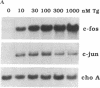
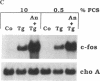
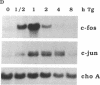
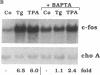
Abstract
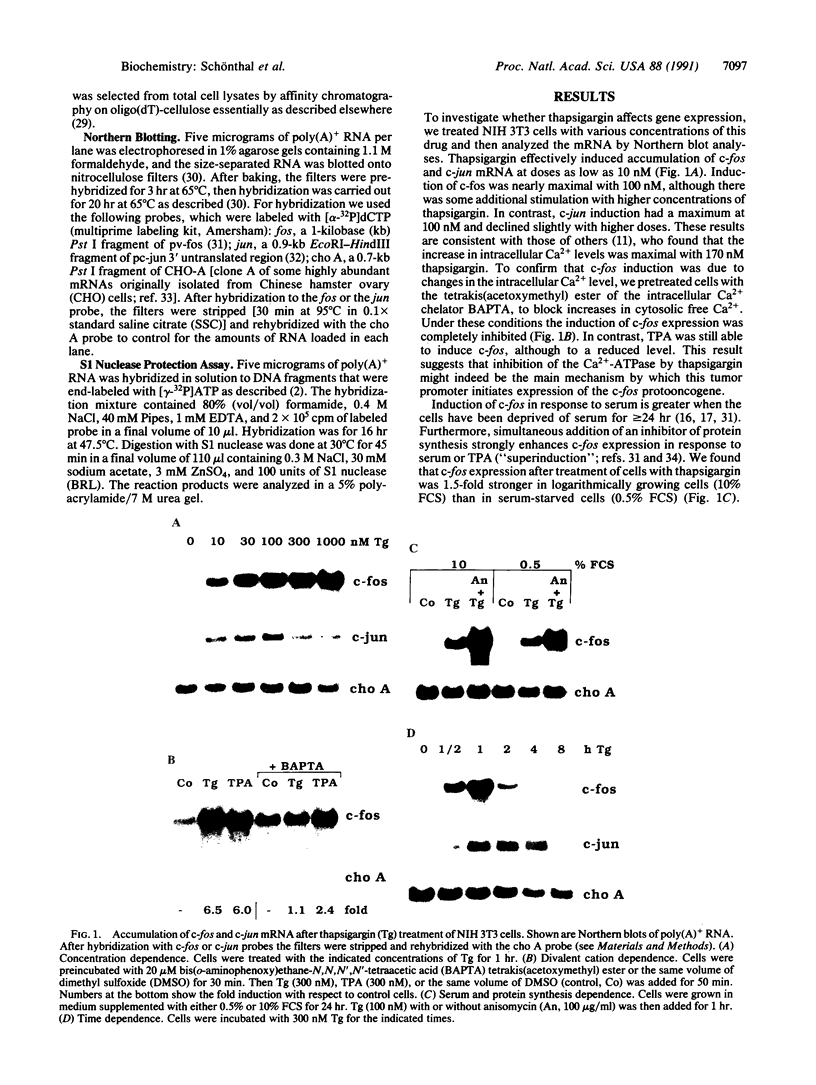
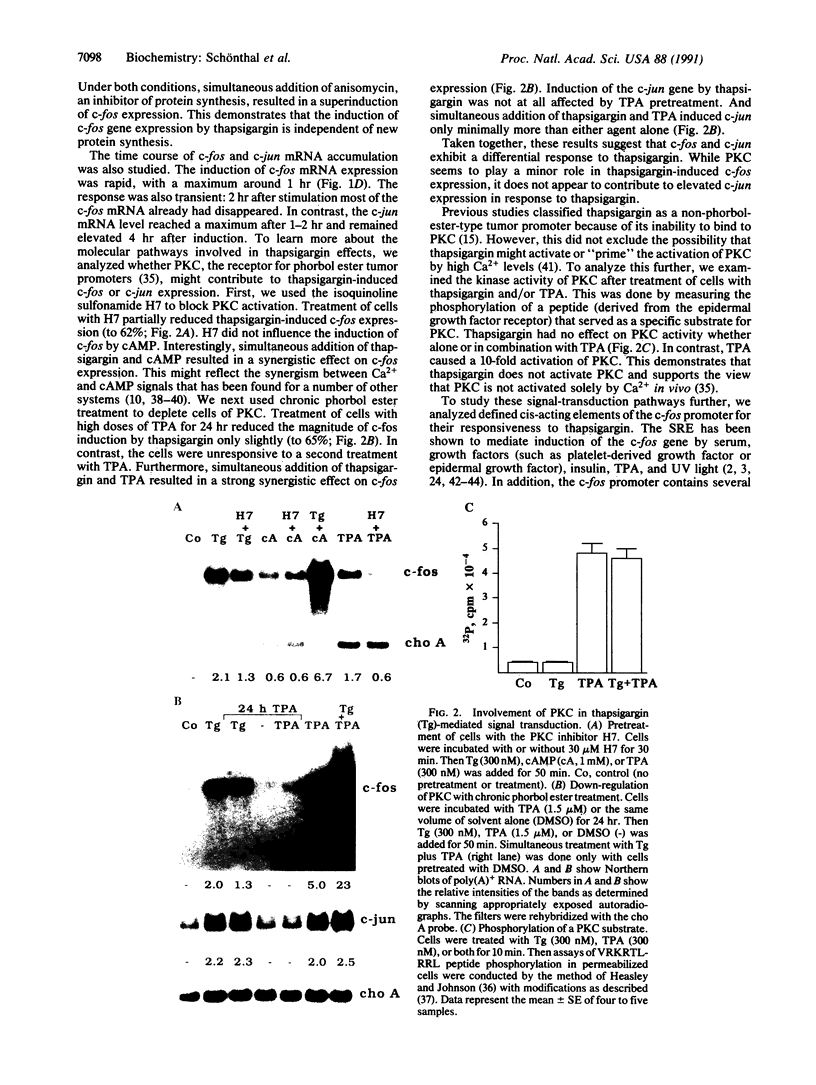
Thapsigargin, a non-phorbol-ester-type tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca(2+)-ATPase. We used this drug to analyze the involvement of Ca2+ and Ca(2+)-ATPases in the control of growth- and transformation-related genes. Here we show that treatment of mouse NIH 3T3 fibroblasts with thapsigargin induced rapid expression of the c-fos and c-jun protooncogenes. Inhibition or depletion of protein kinase C partially diminished the c-fos but not the c-jun response. Furthermore, thapsigargin could synergize with the tumor promoter phorbol 12-myristate 13-acetate to induce c-fos but not c-jun. However, thapsigargin had no effect on basal or phorbol ester-induced protein kinase C activity. Our results indicate that Ca2+ is a potent second messenger that controls expression of growth- and transformation-related genes. Since inhibition of the endoplasmic reticulum Ca(2+)-ATPase results in a strong induction of these genes, our data suggest that this Ca2+ pump may act as a negative regulator of cell growth.
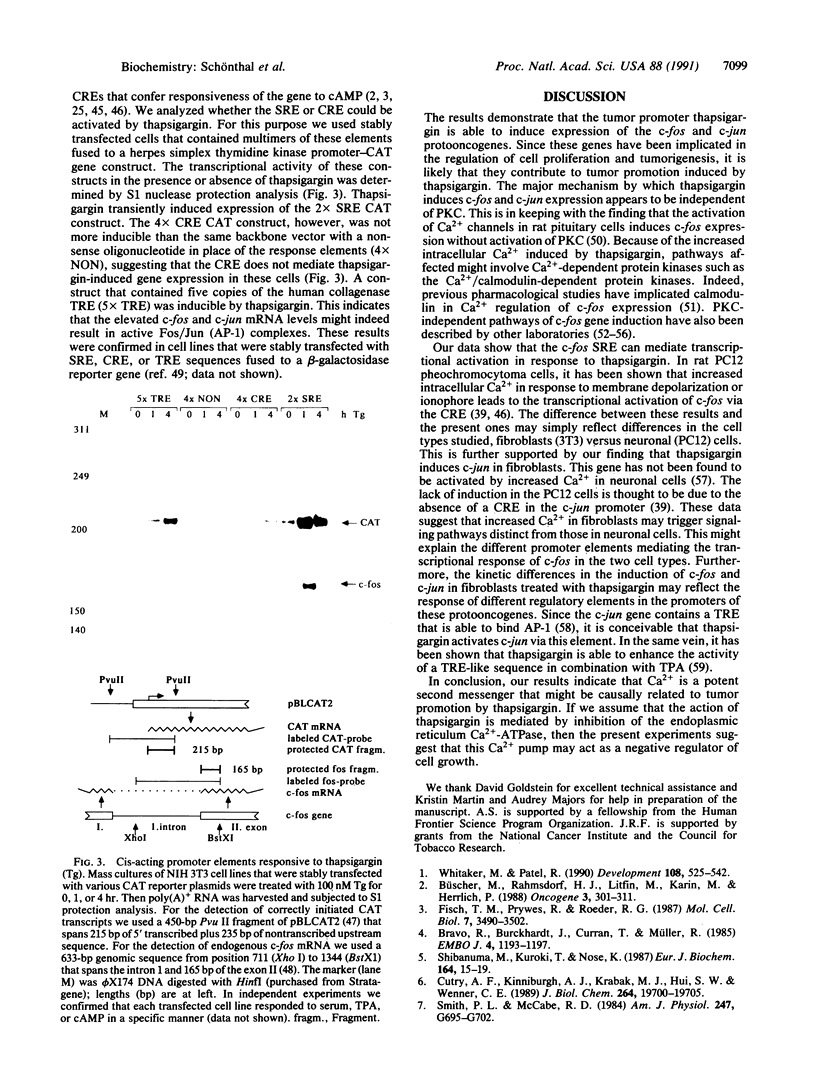
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Badley J. E., Bishop G. A., St John T., Frelinger J. A. A simple, rapid method for the purification of poly A+ RNA. Biotechniques. 1988 Feb;6(2):114–116. [PubMed] [Google Scholar]
- Bandyopadhyay S. K., Bancroft C. Calcium induction of the mRNAs for prolactin and c-fos is independent of protein kinase C activity. J Biol Chem. 1989 Aug 25;264(24):14216–14219. [PubMed] [Google Scholar]
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Berkowitz L. A., Riabowol K. T., Gilman M. Z. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989 Oct;9(10):4272–4281. doi: 10.1128/mcb.9.10.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Stumpo D. J., Huang J. K., Nemenoff R. A., Spach D. H. Protein kinase C-dependent and -independent pathways of proto-oncogene induction in human astrocytoma cells. J Biol Chem. 1987 Jun 5;262(16):7774–7781. [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Stimulation and inhibition of growth by EGF in different A431 cell clones is accompanied by the rapid induction of c-fos and c-myc proto-oncogenes. EMBO J. 1985 May;4(5):1193–1197. doi: 10.1002/j.1460-2075.1985.tb03759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brayden D. J., Hanley M. R., Thastrup O., Cuthbert A. W. Thapsigargin, a new calcium-dependent epithelial anion secretagogue. Br J Pharmacol. 1989 Nov;98(3):809–816. doi: 10.1111/j.1476-5381.1989.tb14609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büscher M., Rahmsdorf H. J., Litfin M., Karin M., Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988 Sep;3(3):301–311. [PubMed] [Google Scholar]
- Cartwright C. A., McRoberts J. A., Mandel K. G., Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Angel P., Karin M. Jun-B differs in its biological properties from, and is a negative regulator of, c-Jun. Cell. 1989 Dec 22;59(6):979–986. doi: 10.1016/0092-8674(89)90754-x. [DOI] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., Egléme C., Greenwood H., Hickman M. E., Kirkland S. C., MacVinish L. J. Calcium- and cyclic AMP-dependent chloride secretion in human colonic epithelia. Br J Pharmacol. 1987 Jul;91(3):503–515. doi: 10.1111/j.1476-5381.1987.tb11243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutry A. F., Kinniburgh A. J., Krabak M. J., Hui S. W., Wenner C. E. Induction of c-fos and c-myc proto-oncogene expression by epidermal growth factor and transforming growth factor alpha is calcium-independent. J Biol Chem. 1989 Nov 25;264(33):19700–19705. [PubMed] [Google Scholar]
- Erlij D., Gersten L., Sterba G., Schoen H. F. Role of prostaglandin release in the response of tight epithelia to Ca2+ ionophores. Am J Physiol. 1986 Apr;250(4 Pt 1):C629–C636. doi: 10.1152/ajpcell.1986.250.4.C629. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Simon M. C., Roeder R. G. Multiple sequence elements in the c-fos promoter mediate induction by cAMP. Genes Dev. 1989 Feb;3(2):198–211. doi: 10.1101/gad.3.2.198. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kaibuchi K., Oku N., Hori Y., Takai Y. Activation of the c-fos serum-response element by the activated c-Ha-ras protein in a manner independent of protein kinase C and cAMP-dependent protein kinase. J Biol Chem. 1990 Jan 15;265(2):774–780. [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hakii H., Fujiki H., Suganuma M., Nakayasu M., Tahira T., Sugimura T., Scheuer P. J., Christensen S. B. Thapsigargin, a histamine secretagogue, is a non-12-O-tetradecanoylphorbol-13-acetate (TPA) type tumor promoter in two-stage mouse skin carcinogenesis. J Cancer Res Clin Oncol. 1986;111(3):177–181. doi: 10.1007/BF00389230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lamph W. W., Wamsley P., Sassone-Corsi P., Verma I. M. Induction of proto-oncogene JUN/AP-1 by serum and TPA. Nature. 1988 Aug 18;334(6183):629–631. doi: 10.1038/334629a0. [DOI] [PubMed] [Google Scholar]
- Lenormand P., Muldoon L. L., Enslen H., Rodland K. D., Magun B. E. Two tumor promoters, 12-O-tetradecanoylphorbol-13-acetate and thapsigargin, act synergistically via distinct signaling pathways to stimulate gene expression. Cell Growth Differ. 1990 Dec;1(12):627–635. [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehmet H., Morris C., Rozengurt E. Multiple synergistic signal transduction pathways regulate c-fos expression in Swiss 3T3 cells: the role of cyclic AMP. Cell Growth Differ. 1990 Jun;1(6):293–298. [PubMed] [Google Scholar]
- Meinkoth J., Alberts A. S., Feramisco J. R. Construction of mammalian cell lines with indicator genes driven by regulated promoters. Ciba Found Symp. 1990;150:47–56. doi: 10.1002/9780470513927.ch4. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986 Aug 7;322(6079):552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Rahmsdorf H. J., Schönthal A., Angel P., Litfin M., Rüther U., Herrlich P. Posttranscriptional regulation of c-fos mRNA expression. Nucleic Acids Res. 1987 Feb 25;15(4):1643–1659. doi: 10.1093/nar/15.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder K., Nathans D. Induction of protooncogene c-jun by serum growth factors. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8464–8467. doi: 10.1073/pnas.85.22.8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Visvader J., Ferland L., Mellon P. L., Verma I. M. Induction of proto-oncogene fos transcription through the adenylate cyclase pathway: characterization of a cAMP-responsive element. Genes Dev. 1988 Dec;2(12A):1529–1538. doi: 10.1101/gad.2.12a.1529. [DOI] [PubMed] [Google Scholar]
- Schönthal A. Nuclear protooncogene products: fine-tuned components of signal transduction pathways. Cell Signal. 1990;2(3):215–225. doi: 10.1016/0898-6568(90)90049-g. [DOI] [PubMed] [Google Scholar]
- Schönthal A., Tsukitani Y., Feramisco J. R. Transcriptional and post-transcriptional regulation of c-fos expression by the tumor promoter okadaic acid. Oncogene. 1991 Mar;6(3):423–430. [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Kuroki T., Nose K. Inhibition of proto-oncogene c-fos transcription by inhibitors of protein kinase C and ion transport. Eur J Biochem. 1987 Apr 1;164(1):15–19. doi: 10.1111/j.1432-1033.1987.tb10985.x. [DOI] [PubMed] [Google Scholar]
- Smith P. L., McCabe R. D. A23187-induced changes in colonic K and Cl transport are mediated by separate mechanisms. Am J Physiol. 1984 Dec;247(6 Pt 1):G695–G702. doi: 10.1152/ajpgi.1984.247.6.G695. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stein B., Rahmsdorf H. J., Steffen A., Litfin M., Herrlich P. UV-induced DNA damage is an intermediate step in UV-induced expression of human immunodeficiency virus type 1, collagenase, c-fos, and metallothionein. Mol Cell Biol. 1989 Nov;9(11):5169–5181. doi: 10.1128/mcb.9.11.5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Blackshear P. J. Insulin and growth factor effects on c-fos expression in normal and protein kinase C-deficient 3T3-L1 fibroblasts and adipocytes. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9453–9457. doi: 10.1073/pnas.83.24.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpo D. J., Stewart T. N., Gilman M. Z., Blackshear P. J. Identification of c-fos sequences involved in induction by insulin and phorbol esters. J Biol Chem. 1988 Feb 5;263(4):1611–1614. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Trilivas I., Brown J. H. Increases in intracellular Ca2+ regulate the binding of [3H]phorbol 12,13-dibutyrate to intact 1321N1 astrocytoma cells. J Biol Chem. 1989 Feb 25;264(6):3102–3107. [PubMed] [Google Scholar]
- Trilivas I., McDonough P. M., Brown J. H. Dissociation of protein kinase C redistribution from the phosphorylation of its substrates. J Biol Chem. 1991 May 5;266(13):8431–8438. [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]