Abstract
Propionibacterium acnes , plays an important role in acne vulgaris and other diseases. However, understanding of the exact mechanisms of P. acnes pathogenesis is limited. Few studies have investigated its proteome, which is essential for vaccine development. Here, we comprehensively investigate the proteome of P. acnes strain ATCC 6919, including secreted, cell wall, membrane, and cytosolic fractions in three types of growth media. A total of 531 proteins were quantified using an Orbitrap mass spectrometer and bioinformatically categorized for localization and function. Several, including PPA1939, a highly expressed surface and secreted protein, were identified as potential vaccine candidates.
Keywords: Propionibacterium acnes, acne, proteomics, mass spectrometry, cell wall
Graphical abstract

Introduction
Acne vulgaris is an inflammatory disease of the pilosebaceous unit. With at least 85% prevalence among 12–24 year olds, it can cause long-term scarring and have a major psychological impact on individuals [1]. The gram-positive, anaerobic species Propionibacterium acnes has been traditionally implicated in the development of acne vulgaris [2]. It is also a significant organism in infections of the prostate [3], prosthetic joints [4], other surgical implants [5], spinal discs [6], and ophthalmic infections [7]. Unfortunately, the bacteria in many cases are resistant to antibiotic therapy [8, 9], and other treatments often have low patient compliance [10]. Thus, the need exists for novel approaches to develop treatments that are more effective against P. acnes and have fewer side effects.
A vaccine may be an efficient means to protect against multiple types of infections caused by P. acnes [11, 12]. Several recent studies have investigated this possibility. Antibodies generated by mouse intranasal vaccination of heat-killed P. acnes reduced P. acnes-induced IL-8 inflammation and cytotoxicity in sebocytes [13], though this study used an in vitro experimental system. Vaccination with heat-killed P. acnes reduced the severity of disease and inflammation in a P. acnes ear infection mouse model. The same group generated antibodies against P. acnes surface sialidase [14], which had similar effects in sebocytes. Vaccination with the sialidase [14], as well as with Christie–Atkins–Munch-Peterson (CAMP) factor 2 [15, 16], also successfully reduced inflammation in mouse ear infections. However, this mouse model is not necessarily representative of the environment in acne vulgaris, in which an inflammatory response may act to either quickly clear P. acnes, or worsen the disease state. A better in vivo model is needed to further investigate vaccines.
In addition to its potential protective effects against acne, a P. acnes-based vaccine may also have other beneficial effects. Heat-killed P. acnes reduced atopic dermatitis in a mouse model, and increased the number of Th1 and Treg cells in the spleen [17]. A heat-killed P. acnes vaccine was also cross-protective against Actinobacillus pleuropneumoniae infection in mouse and pig models, inducing cross-reactive antibodies [18]. Specific P. acnes proteins could also induce cross-reactive antibodies and protection in the A. pleuropneumoniae mouse model [19]. Intratumoral injection of live P. acnes was successful in increasing the antitumor Th1 immune response in a melanoma mouse model [20]. A P. acnes vaccine also increased Th1, improving glomerulosclerosis in a mouse model [21]. Several vaccine studies have also utilized P. acnes as an adjuvant. A microparticle preparation of P. acnes cell wall increased Th1 response to vaccination [22], and heat-killed P. acnes increased activation of B-1 lymphocytes [23].
P. acnes remains a largely understudied organism, with little information available to investigate additional vaccine candidates. Only four studies have covered the P. acnes proteome, none of which were comprehensive. Holland et al. examined the secreted proteome of several types of P. acnes, discovering interesting differences between types [24]. Dekio et al. identified several proteins expressed by P. acnes in anaerobic and microaerophilic conditions, but not aerobic conditions [25]. Mak et al. assessed the surface proteome of P. acnes using trypsin shaving, comparing it to other Propionibacterium species [26]. Bek-Thomsen et al. examined the proteome of sebaceous follicular casts, which included several P. acnes proteins [27]. However, none of these studies used a quantitative method, and only a limited number of proteins could be detected. A more comprehensive picture of its proteome, including proteins from all fractions of the cell, may contribute to our understanding of the molecular mechanisms of P. acnes disease pathogenesis, in addition to suggesting additional vaccine candidates. Here, we present a comprehensive study of the proteome, including surface proteins, secreted proteins, and intracellular proteins, of P. acnes strain ATCC 6919 (phylotype IA-2, a group enriched in acne vulgaris patients [28, 29]) grown in three types of media.
Methods
Bacterial Culture
P. acnes strain ATCC 6919 (NCTC 737), a commonly used laboratory strain originally isolated from an acne patient, was inoculated from glycerol stocks into 10 mL of Reinforced Clostridial Media (RCM) (Oxoid) and grown at 37°C using AnaeroPack system sachets (Remel). When bacteria reached the exponential phase of growth (optical density of 0.1–0.3 at 600 nm wavelength with 1 cm path length) after 5–6 days, bacteria were collected by centrifugation and divided evenly into 50 mL of RCM, 50 mL of Brain-Heart Infusion Broth (BHI) (Oxoid), and 50 mL of BHI supplemented with 5% egg yolk (Sigma) after autoclaving (EBHI). Cultures were again incubated at 37°C for approximately 40 hours using anaerobic sachets, with shaking at 200 rpm for the cultures in BHI and EBHI. P. acnes was harvested in the late exponential phase (optical density of ~1.0 at 600 nm wavelength with 1 cm path length) for protein fraction preparation.
Fraction Preparation
P. acnes samples were pelleted by centrifugation at 4,000g for 10 minutes for the BHI and BHI-E samples and 30 minutes for the RCM sample. The supernatant, containing the secreted proteins, was collected and filtered through 0.2 μm pores, yielding the Cell Secretion (CS) fraction. The pellets were washed thrice with phosphate buffered saline (PBS), and divided into four equally sized samples.
To protoplast the bacteria and release cell wall proteins, a technique was followed similar to one used by Gallis et al [30]. One sample was resuspended in 200 μL of solution containing 10 mM pH 7 phosphate buffer, 600 mM KCl, 10 mM MgCl2, and 1 mg/mL egg white lysozyme (Pierce). Another sample was resuspended in 200 μL of solution containing 50 mM Tris-HCl, 250 mM sucrose, 10 mM MgCl2, 30mM KCl, and 1 mg/mL egg white lysozyme. These two samples were incubated with rotation at 37°C for 4 hours to allow for lysozyme digestion of cell walls. Samples were then centrifuged for 5 minutes at 1,000g with the supernatant retained. Samples were than centrifuged for 5 minutes at 20,000g, and supernatants from the two fractions were combined. The sample was filtered through 0.2 μm pores, yielding the Cell Wall (CW) fraction.
The remaining two samples were subjected to beadbeating with a micro-MiniBeadbeater (Biospec Products) for five minutes with cooling every minute. Samples were than sonicated. One of these samples was designated the Total Cell Extract (TCE) fraction. The other sample was centrifuged at first 1,000g for 5 minutes and then at 8,000g for five minutes, with the supernatant retained each time. The sample was then centrifuged at 20,000g for 15 minutes. The supernatant was designated the Cell Cytosolic (CC) fraction. Membranes were obtained using a similar protocol to one developed by Zuobi-Hasona & Brady [31]. The pellet was washed three times with PBS with centrifugation at 20,000g for 15 minutes each, and it was than resuspended in 100 μL of PBS. This was designated the Cell Membrane (CM) fraction.
Mass Spectrometry
10 μg of protein from the CC and TCE fractions as quantified by Bradford assay and all of the CS, CW, and CM protein samples were adjusted to 20% trichloroacetic acid and incubated at 4°C for 30 minutes. Samples were centrifuged at 20,000g for 5 minutes, and the pellet was washed with 200 μL of cold acetone. The pellet was resuspended in a solution of 50% aqueous 100 mM ammonium bicarbonate and 50% acetonitrile. Samples were reduced with 25mM tris-(2-carboxyethyl)-phosphine for 30 minutes at 37°C and then alkylated with 75 mM iodoacetamide for 1 hour at room temperature in the dark. Samples were diluted to 5% acetonitrile in pH 8 100 mM ammonium bicarbonate buffer and digested for 16 hours with 500 ng trypsin (Promega). Samples were then centrifuged at 20,000g for 10 minutes twice, with the supernatant retained. Samples underwent liquid chromatography and tandem mass spectrometry using an LTQ Orbitrap XL mass spectrometer (Thermo Fisher) with NanoLC-2D HPLC (Eksigent). This method has been shown to be accurate for label-free quantification of proteins [32, 33]. Reverse phase chromatography on a reverse phase column (New Objective C18, 15 cm, 75 μM diameter) was conducted at 500 nL/min for loading and analytical separation with Buffer A containing aqueous 0.1% formic acid and Buffer B containing 0.1% formic acid in acetonitrile. Peptides were eluted using a gradient of 3–40% Buffer B over 3 hours. The Orbitrap was used in MS/MS mode with a high-resolution full precursor scan and ten low resolution MS/MS events on the linear trap during the full scan., The threshold intensity for Collision Induced Dissociation was 5000 and the allowed mass range was 350–2000 Da.
Data Analysis
Raw spectral data were processed with RawXtract, and identified peptides were analyzed with the ProLuCID algorithm (V1.3.3) using the database of P. acnes reference strain KPA171202 [34]. No additional unique protein hits were found when using the type IA P. acnes strain 266 database. Scaffold 4.4.1.1 (Proteome Software) was used for identification and quantification of proteins with a false discovery rate of 5%, allowing for more true identifications in the smaller CS and CW datasets than the standard 1%. Only proteins with at least two unique peptides were counted. Protein quantification was determined by normalized spectral abundance factors to obtain relative quantification of protein in each sample. All quantities are reported in fmol protein per microgram of total protein detected. Protein localization was predicted using the PSORTb 3.0 tool [35], and signal peptides were predicted using SignalP 4.1 [36]. PSORTb assigns scores for extracellular, cell wall, membrane, or cytoplasmic localization, with the sum adding up to 10. A “non-cytoplasmic” PSORTb localization indicates a zero score for cytoplasmic localization, while “unknown” indicates no localization prediction. In Table S1, only the highest score for any fraction is shown. For SignalP, a score of over 0.45 was indicative of a signal peptide (Y), with scores of 0.35–0.45 listed as probable signal peptides (P), scores of 0.25–0.35 listed as maybe a signal peptide (M), and scores of below 0.25 listed as no signal peptide (N). NCBI’s BLAST tool [37] was used to search for homologs with known function to assist with functional annotation of protein lists.
Results
Identification of Propionibacterium acnes Proteins
To investigate the proteome of P. acnes in different environments, we assessed its proteome in Reinforced Clostridial Media (RCM) and Brain-Heart Infusion Broth (BHI), common media used in laboratories. Additionally, we devised EBHI media, BHI supplemented with 5% egg yolk, to approximate the lipid-rich environment of the pilosebacous unit. In total, 531 proteins from P. acnes were identified and quantified (Table S1), representing slightly over 20% of the total number of predicted proteins in its genome. Of these, 471 were detected in the fractions from P. acnes grown in Reinforced Clostridial Media RCM, 359 in fractions from BHI, and 415 in fractions from EBHI. Most proteins were found in all three growth media groups, including nearly all from BHI fractions (Figure 1A).
Figure 1. Identified Proteins.
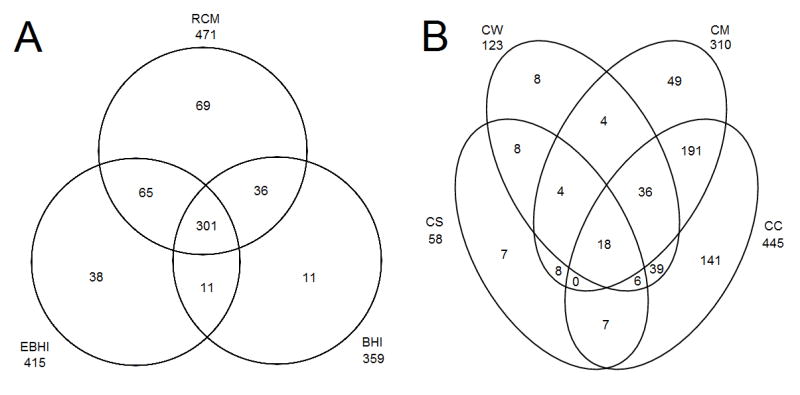
Comparison of proteins identified in different growth media (A) and different cell fractions (B). Data from one of two similar experiments. Abbreviations: RCM - Reinforced Clostridial Media, BHI - Brain Heart Infusion Broth, EBHI - 5% Egg Yolk Supplemented Brain Heart Infusion Broth, CS - Cell Secretion, CW - Cell Wall, CM - Cell Membrane, CC - Cell Cytosolic.
Cell Secretion (CS) fractions together across the three different media contained 58 distinct proteins (Figure 1B). However, very few proteins were detected in CS fractions from BHI and EBHI media due to a large amount of precipitate after treatment with trichloroacetic acid that could not be separated from protein. Cell Wall (CW) fractions contained 123 distinct proteins across the three different media. Cell Membrane (CM) fractions contained 310 proteins, though the BHI CM fraction had only 34, significantly fewer than the RCM and EBHI fractions. Cell Cytosolic (CC) fractions contained 445 proteins. All fractions had significant overlap of proteins with other fractions, but few proteins were present in all four of these fractions (Figure 1B). Additionally, the Total Cell Extract (TCE) fraction contained 347 proteins, of which only 5 were unique to this fraction. The TCE fraction had a very high degree of overlap with the CC fraction.
Protein Localization
To assess the purity of our protein fractions, we utilized PSORTb to predict protein localization and SignalP to predict signal peptides, which are indicative of a non-cytoplasmic localization. Both PSORTb and SignalP were in fairly good agreement for non-cytoplasmic localization, with SignalP more stringent in its assessments. Of the PSORTb predicted cytoplasmic proteins, only 12 out of 384 proteins (3.1%) had a signal peptide or probable signal peptide. Of the PSORTb predicted non-cytoplasmic proteins (including predicted cell wall, extracellular, and membrane proteins), 35 out of 94 proteins (37.2%) had a signal peptide or probable signal peptide. For PSORTb unknown proteins, 17 out of 53 proteins (32.1%) had a signal peptide or probable signal peptide, indicating that the unknown proteins may contain nearly the same proportion of true non-cytoplasmic proteins and PSORTb predicted non-cytoplasmic proteins.
The CS fractions were highly enriched for non-cytoplasmic proteins according to PSORTb, with 31 out of 58 proteins (53.4%) having non-cytoplasmic (including unknown) localization prediction. In the 20 most abundant CS proteins, the level of enrichment was even higher (85%) (Table 1). The CW fractions were also enriched for non-cytoplasmic proteins, with 53 out of 123 proteins (43.1%) having non-cytoplasmic localization prediction. The level of enrichment was also higher (60%) for the 20 most abundant CW proteins (Table 2), not including a phosphocarrier protein that may have a signal peptide and act as a cell surface transporter. The CM fractions had 80 out of 310 proteins (25.8%) with non-cytoplasmic localization prediction. This is only slightly higher than the proportion in the CC fraction (22.7%). The proportion of non-cytoplasmic proteins was not further enriched in the most abundant CM proteins. In the CC fraction, all of the 20 most abundant proteins had cytoplasmic localization (Table 3).
Table 1.
The 20 Most Abundant Secreted Proteins
| Protein | Accession (gi) | MW (kDa) | RCM fmol | BHI fmol | EBHI fmol | SignalP | PSORTb | Functional Group |
|---|---|---|---|---|---|---|---|---|
| protein PPA1939 | 50843388 | 17 | 6222 | 58824 | 6757 | Y | Unknown | unknown |
| adhesion | 50843565 | 42 | 50 | 0 | 19238 | M | Unknown | adhesion |
| cAMP factor | 50842175 | 29 | 3408 | 0 | 2660 | Y | Extracellular | digestion |
| protein PPA2239 | 50843674 | 41 | 1996 | 0 | 0 | Y | Non-Cytoplasmic | digestion |
| protein PPA2271 | 50843708 | 52 | 1415 | 0 | 0 | Y | Unknown | digestion |
| endoglycoceramidase | 50842131 | 57 | 1351 | 0 | 0 | Y | Non-Cytoplasmic | digestion |
| protein PPA1746 | 50843206 | 22 | 1218 | 0 | 0 | Y | Non-Cytoplasmic | unknown |
| NPL/P60 protein | 50842209 | 41 | 1100 | 0 | 0 | Y | Membrane | digestion |
| cell wall hydrolase | 50843410 | 43 | 986 | 0 | 0 | M | Extracellular | digestion |
| protein PPA1745 | 50843205 | 90 | 979 | 0 | 0 | M | Extracellular | digestion |
| cAMP factor | 50842820 | 30 | 685 | 0 | 0 | Y | Extracellular | digestion |
| chaperone GroEL | 50841936 | 57 | 662 | 0 | 0 | N | Cytoplasmic | protein folding |
| triacylglycerol lipase | 50843543 | 36 | 642 | 0 | 0 | Y | Non-Cytoplasmic | digestion |
| protein PPA0533 | 50842017 | 20 | 599 | 0 | 0 | Y | Non-Cytoplasmic | unknown |
| co-chaperonin GroES | 50843233 | 11 | 595 | 0 | 0 | N | Cytoplasmic | protein folding |
| endoglycoceramidase | 50843544 | 54 | 571 | 0 | 0 | Y | Non-Cytoplasmic | digestion |
| fine tangled pili | 50843572 | 19 | 436 | 0 | 0 | N | Cytoplasmic | mobility |
| lipase/acylhydrolase | 50843480 | 30 | 377 | 0 | 0 | P | Unknown | digestion |
| regulatory protein | 50842205 | 39 | 325 | 0 | 0 | P | Unknown | translation |
| protein PPA1715 | 50843175 | 49 | 309 | 0 | 0 | Y | Non-Cytoplasmic | unknown |
Table shows quantity in femtomoles per microgram of total protein detected. SignalP predictions are yes (Y), probably (P), maybe (M), and no (N). Abbreviations: RCM - Reinforced Clostridial Media, BHI - Brain Heart Infusion Broth, EBHI - 5% Egg Yolk Supplemented Brain Heart Infusion Broth. Data from one of two similar experiments.
Table 2.
The 20 Most Abundant Cell Wall Proteins
| Protein | Accession (gi) | MW (kDa) | RCM fmol | BHI fmol | EBHI fmol | SignalP | PSORTb | Functional Group |
|---|---|---|---|---|---|---|---|---|
| co-chaperonin GroES | 50843233 | 11 | 5024 | 251 | 2008 | N | Cytoplasmic | protein folding |
| protein PPA1939 | 50843388 | 17 | 2799 | 1799 | 1555 | Y | Unknown | unknown |
| membrane lipoprotein | 50843218 | 34 | 91 | 4124 | 1924 | M | Membrane | transportation |
| adhesion | 50843565 | 42 | 2553 | 0 | 3283 | M | Unknown | adhesion |
| protein PPA2271 | 50843708 | 52 | 800 | 1948 | 1539 | Y | Unknown | digestion |
| protein PPA2334 | 50843769 | 126 | 0 | 4210 | 0 | N | Cytoplasmic | unknown |
| DNA-binding HU | 50843144 | 10 | 1948 | 749 | 1427 | N | Cytoplasmic | miscellaneous |
| CsbD-like protein | 50842907 | 7 | 2477 | 635 | 743 | N | Unknown | unknown |
| protein PPA1281 | 50842762 | 30 | 1297 | 219 | 1754 | M | Non-Cytoplasmic | unknown |
| peptide transporter | 50843590 | 61 | 1311 | 0 | 1813 | Y | Cell Wall | transportation |
| phosphocarrier HPr | 50841838 | 9 | 1424 | 508 | 240 | M | Cytoplasmic | transportation |
| protein PPA1018 | 50842501 | 7 | 1033 | 271 | 696 | N | Unknown | miscellaneous |
| protein PPA1715 | 50843175 | 49 | 1265 | 384 | 96 | Y | Non-Cytoplasmic | unknown |
| rare lipoprotein A | 50843612 | 37 | 0 | 1553 | 113 | Y | Non-Cytoplasmic | cell wall structure |
| protein PPA0542 | 50842026 | 18 | 510 | 0 | 960 | Y | Non-Cytoplasmic | cell unknown |
| 50S ribosomal L29 | 50843310 | 9 | 1052 | 246 | 139 | N | Cytoplasmic | translation |
| adhesion | 50843645 | 48 | 45 | 0 | 1356 | P | Cell Wall | adhesion |
| tRNA synthetase | 50842977 | 64 | 0 | 0 | 1369 | N | Cytoplasmic | synthesis |
| 30S ribosomal S15 | 50842951 | 10 | 604 | 238 | 526 | N | Cytoplasmic | translation |
| 30S ribosomal S18 | 50843663 | 9 | 155 | 333 | 766 | M | Cytoplasmic | translation |
Table shows quantity in femtomoles per microgram of total protein detected. SignalP predictions are yes (Y), probably (P), maybe (M), and no (N). Abbreviations: RCM - Reinforced Clostridial Media, BHI - Brain Heart Infusion Broth, EBHI - 5% Egg Yolk Supplemented Brain Heart Infusion Broth. Data from one of two similar experiments.
Table 3.
The 20 Most Abundant Cytoplasmic Proteins
| Protein | Accession (gi) | MW (kDa) | RCM fmol | BHI fmol | EBHI fmol | SignalP | PSORTb | Functional Group |
|---|---|---|---|---|---|---|---|---|
| GAPDH | 50842303 | 36 | 943 | 1270 | 1013 | N | Cytoplasmic | metabolism |
| co-chaperonin GroES | 50843233 | 11 | 1206 | 1108 | 605 | N | Cytoplasmic | protein folding |
| phosphopyruvate hydratase | 50842029 | 46 | 680 | 1193 | 699 | N | Cytoplasmic | metabolism |
| 50S ribosomal L7/L12 | 50843339 | 14 | 546 | 816 | 695 | N | Cytoplasmic | translation |
| elongation factor Tu | 50843327 | 44 | 649 | 795 | 480 | N | Cytoplasmic | translation |
| fructose-bisphosphate aldolase | 50843457 | 37 | 430 | 605 | 681 | N | Cytoplasmic | metabolism |
| phosphoglyceromutase | 50841849 | 28 | 519 | 573 | 523 | N | Cytoplasmic | metabolism |
| aspartate aminotransferase | 50841624 | 39 | 443 | 478 | 568 | N | Cytoplasmic | synthesis |
| molecular chaperone GroEL | 50843232 | 56 | 755 | 368 | 349 | N | Cytoplasmic | protein folding |
| DNA-binding protein HU | 50843144 | 10 | 351 | 539 | 517 | N | Cytoplasmic | DNA structure |
| molecular chaperone GroEL | 50841936 | 57 | 637 | 261 | 382 | N | Cytoplasmic | protein folding |
| phosphoglycerate kinase | 50842304 | 42 | 262 | 476 | 456 | N | Cytoplasmic | metabolism |
| malate dehydrogenase | 50843200 | 35 | 367 | 298 | 427 | N | Cytoplasmic | metabolism |
| molecular chaperone DnaK | 50843484 | 66 | 379 | 265 | 430 | M | Cytoplasmic | protein folding |
| elongation factor Ts | 50842998 | 28 | 249 | 374 | 378 | N | Cytoplasmic | translation |
| hypothetical protein PPA0277 | 50841766 | 12 | 45 | 555 | 311 | N | Cytoplasmic | DNA structure |
| polynucleotide phosphorylase | 50842950 | 79 | 225 | 301 | 356 | N | Cytoplasmic | miscellaneous |
| fine tangled pili | 50843572 | 19 | 381 | 189 | 282 | N | Cytoplasmic | mobility |
| ornithine carbamoyltransferase | 50842070 | 37 | 386 | 179 | 234 | N | Cytoplasmic | synthesis |
| triosephosphate isomerase | 50842305 | 27 | 213 | 241 | 330 | N | Cytoplasmic | metabolism |
Table shows quantity in femtomoles per microgram of total protein detected. SignalP predictions are yes (Y), probably (P), maybe (M), and no (N). Abbreviations: RCM - Reinforced Clostridial Media, BHI - Brain Heart Infusion Broth, EBHI - 5% Egg Yolk Supplemented Brain Heart Infusion Broth. Data from one of two similar experiments.
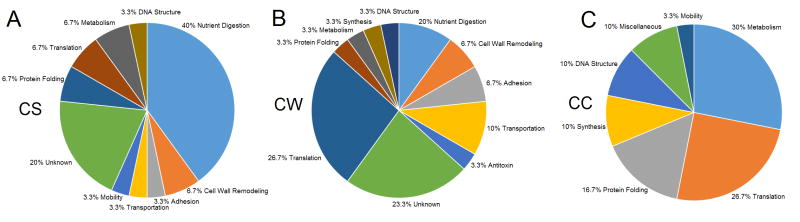
Functional Analysis
NCBI’s BLAST was used to assess the function of proteins by homology. Similar proteins were identified, together with conserved domains, and this information was used to assess a possible function of 176 proteins (Table S1). In the CS fractions, 40% of the 30 most abundant proteins were associated with digestion of protein, lipid, or carbohydrate nutrients (Figure 2A). Proteins of unknown function represented 20% of the CS proteins, and 16.7% were possible surface proteins (involved in cell wall remodeling, adhesion, transportation, and mobility) that had been released. The remainder in the CS fraction were cytosolic proteins of varying function. In the CW fractions, 36.7% of the most abundant 30 proteins were associated with varying cell surface-related activities including nutrient digestion, adhesion, transportation, cell wall remodeling, as well as one protein with putative antitoxin properties (Figure 2B). Comparatively, 23.3% of the CS proteins were unknown, and the rest were cytosolic proteins, with ribosomal proteins particularly well-represented. The CC fraction was dominated by proteins involved in metabolism, translation, and protein folding, with significant numbers of proteins involved in synthesis and DNA structure (Figure 2C).
Figure 2. Protein Functional Analysis.
Functional analysis of the 30 most abundant proteins in the secreted fraction (A), cell wall fraction (B), and cytosolic fraction (C). Abbreviations: CS - Cell Secretion, CW - Cell Wall, CC - Cell Cytosolic.
Discussion
By examining the proteome in three different types of growth media, we can gain insight into which proteins may be expressed in a wide variety of environments in vivo, including the pilosebaceous unit in acne and those environments in other P. acnes infections. RCM and BHI are common media used to culture P. acnes in vivo, but these do not provide a lipid-rich environment to approximate the pilosebacous gland. Thus, we investigated a third media, EBHI, which uses egg yolk as a source of lipids. Of note, a large number of proteins were expressed by P. acnes in all three types of media, indicating that its proteome is largely conserved between different environments. This may be due to the small ecological niche that P. acnes occupies, reducing its need for an ability to significantly change its proteome for different environments. Since most of those conserved proteins identified in vitro across media types would likely be expressed in vivo, our findings may also apply to the P. acnes proteome in the pilosebacous unit, and thus easing the identification of good vaccine candidates. Indeed, the proteins detected in vivo by Bek-Thomsen et al. were among the most abundant in our in vitro fractions [27]. Nevertheless, we did detect a few interesting differences between media types. In BHI media, we found lower expression of some adhesion proteins (50843565, 50843645) and lipases (50843205, 50843480, 50843543), perhaps reflective of the increased nutrient density and variety in EBHI and especially RCM. One CAMP factor was also not found in the CS and CW fractions of BHI, but it was abundant in RCM and EBHI.
The CS and CW fractions contained many proteins with “unknown” or “non-cytoplasmic” localization prediction according to PSORTb. It seems likely that the bulk of these proteins are true secreted proteins or cell wall proteins, implying that the PSROTb program can be further refined for greater predictive accuracy for these types of proteins. Also many proteins with predicted membrane localization were detected in the CW fractions, including several transporters. While lysozyme digestion of the cell wall should have left the membrane intact, it is possible that some membrane proteins may have components embedded in the cell wall that were released upon cell wall disruption.
There were several proteins of predicted cytoplasmic localization in our CS and CW fractions, several of which have been seen in previous studies [24, 26]. While some of these are likely contaminants, natural autolysis of P. acnes could result in many of these proteins being released into the media and later binding to the surface of the bacteria. Additionally, these proteins may have dual roles outside of the cytoplasm, and may be secreted by non-traditional pathways, accounting for their cytoplasmic localization prediction. Furthermore, many abundant CC proteins were not found in CS and CW fractions, indicating that those possible contaminating proteins represent only a subfraction of likely contaminants and thus, are largely not contaminants at all. Finally, functional analysis of CS and CW proteins indicates that these prediction programs are likely accurate, since they predicted that proteins with expected secreted and cell wall functions were non-cytoplasmic.
More refined methods are needed to obtain pure CM fractions of P. acnes. Even protocols utilizing ultracentrifugation yielded no improvement in purity. However, it should be noted that while the CC and TCE fractions have a very high degree of overlap, the CM fraction has a fair number of unique protein identifications in comparison, implying that this protein fraction is distinctly different from a purely cytosolic fraction, which would be the case if contaminants completely dominated the CM fraction.
The RCM CS fraction shared many highly expressed proteins in common with a study on the P. acnes secretome by Holland et al. [24]. While not directly comparable due to the different type of media used, the fact that similar highly expressed proteins were found in both studies and in a separate in vivo study [27] supports both methods used. While Holland et al.’s method of 2D gel digestion allowed for use of BHI, and their earlier exponential phase cultures allowed for higher purity (fewer cytosolic proteins, likely due to less autolysis), our use of in-solution digestion followed by assessment with an Orbitrap mass spectrometer allowed for many more proteins to be both identified and quantified. These same conclusions apply to the study of the cell wall proteome by Mak et al. [26], where many of the same highly expressed P. acnes proteins were also detected in both studies.
With regard the existing vaccine candidates, the most highly expressed CAMP factor (50842175) detected in our fractions was the same protein used in a vaccine to reduce inflammation in a mouse ear infection model [16]. In contrast, the surface sialidase, which conferred similar vaccine protection [14], was not detected in our protein fractions or in the in vivo study of Bek-Thomsen et al. [27]. The CAMP factor protein may therefore have a higher probability of in vivo expression in the pilosebaceous environment. Several proteins from P. acnes were found to confer vaccine protection in mice against A. pleuropneumoniae [19]. The most efficient of these was a single-stand DNA binding protein (50843664), which was detected at moderate level in our CC fraction. Phage shock protein A (50842186), the second-most efficient vaccine in the mouse model, was detected at a somewhat lower level in the CC fraction. All other proteins in their study were also detected in our CC fraction in varying quantity, indicating that they may be also be used in a P. acnes-based vaccine as well.
Several highly expressed surface and secreted proteins detected in our study are of potential future interest for functional studies or vaccine candidates. These include the PPA1939 protein (50843388) of unknown function, which was the most abundant secreted protein, and among the most abundant in the cell wall. Other surface proteins that are highly expressed and may make good vaccine candidates include DsA1, an adhesion/S-layer protein (50843565), and a probable transporter lipoprotein (50843218). Since P. acnes is a commensal organism and acne is an inflammatory disease, it may be preferable for an acne vaccine candidate to induce a strong Treg response, rather than the more common Th1, Th17, or antibody response. Thus, several vaccine candidates would need thorough assessment using human cells to find one suitable for use in acne. These same highly expressed surface and secreted proteins may be potential virulence factors. Further research to determine their function may allow for greater understanding of the mechanisms of pathogenesis in acne.
We investigated P. acnes strain ATCC 6919, a MLST phylotype IA1 [9, 29] and whole-genome phylotype IA-2 [38] strain of P. acnes. Many other phylotypes are of potential interest, since they have been recently shown to have differing disease associations, including for acne vulgaris [28, 29, 39]. We are currently characterizing the full proteome of several of these phylotypes.
Conclusions
Our study presents a comprehensive overview of the P. acnes proteome, with proteins identified and quantified using an Orbitrap mass spectrometer. In addition to cytoplasmic proteins, we also identified several dozen secreted and cell wall proteins, which were analyzed for predicted localization and function. Our identified cell wall proteins, due to their surface localization, represent potential vaccine candidates.
Supplementary Material
Highlights.
We quantify the secreted, cell wall, membrane, and cytosolic proteome of P. acnes.
531 proteins were identified and analyzed for localization and function.
Several surface proteins were identified as potential vaccine candidates.
Acknowledgments
This research was supported by NIH grant RO1-AR-053542 to JK, an Annenberg Foundation Grant to JK, an ADA Medical Student Fellowship to YY, an AARS Clinical Research Grant to YY, and a Carolyn L. Kuckein Student Research Fellowship to YY.
Footnotes
Conflicts of Interest
JK has consulted for Allergan, Leo Pharma, Anacor, and TPG.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2013;168(3):474–485. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 2.Beylot C, Auffret N, Poli F, Claudel JP, et al. Propionibacterium acnes: an update on its role in the pathogenesis of acne. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014;28(3):271–278. doi: 10.1111/jdv.12224. [DOI] [PubMed] [Google Scholar]
- 3.Mak TN, Yu SH, De Marzo AM, Bruggemann H, Sfanos KS. Multilocus sequence typing (MLST) analysis of Propionibacterium acnes isolates from radical prostatectomy specimens. Prostate. 2013;73(7):770–777. doi: 10.1002/pros.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubin GG, Portillo ME, Trampuz A, Corvec S. Propionibacterium acnes, an emerging pathogen: From acne to implant-infections, from phylotype to resistance. Medecine et maladies infectieuses. 2014 doi: 10.1016/j.medmal.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Portillo ME, Corvec S, Borens O, Trampuz A. Propionibacterium acnes: an underestimated pathogen in implant-associated infections. Biomed Res Int. 2013;2013:804391. doi: 10.1155/2013/804391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rollason J, McDowell A, Albert HB, Barnard E, et al. Genotypic and antimicrobial characterisation ofPropionibacterium acnes isolates from surgically excised lumbar disc herniations. Biomed Res Int. 2013;2013:530382. doi: 10.1155/2013/530382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schimel AM, Miller D, Flynn HW., Jr Endophthalmitis isolates and antibiotic susceptibilities: a 10-year review of culture-proven cases. American journal of ophthalmology. 2013;156(1):50–52. e51. doi: 10.1016/j.ajo.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Lomholt HB, Kilian M. Clonality and anatomic distribution on the skin of antibiotic resistant and sensitive Propionibacterium acnes. Acta dermato-venereologica. 2014 doi: 10.2340/00015555-1794. [DOI] [PubMed] [Google Scholar]
- 9.McDowell A, Barnard E, Nagy I, Gao A, et al. An expanded multilocus sequence typing scheme for Propionibacterium acnes: investigation of 'pathogenic', 'commensal' and antibiotic resistant strains. PLoS One. 2012;7(7):e41480. doi: 10.1371/journal.pone.0041480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder S, Crandell I, Davis SA, Feldman SR. Medical adherence to acne therapy: a systematic review. American journal of clinical dermatology. 2014;15(2):87–94. doi: 10.1007/s40257-014-0063-y. [DOI] [PubMed] [Google Scholar]
- 11.Simonart T. Immunotherapy for acne vulgaris: current status and future directions. American journal of clinical dermatology. 2013;14(6):429–435. doi: 10.1007/s40257-013-0042-8. [DOI] [PubMed] [Google Scholar]
- 12.Kim J. Acne vaccines: therapeutic option for the treatment of acne vulgaris? J Invest Dermatol. 2008;128(10):2353–2354. doi: 10.1038/jid.2008.221. [DOI] [PubMed] [Google Scholar]
- 13.Nakatsuji T, Liu YT, Huang CP, Zoubouis CC, et al. Antibodies elicited by inactivated propionibacterium acnes-based vaccines exert protective immunity and attenuate the IL-8 production in human sebocytes: relevance to therapy for acne vulgaris. J Invest Dermatol. 2008;128(10):2451–2457. doi: 10.1038/jid.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatsuji T, Liu YT, Huang CP, Zouboulis CC, et al. Vaccination targeting a surface sialidase of P.acnes: implication for new treatment of acne vulgaris. PLoS One. 2008;3(2):e1551. doi: 10.1371/journal.pone.0001551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakatsuji T, Tang DC, Zhang L, Gallo RL, Huang CM. Propionibacterium acnes CAMP factor and host acid sphingomyelinase contribute to bacterial virulence: potential targets for inflammatory acne treatment. PLoS One. 2011;6(4):e14797. doi: 10.1371/journal.pone.0014797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu PF, Nakatsuji T, Zhu W, Gallo RL, Huang CM. Passive immunoprotection targeting a secreted CAMP factor of Propionibacterium acnes as a novel immunotherapeutic for acne vulgaris. Vaccine. 2011;29(17):3230–3238. doi: 10.1016/j.vaccine.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitagawa H, Yamanaka K, Kakeda M, Inada H, et al. Propionibacterium acnes vaccination induces regulatory T cells and Th1 immune responses and improves mouse atopic dermatitis. Exp Dermatol. 2011;20(2):157–158. doi: 10.1111/j.1600-0625.2010.01180.x. [DOI] [PubMed] [Google Scholar]
- 18.Lei L, Sun C, Lu S, Feng X, et al. Selection of serotype-specific vaccine candidate genes in Actinobacillus pleuropneumoniae and heterologous immunization with Propionibacterium acnes. Vaccine. 2008;26(49):6274–6280. doi: 10.1016/j.vaccine.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Sun C, Yang F, Yang S, et al. Identification of proteins of Propionibacterium acnes for use as vaccine candidates to prevent infection by the pig pathogen Actinobacillus pleuropneumoniae. Vaccine. 2013;31(45):5269–5275. doi: 10.1016/j.vaccine.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda K, Yamanaka K, Linan W, Miyahara Y, et al. Intratumoral injection of Propionibacterium acnes suppresses malignant melanoma by enhancing Th1 immune responses. PLoS One. 2011;6(12):e29020. doi: 10.1371/journal.pone.0029020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis VO, Silva JC, Souza GT, Semedo P, et al. The polysaccharide fraction of Propionibacterium acnes modulates the development of experimental focal segmental glomerulosclerosis. Immunobiology. 2012;217(9):831–841. doi: 10.1016/j.imbio.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Girvan RC, Knight DA, O'Loughlin CJ, Hayman CM, et al. MIS416, a non-toxic microparticle adjuvant derived from Propionibacterium acnes comprising immunostimulatory muramyl dipeptide and bacterial DNA promotes cross-priming and Th1 immunity. Vaccine. 2011;29(3):545–557. doi: 10.1016/j.vaccine.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Mussalem JS, Squaiella-Baptistao CC, Teixeira D, Yendo TM, et al. Adjuvant effect of killed Propionibacterium acnes on mouse peritoneal B-1 lymphocytes and their early phagocyte differentiation. PLoS One. 2012;7(3):e33955. doi: 10.1371/journal.pone.0033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holland C, Mak TN, Zimny-Arndt U, Schmid M, et al. Proteomic identification of secreted proteins of Propionibacterium acnes. BMC Microbiol. 2010;10:230. doi: 10.1186/1471-2180-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dekio I, Culak R, Fang M, Ball G, et al. Correlation between phylogroups and intracellular proteomes of Propionibacterium acnes and differences in the protein expression profiles between anaerobically and aerobically grown cells. Biomed Res Int. 2013;2013:151797. doi: 10.1155/2013/151797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mak TN, Schmid M, Brzuszkiewicz E, Zeng G, et al. Comparative genomics reveals distinct host-interacting traits of three major human-associated propionibacteria. BMC genomics. 2013;14:640. doi: 10.1186/1471-2164-14-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bek-Thomsen M, Lomholt HB, Scavenius C, Enghild JJ, Bruggemann H. Proteome analysis of human sebaceous follicle infundibula extracted from healthy and acne-affected skin. PLoS One. 2014;9(9):e107908. doi: 10.1371/journal.pone.0107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitz-Gibbon S, Tomida S, Chiu BH, Nguyen L, et al. Propionibacterium acnes strain populations in the human skin microbiome associated with acne. J Invest Dermatol. 2013;133(9):2152–2160. doi: 10.1038/jid.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDowell A, Nagy I, Magyari M, Barnard E, Patrick S. The opportunistic pathogen Propionibacterium acnes: insights into typing, human disease, clonal diversification and CAMP factor evolution. PLoS One. 2013;8(9):e70897. doi: 10.1371/journal.pone.0070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallis HA, Miller SE, Wheat RW. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infection and immunity. 1976;13(5):1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuobi-Hasona K, Brady LJ. Isolation and solubilization of cellular membrane proteins from bacteria. Methods in molecular biology. 2008;425:287–293. doi: 10.1007/978-1-60327-210-0_23. [DOI] [PubMed] [Google Scholar]
- 32.Graham C, McMullan G, Graham RL. Proteomics in the microbial sciences. Bioengineered bugs. 2011;2(1):17–30. doi: 10.4161/bbug.2.1.14413. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Adams RM, Chourey K, Hurst GB, et al. Systematic comparison of label-free, metabolic labeling, and isobaric chemical labeling for quantitative proteomics on LTQ Orbitrap Velos. Journal of proteome research. 2012;11(3):1582–1590. doi: 10.1021/pr200748h. [DOI] [PubMed] [Google Scholar]
- 34.Bruggemann H, Henne A, Hoster F, Liesegang H, et al. The complete genome sequence of Propionibacterium acnes, a commensal of human skin. Science. 2004;305(5684):671–673. doi: 10.1126/science.1100330. [DOI] [PubMed] [Google Scholar]
- 35.Yu NY, Wagner JR, Laird MR, Melli G, et al. PSORTb 3. 0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26(13):1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4. 0: discriminating signal peptides from transmembrane regions. Nature methods. 2011;8(10):785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. Journal of molecular biology. 1990;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 38.Tomida S, Nguyen L, Chiu BH, Liu J, et al. Pan-genome and comparative genome analyses of Propionibacterium acnes reveal its genomic diversity in the healthy and diseased human skin microbiome. MBio. 2013;4(3):e00003–00013. doi: 10.1128/mBio.00003-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Champer J, Garban H, Kim J. Typing of Propionibacterium acnes: a review of methods and comparative analysis. Br J Dermatol. 2015 doi: 10.1111/bjd.13667. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.