Abstract
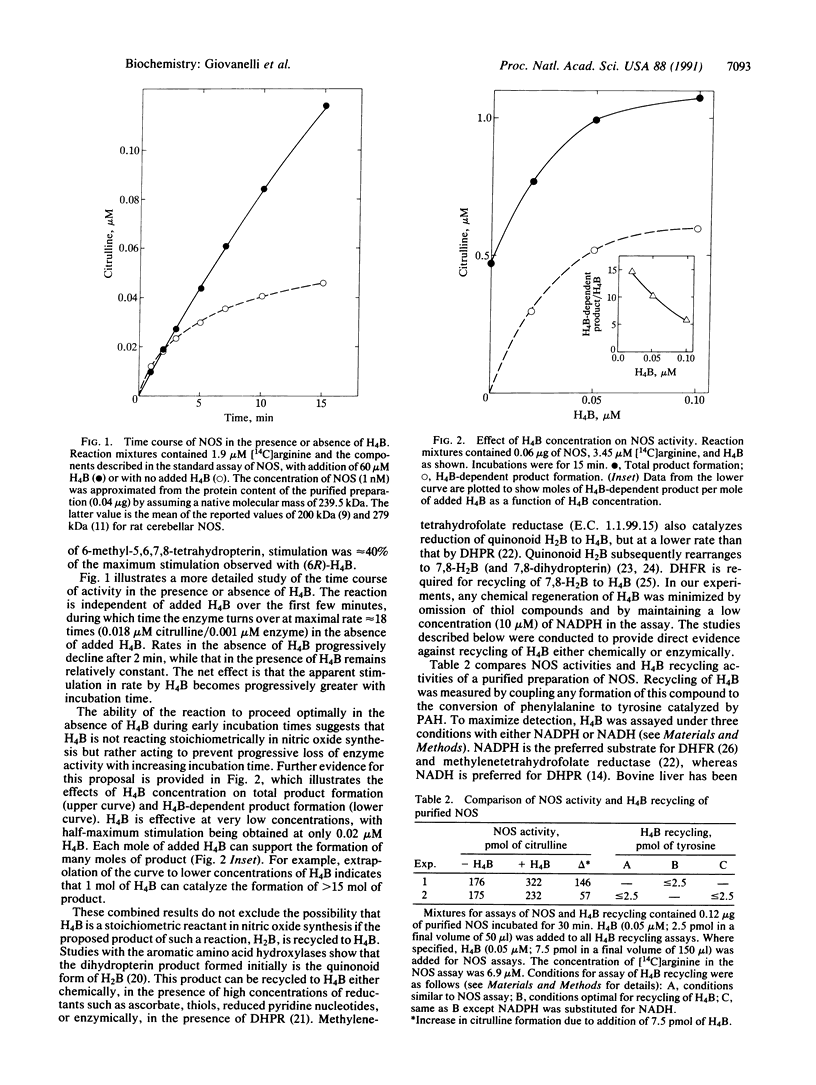
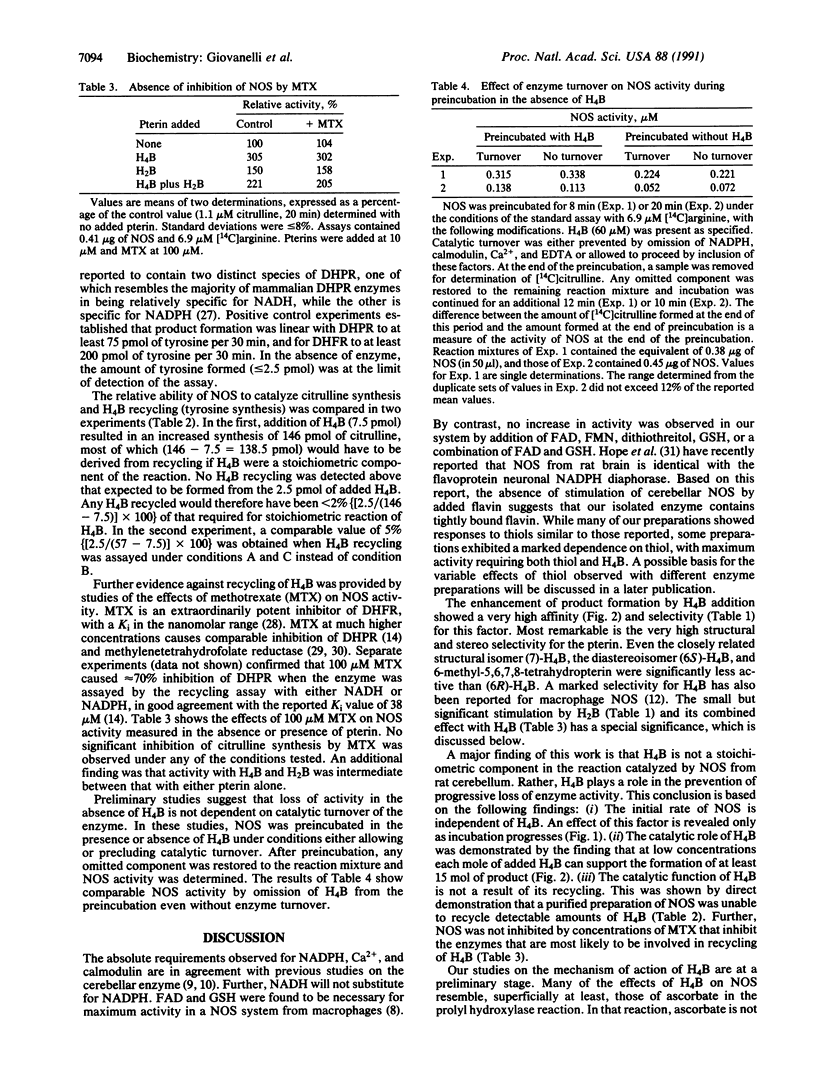
Studies with purified nitric oxide synthase from rat cerebellum have confirmed previous reports that product formation is enhanced by tetrahydrobiopterin [H4B; 6-(L-erythro-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin]. The effect of the natural isomer, (6R)-H4B, is observed at extremely low (less than 0.1 microM) concentrations and is remarkably selective. At these concentrations, only the diastereoisomer (6S)-H4B, the structural isomer 7-(L-erythro-1,2-dihydroxypropyl)-5,6,7,8-tetrahydropterin, and 7,8-dihydrobiopterin showed detectable effects. Our observations are inconsistent with a stoichiometric role for H4B in the oxygenation of arginine [e.g., Stuehr, D. J., Kwon, N. S., Nathan, C. F., Griffith, O. W., Feldman, P. L. & Wiseman, J. (1991) J. Biol. Chem. 266, 6259-6263]. Activity is initially independent of added H4B; enhanced product formation with H4B is observed only as incubation progresses. The effect of H4B is catalytic, with each mole of added H4B supporting the formation of greater than 15 mol of product. Recycling of H4B was excluded by direct measurement during nitric oxide synthesis and by the demonstration that nitric oxide synthase is not inhibited by methotrexate. These combined results exclude H4B as a stoichiometric reactant and suggest that H4B enhances product formation by protecting enzyme activity against progressive loss. Preliminary studies indicate that the decreased activity in the absence of added H4B does not depend on catalytic turnover of the enzyme. The role of H4B may be allosteric or it may function to maintain some group(s) on the enzyme in a reduced state required for activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer M. C., Vonderschmitt D. J., Scrimgeour K. G. Mechanism of oxidation of tetrahydropterins. Can J Biochem. 1972 Nov;50(11):1174–1182. doi: 10.1139/o72-160. [DOI] [PubMed] [Google Scholar]
- Armarego W. L., Randles D., Taguchi H. Peroxidase catalysed aerobic degradation of 5,6,7,8-tetrahydrobiopterin at physiological pH. Eur J Biochem. 1983 Oct 3;135(3):393–403. doi: 10.1111/j.1432-1033.1983.tb07666.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craine J. E., Hall E. S., Kaufman S. The isolation and characterization of dihydropteridine reductase from sheep liver. J Biol Chem. 1972 Oct 10;247(19):6082–6091. [PubMed] [Google Scholar]
- Davis M. D., Kaufman S., Milstien S. The auto-oxidation of tetrahydrobiopterin. Eur J Biochem. 1988 Apr 15;173(2):345–351. doi: 10.1111/j.1432-1033.1988.tb14004.x. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Vanhoutte P. M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989 Jul;3(9):2007–2018. [PubMed] [Google Scholar]
- Förstermann U., Pollock J. S., Schmidt H. H., Heller M., Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1788–1792. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik J., Flatmark T. Isolation and characterization of tetrahydropterin oxidation products generated in the tyrosine 3-monooxygenase (tyrosine hydroxylase) reaction. Eur J Biochem. 1987 Oct 1;168(1):21–26. doi: 10.1111/j.1432-1033.1987.tb13381.x. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro L. J. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989 Jan;3(1):31–36. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- KAUFMAN S. STUDIES ON THE STRUCTURE OF THE PRIMARY OXIDATION PRODUCT FORMED FROM TETRAHYDROPTERIDINES DURING PHENYLALAMINE HYDROXYLATION. J Biol Chem. 1964 Jan;239:332–338. [PubMed] [Google Scholar]
- Kaufman S. Metabolism of the phenylalanine hydroxylation cofactor. J Biol Chem. 1967 Sep 10;242(17):3934–3943. [PubMed] [Google Scholar]
- Kaufman S. Phenylalanine 4-monooxygenase from rat liver. Methods Enzymol. 1987;142:3–17. doi: 10.1016/s0076-6879(87)42003-x. [DOI] [PubMed] [Google Scholar]
- Kaufman S. Regulation of the activity of hepatic phenylalanine hydroxylase. Adv Enzyme Regul. 1986;25:37–64. doi: 10.1016/0065-2571(86)90007-5. [DOI] [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Kinetic characteristics of nitric oxide synthase from rat brain. Biochem J. 1990 Jul 1;269(1):207–210. doi: 10.1042/bj2690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuga K., Yui Y., Hattori R., Eizawa H., Hiki K., Kawai C. Stabilizing factor(s) of nitric oxide (NO) synthetase. Biochem Biophys Res Commun. 1990 Oct 30;172(2):705–708. doi: 10.1016/0006-291x(90)90731-2. [DOI] [PubMed] [Google Scholar]
- Kwon N. S., Nathan C. F., Stuehr D. J. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989 Dec 5;264(34):20496–20501. [PubMed] [Google Scholar]
- MATHEWS C. K., HUENNEKENS F. M. FURTHER STUDIES ON DIHYDROFOLIC REDUCTASE. J Biol Chem. 1963 Oct;238:3436–3442. [PubMed] [Google Scholar]
- Marota J. J., Shiman R. Stoichiometric reduction of phenylalanine hydroxylase by its cofactor: a requirement for enzymatic activity. Biochemistry. 1984 Mar 13;23(6):1303–1311. doi: 10.1021/bi00301a044. [DOI] [PubMed] [Google Scholar]
- Matthews R. G., Kaufman S. Characterization of the dihydropterin reductase activity of pig liver methylenetetrahydrofolate reductase. J Biol Chem. 1980 Jul 10;255(13):6014–6017. [PubMed] [Google Scholar]
- Mayer B., John M., Böhme E. Purification of a Ca2+/calmodulin-dependent nitric oxide synthase from porcine cerebellum. Cofactor-role of tetrahydrobiopterin. FEBS Lett. 1990 Dec 17;277(1-2):215–219. doi: 10.1016/0014-5793(90)80848-d. [DOI] [PubMed] [Google Scholar]
- Moncada S., Radomski M. W., Palmer R. M. Endothelium-derived relaxing factor. Identification as nitric oxide and role in the control of vascular tone and platelet function. Biochem Pharmacol. 1988 Jul 1;37(13):2495–2501. doi: 10.1016/0006-2952(88)90236-5. [DOI] [PubMed] [Google Scholar]
- Myllylä R., Kuutti-Savolainen E. R., Kivirikko K. I. The role of ascorbate in the prolyl hydroxylase reaction. Biochem Biophys Res Commun. 1978 Jul 28;83(2):441–448. doi: 10.1016/0006-291x(78)91010-0. [DOI] [PubMed] [Google Scholar]
- Nakanishi N., Hasegawa H., Watabe S. A new enzyme, NADPH-dihydropteridine reductase in bovine liver. J Biochem. 1977 Mar;81(3):681–685. doi: 10.1093/oxfordjournals.jbchem.a131504. [DOI] [PubMed] [Google Scholar]
- OSBORN M. J., FREEMAN M., HUENNEKENS F. M. Inhibition of dihydrofolic reductase by aminopterin and amethopterin. Proc Soc Exp Biol Med. 1958 Feb;97(2):429–431. doi: 10.3181/00379727-97-23764. [DOI] [PubMed] [Google Scholar]
- Schmidt H. H., Pollock J. S., Nakane M., Gorsky L. D., Förstermann U., Murad F. Purification of a soluble isoform of guanylyl cyclase-activating-factor synthase. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):365–369. doi: 10.1073/pnas.88.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F. FAD and GSH participate in macrophage synthesis of nitric oxide. Biochem Biophys Res Commun. 1990 Apr 30;168(2):558–565. doi: 10.1016/0006-291x(90)92357-6. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Nathan C. F., Griffith O. W., Feldman P. L., Wiseman J. N omega-hydroxy-L-arginine is an intermediate in the biosynthesis of nitric oxide from L-arginine. J Biol Chem. 1991 Apr 5;266(10):6259–6263. [PubMed] [Google Scholar]
- de Jong L., Albracht S. P., Kemp A. Prolyl 4-hydroxylase activity in relation to the oxidation state of enzyme-bound iron. The role of ascorbate in peptidyl proline hydroxylation. Biochim Biophys Acta. 1982 Jun 4;704(2):326–332. doi: 10.1016/0167-4838(82)90162-5. [DOI] [PubMed] [Google Scholar]



