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang H, Liu W et al (2005) Specific antihepatocellular carcinoma T cells generated by dendritic cells pulsed with hepatocellular carcinoma cell line HepG2 total RNA. Cell Immunol 238:61–66 [DOI] [PubMed] [Google Scholar]
- Zhou P, Liang P, Dong B et al (2011) Phase I clinical study of combination therapy with microwave ablation and cellular immunotherapy in hepatocellular carcinoma. Cancer Biol Ther 11(5):450–456 [DOI] [PubMed] [Google Scholar]