Abstract
Oral tongue squamous cell carcinoma (TSCC) is a complex disease with extensive genetic and epigenetic defects, including microRNA deregulation. The aims of the present study were to test the feasibility of performing the microRNA profiling analysis on archived TSCC specimens and to assess the potential diagnostic utility of the identified microRNA biomarkers for the detection of TSCC. TaqMan array-based microRNA profiling analysis was performed on 10 archived TSCC samples and their matching normal tissues. A panel of 12 differentially expressed microRNAs was identified. Eight of these differentially expressed microRNAs were validated in an independent sample set. A random forest (RF) classification model was built with miR-486-3p, miR-139-5p, and miR-21, and it was able to detect TSCC with a sensitivity of 100% and a specificity of 86.7% (overall error rate = 6.7%). As such, this study demonstrated the utility of the archived clinical specimens for microRNA biomarker discovery. The feasibility of using microRNA biomarkers (miR-486-3p, miR-139-5p, and miR-21) for the detection of TSCC was confirmed.
Keywords: microRNA, biomarker, miR-486-3p, miR-139-5p, miR-21, formalin-fixed paraffin-embedded, tongue squamous cell carcinoma
Introduction
Head and neck/oral cancer (HNOC) is the sixth most common cancer worldwide, accounting for 4% of cancers in men and 2% of cancers in women,1 with an incidence of approximately 600,000 cases per year and a mortality rate of approximately 50%.2 In some parts of the world, including Southern China and the Indian subcontinent, head and neck squamous cell carcinoma (HNSCC) is still a major cancer problem. Tongue squamous cell carcinoma (TSCC) is significantly more aggressive compared to other HNOCs, with a propensity for rapid spreading and local invasion3 with a distinct pattern of lymph nodal metastasis,4,5 and high recurrence rate.6 Despite recent advances in clinical strategies for treating TSCCs, the overall survival has improved only marginally. This is because TSCCs are often detected at later stages. Advances in cancer screening strategies and early detection methods are required for improvement in the prognosis of TSCC patients.
Like most of the other human cancers, TSCC is a disease involving multistep dynamic changes in the genome. While many recent studies have attempted to identify molecular biomarkers for the screening and early diagnosis of TSCC, most of these studies are based on coding genes. The potential value of utilizing microRNAs as biomarkers for TSCC detection is still not entirely clear. Micro RNAs are endogenous noncoding single-stranded small RNA molecules (18–25 nt). A number of microRNA genes have recently been characterized as oncogenes or tumor-suppressor genes, and their deregulations have been detected in many cancers, including HNOCs.7–11 MicroRNAs are important gene expression regulators, and they control the expression of their target genes by posttranscriptional mechanisms. Deregulation of these cancerous microRNA genes (eg, overexpression or deletion) contributes to tumorigenesis by promoting proliferation, survival, and invasion.12,13 MicroRNA deregulation is a frequent event in HNSCC. A number of recent reports demonstrated the feasibility of utilizing microRNAs as biomarkers to detect cancer cases from noncancerous specimens, with varying degrees of success.14,15 This type of microRNA biomarker-based approach can enhance the standard diagnostic technique of histopathologic examination.
In this study, we identified a panel of differentially expressed microRNAs based on the TaqMan array microRNA profiling analysis of archived TSCC samples and normal matched tissue samples. Using a statistical model based on three microRNA biomarkers (miR-486-3p, miR-139-5p, and miR-21), we were able to identify TSCC cases from an independent validation set, with a sensitivity of 100% (15/15), a specificity of 86.7% (13/15), and an overall error rate of 6.7%.
Patients and Methods
Patient cohorts
The following two TSCC patient cohorts were used in this study: (1) the training set: specimens from 10 TSCC cases and the adjacent matched normal tissue samples were used for microRNA differential expression profiling analysis and (2) the validation set: microRNA expression data (deep sequencing-based profiling results) and the associated demographic and clinical information of 15 TSCC cases that have matched normal samples were obtained from The Cancer Genome Atlas repository (TCGA; https://tcga-data.nci.nih.gov). Demographic and clinical information of the patients is presented in Table 1. This investigation was approved by the Institutional Review Board (IRB) of the University of Illinois at Chicago.
Table 1.
Clinical characterization of the TSCC cohorts.
| TRAINING SET (n = 10 PAIRS) |
VALIDATION SETa (n = 15 PAIRS) |
|
|---|---|---|
| Age | ||
| Median (range) | 47 (43–81) | 61 (32–87) |
| Gender | ||
| Male (%) | 50.0 | 53.3 |
| Female (%) | 50.0 | 46.7 |
| Anatomic site | ||
| Tongue (%) | 90.0 | 86.7 |
| Base of tongue (%) | 0.0 | 13.3 |
| Tongue, tonsil (%) | 10.0 | 0.0 |
| pT | ||
| pT3–4 (%) | 30.0 | 46.7 |
| pT1–2 (%) | 70.0 | 53.3 |
| pN | ||
| Positive (%) | 50.0 | 53.3 |
| Negative (%) | 50.0 | 33.3 |
| Data not available (%) | 0.0 | 13.3 |
Note:
The data for the validation set were extracted from The Cancer Genome Atlas (TCGA) Data Portal.
Laser-capture microdissection and RNA isolation
Laser-capture microdissection (LCM) procedure was performed as described previously.16,17 In brief, 7 μm sections were cut with a microtome and mounted onto Leica RNase-free PEN slides (Leica). The paraffin sections were deparaffinized and lightly stained with toluidine blue. The tumor and noncancerous epithelial cells were selectively procured using a Leica LMD7000 Laser Microdissection System. The LCM-captured cells were collected into Eppendorf caps containing 50 μL of digestion buffer (from RecoverAll kit).
Total RNA was extracted using RecoverAll (Thermo Fisher Scientific), following the manufacturer’s protocol with the exception of increased DNase digestion for 60 minutes at 37°C. RNA samples were quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies).
microRNA expression analysis by TaqMan low-density array and by TaqMan assay
MicroRNA profiling analysis was performed using the TaqMan low-density array (TLDA; Applied Biosystems), following the manufacturer’s protocol with minor modifications as previously described.18 In brief, 20 ng of RNA was used as the input for cDNA generation. Eight distinct pools of RT primers were used for analysis of 370 distinct microRNAs. Following dilution, 14 cycles of preamplification with the Megaplex pool protocol for the array were performed on the cDNA. Following dilution, the cDNAs were loaded onto the arrays (Human miRNA Array v1.0; Applied Biosystems). This facilitated analysis of 386 wells; 370 distinct microRNAs were analyzed in singlicate and two housekeeping snoRNAs with eight replicates for each. Individual TaqMan assays were also performed for selected microRNAs in triplicates for the validation study. To control for potential variations in RNA samples isolated from each case,19 we also assessed U6 snRNA with TaqMan assay (Thermo Fisher Scientific). The polymerase chain reaction (PCR) was performed on an ABI 7900HT real-time PCR system (Thermo Fisher Scientific). Ct (crosses threshold) values were determined for all samples and genes, and delta Ct (ΔCt) was computed using U6 snRNA as an internal control.20
Data analysis and statistical methods
MicroRNA differential expression analysis was performed using Cyber-T,21,22 and hierarchical clustering and principal component (PC) analysis were performed using ClustVis.23 Other statistical analyses were performed using the S-Plus 6.0 and R 3.2.2. The differences between groups were evaluated by Wilcoxon signed-rank test. Receiver-operating characteristic (ROC) curve analysis was performed, and the area under the ROC (AUROC) was computed for assessing the predictive power of the selected biomarkers. To select the combination of biomarkers that provides the best prediction, the random forest (RF) classification model was utilized. The Mean Decrease Gini was computed to assess the relative importance of microRNA biomarkers toward an RF classification model. Since the structure of the data for the training set and validation set are different (ΔCt value for the quantitative PCR measurement, and reads per million miRNA mapped for the deep sequencing-based quantification, respectively), a simple transformation was performed on all data before the statistical modeling [(x − μ(normal))/σ(normal), where μ(normal) is the mean of the normal group and σ(normal) is the standard deviation of the normal group].
Results
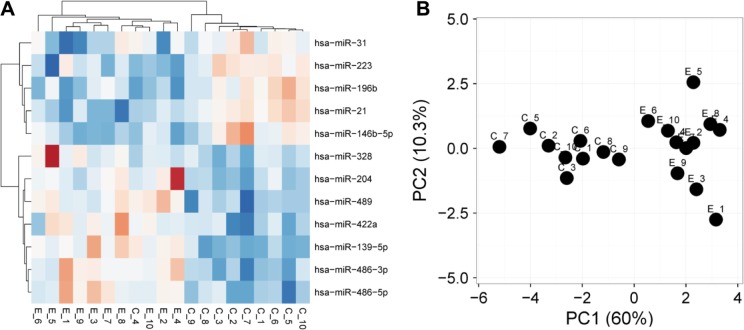
LCM was performed on 10 archived TSCC samples and matched noncancerous tissue samples, and microRNA profiling analysis was performed using TaqMan arrays. As shown in Table 2 and Supplementary Table 1, we identified a panel of 12 differentially expressed microRNAs. Hierarchical clustering (Fig. 1A) and PC analysis (Fig. 1B) suggested that this panel of microRNAs could provide classification values distinguishing TSCC samples from normal tissue samples.
Table 2.
Receiver-operating characteristic (ROC) curve analysis of TSCC-associated microRNAs.a
| TRAINING SAMPLE SET | VALIDATION SAMPLE SETb | |||
|---|---|---|---|---|
| WILCOXON (P VALUE) |
ROC (AUC) |
WILCOXON (P VALUE) |
ROC (AUC) |
|
| miR-486-3p | 0.0006 | 0.9333 | 0.0619 | 0.7022 |
| miR-21 | 0.0021 | 0.9000 | <0.0001 | 0.9911 |
| miR-486-5p | 0.0015 | 0.9111 | 0.0086 | 0.7778 |
| miR-139-5p | 0.0006 | 0.9333 | 0.0002 | 0.8756 |
| miR-204 | 0.0133 | 0.8333 | <0.0001 | 1.0000 |
| miR-489 | 0.0041 | 0.8778 | 0.8985 | 0.4844 |
| miR-223 | 0.0057 | 0.8667 | 0.1064 | 0.6756 |
| miR-196b | 0.0057 | 0.8667 | <0.0001 | 0.9822 |
| miR-31 | 0.0435 | 0.7778 | 0.0050 | 0.7956 |
| miR-422a | 0.0042 | 0.8778 | 0.0384 | 0.6333 |
| miR-328 | 0.2428 | 0.6667 | 0.2854 | 0.6178 |
| miR-146b-5p | 0.0279 | 0.8000 | 0.0003 | 0.8667 |
Notes:
The microRNA data for the training sample set and validation sample set were assessed with different platforms (TaqMan assay and deep sequencing, respectively). To enable the comparison between these datasets, transformation was performed as described in “Patients and methods” section.
The miRSeq dataset for 15 TSCC and paired normal tissue samples was downloaded from TCGA data portal. The levels of microRNAs were extracted as reads per million miRNA mapped.
Figure 1.
MicroRNAs profiling on TSCC samples. Laser-capture microdissection was performed to acquire tumor cells from 10 cases of archived TSCC samples and matched normal samples. MicroRNA profiling was performed on these samples using TaqMan microRNA arrays. A signature gene set of 12 microRNAs was created as described in the “Patients and methods” section. Hierarchical clustering (A) and principal component (PC) analysis (B) were performed based on this signature set.
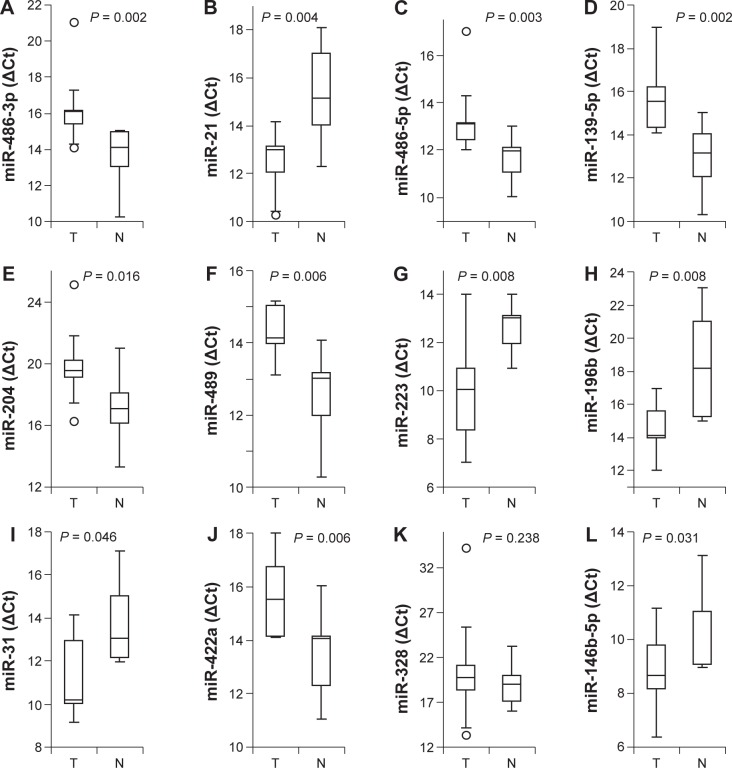
Of the 12 identified microRNAs, the differential expression was validated for 11 microRNAs (miR-486-3p, miR-21, miR-486-5p, miR-139-5p, miR-204, miR-489, miR-223, miR-196b, miR-31, miR-422a, and miR-146b-5p) using the individual TaqMan assays on the same sample set (Fig. 2). Differential expression of miR-328 was not validated (P = 0.238; Fig. 2K). To further validate the differential expression of the identified microRNAs, microRNA expression profiling results on 15 TSCC cases and the paired normal tissue samples were obtained from TCGA. Of the 12 microRNAs tested, the differential expression was validated for 8 microRNAs (miR-21, miR-486-5p, miR-139-5p, miR-204, miR-196b, miR-31, miR-422a, and miR-146b-5p) based on the TCGA dataset (Table 2 and Supplementary Table 2). Differential expression of miR-489, miR-223, and miR-328 was not validated. Apparent differential expression of miR-486-3p was also observed, but the difference is not statistically significant (P = 0.0619).
Figure 2.
MicroRNAs differential expression on TSCC samples. The TaqMan-based qPCR was performed to assess the levels of miR-486-3p (A), miR-21 (B), miR-486-5p (C), miR-139-5p (D), miR-204 (E), miR-489 (F), miR-223 (G), miR-196b (H), miR-31 (I), miR-422a (J), miR-328 (K), and miR-146b-5p (L) on TSCC samples and normal samples. The boxes represent 25th to 75th percentile of the observations, and the lines in the middle of the box represent the median. The whiskers represent maximum (or minimum) observations below (or above) the 1.5 times of the interquartile range, respectively. Outliers are also indicated in the plots as black circles.
The ROC analysis was performed to assess the predictive powers of the identified microRNA biomarkers using both sample sets. The AUROC for miR-486-3p, miR-21, miR-486-5p, miR-139-5p, miR-204, miR-489, miR-223, miR-196b, miR-31, miR-422a, miR-328, and miR-146b-5p were 0.9333, 0.9000, 0.9111, 0.9333, 0.8333, 0.8778, 0.8667, 0.8667, 0.7778, 0.8778, 0.6667, and 0.8000 for the training samples set, and 0.7022, 0.9911, 0.7778, 0.8756, 1.0000, 0.4844, 0.6756, 0.9822, 0.7956, 0.6333, 0.6178, and 0.8667 for the validation sample set (TCGA), respectively (Table 2).
The RF model was built for selecting the best biomarker combination that can provide the highest prediction power. The Gini importance values were computed to evaluate the relative importance of the biomarkers, and miR-486-3p, miR-139-5p, and miR-21 have the highest relative importance (Fig. 3). The RF classification model based on these top three microRNA biomarkers was used to classify normal and TSCC cases of the validation sample set (n = 30). As shown in Table 3, the overall error rate of this classification model is 6.7% [sensitivity = 100% (15/15) and specificity = 86.7% (13/15)].
Figure 3.
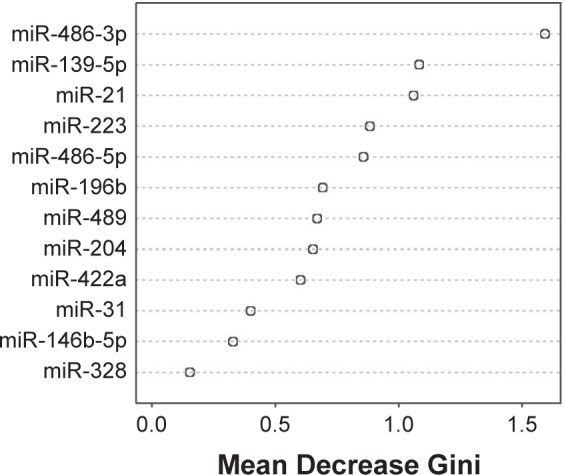
Ranking the microRNA biomarkers by random forest model. The relative importance of microRNA biomarkers toward a random forest classification model was assessed by computing the Gini importance, and the microRNAs were ranked by Mean Decrease Gini.
Table 3.
Random forest classification model for TSCC prediction.a
| TRUTH | ||
|---|---|---|
| NORMAL | TSCC | |
| Prediction | ||
| Normal | 13 | 0 |
| TSCC | 2 | 15 |
Notes:
Random forest classification model based on top three microRNA biomarkers (miR-486-3p, miR-139-5p, and miR-21) was used to predict normal and TSCC cases of the validation sample set (n = 30). The sensitivity of this classification model is 100% (15/15), and the specificity is 86.7% (13/15).
Discussion
While mechanistic studies of the roles of microRNA in tumorigenesis are gaining momentum, translational studies utilizing microRNAs as biomarkers are still in their infancy. Nevertheless, microRNAs are remarkably stable in cells, tissue specimens (archived or fresh), and a number of biofluids, and as such, microRNA-based biomarkers are less prone to minor differences in sample processing, which offers a great advantage over other classes of biomarkers. In the clinical setting, the storing of formalin-fixed paraffin-embedded (FFPE) tissue blocks is the standard method for pathology departments to archive almost all tissue samples, which can then be linked with clinical databases, including disease diagnoses and patients’ follow-up information. As such, reliable biomarkers based on FFPE samples will greatly facilitate large-scale clinical cohort-based studies and provide an extremely powerful tool for cancer research. Hence, it may be the only way to make the integration of new biomarkers into the large-scale and clinical trial studies possible. However, it has been assumed that FFPE samples have insufficient quality of RNA, and, as a result, these samples have not been routinely used in biomarker studies. In recent years, it has been shown that RNA stability varies among different RNA species and that microRNAs are very stable in relation to other RNA species (eg, mRNA). One explanation is that both ends of the mature microRNA are protected by Argonaute family proteins and the entire microRNA molecule is incorporated into the RNA-induced silencing complex (RISC).24 These protein–microRNA complexes may prevent microRNA from degradation, especially during the formalin fixation process and while in long-term storage in paraffin.
The potential of utilizing microRNA as biomarkers in FFPE samples has recently been explored by several groups.18,25–30 Recent studies indicated that quantitative PCR methods based on small amplicons (such as TaqMan assays) work well with microRNA quantification, even with suboptimal starting materials, eg, samples acquired FFPE tissue blocks.18 Our current study confirms the feasibility of utilizing microRNAs from archived specimens as biomarkers to discriminate TSCC from noncancerous control tissues. We identified a panel of 12 differentially expressed microRNAs using the FFPE tissue blocks of TSCC specimens, including miR-486 (both -3p and -5p), miR-21, miR-139-5p, miR-204, miR-489, miR-223, miR-196b, miR-31, miR-422a, miR-328, and miR-146b-5p. Aberrant expression of miR-486 is a common event in many cancer types31–37 and has been suggested to have potential diagnostic value for lung cancer38 and prognostic values for patients with esophageal squamous cell carcinoma (ESCC) or gastric adenocarcinoma (GC).39 MicroRNA-21 is one of the most well-documented oncogenic microRNAs40,41 and has been suggested as a biomarker for many types of cancer, including HNOC.42–46 Downregulation of miR-139 has been observed in HNOC,7,8,47 and a recent study suggested that miR-139-5p from saliva samples may be a molecular biomarker for the early diagnosis of TSCC.48 While miR-204 has been suggested as a tumor suppressor in HNOC,49–51 conflicting results regarding its expression pattern have been reported in various cancer types. The enhanced expression of miR-204 was observed in insulinomas and acute lymphocytic leukemia;52,53 downregulation of miR-204 was reported in other cancer types, including HNOC, lung cancer, gastric cancer, endometrial cancer, and renal cancer.54–57 Furthermore, conflicting results regarding the miR-204 expression were reported in some other cancer types including breast cancer58–60 and prostate cancer.61,62 Our results here confirmed that miR-204 is downregulated in TSCC, which supports its tumor-suppressor role. Relatively little is known on the role of miR-489 in cancer, and conflicting results regarding the aberrant expression pattern of miR-489 have been reported in HNOC.63,64 We observed downregulation of miR-489 in our training set, but no statistical significant change in miR-489 level in the validation set. Thus, the observed differential expression of miR-489 in HNOC remains controversial, and additional studies with large sample sizes will be needed to fully explore this. Upregulation of miR-223 has been consistently observed in both tumor tissues and plasma samples of HNOC patients,11,65–67 and the diagnostic value of circulating miR-223 as a biomarker for HNOC has recently been suggested.67 MicroRNA-196b (together with miR-196a, the other member of miR-196 family) has been suggested as an OncomiR in HNOC, which promotes invasive phenotype and metastasis,68,69 and may serve as a biomarker for early detection and prognosis.70,71 The role of miR-31 appears to be cancer type specific; although miR-31 inhibits metastasis in breast cancer,72 upregulation of miR-31 is essential to the TGF-beta-induced invasion and metastasis of colon cancer.73 The increases of miR-31 in tumor tissue, saliva, and plasma samples have all been suggested as potential biomarkers of HNOC.74–76 MicroRNA-422a has been suggested as a tumor suppressor, and the reintroduction of miR-422a to cancer cells led to inhibition in cell proliferation, migration, invasion, metastasis, and enhanced chemosensitivity in various cancers.77–79 A recent study suggested that miR-422a deregulation may promote locoregional recurrence of HNOC.80 The potential of utilizing miR-422a as a biomarker has been suggested for several cancer types, including colorectal cancer and osteosarcoma.81–84 Several studies have suggested that miR-328 may serve as a biomarker for glioma, thyroid cancer, and non-small cell lung cancer (NSCLC).85–89 However, the differential expression of miR-328 was not validated in our training set, and no statistical significant change of miR-328 was observed in our validation set. The expression of miR-146b-5p appears to be cancer type specific. In glioma, miR-146b-5p expression is downregulated and acts as a tumor suppressor.90 However, in thyroid cancer and lung cancer, miR-146b-5p acts as an oncogene and has been suggested as a potential diagnostic marker.91,92
Our statistical analysis indicated that miR-486-3p, miR-139-5p, and miR-21 have the best classification power in terms of discriminating TSCC from normal control samples. We constructed an RF model by combining miR-486-3p, miR-139-5p, and miR-21, and we were able to achieve an excellent classification outcome (sensitivity = 100%, specificity = 86.7%, and overall error rate = 6.7%). These results clearly indicated that microRNA molecules acquired from archived TSCC specimens can be accurately measured and employed as molecular biomarkers for the early detection of TSCC. However, our sample size is relatively small. Additional studies with larger sample sizes are required to fully evaluate these microRNA biomarkers in detecting TSCCs.
MicroRNA deregulation has also been identified in premalignant lesions57,75,93,94 and in the field of cancerization.95–98 Thus, microRNA biomarker-based approaches can potentially be implemented as a cancer-screening tool for monitoring the oral premalignant lesions and the field of cancerization. In our future study, we anticipate to expend our investigation by utilizing cancer tissue, premalignant lesions, and histologically normal tissue samples adjacent to cancer, as well as other clinical samples (eg, blood, serum, and/or saliva) from the same TSCC patients. This will allow us to fully explore the feasibility of utilizing microRNA biomarkers for cancer screening.
Conclusion
Our study demonstrated that microRNA deregulation can be accurately measured in archived clinical specimens and can be employed as molecular biomarkers for detecting TSCC. While utilizing microRNAs from snap-frozen specimens as biomarkers can produce better overall error rate,18 in the present study, microRNA biomarkers from archived clinical specimens also resulted in excellent levels of sensitivity and specificity. A specific combination of microRNA biomarkers (miR-486-3p, miR-139-5p, and miR-21) can achieve optimal outcomes in distinguishing TSCC from noncancerous tissue samples. Advances in in situ hybridization (ISH) probes for microRNA detection present us with potential strategies of integrating microRNA-based analysis with histopathologic examination,99–101 which may lead to improvement of early detection and prevention of oral cancer. One of the main limitations of our study is the small sample size. It is possible that there are still other microRNA biomarkers yet to be identified. Additional studies consisting of early-stage TSCC cases with larger sample sizes are required to fully evaluate the feasibility of this microRNA-based approach for early detection.
Supplementary Materials
Supplementary table S1. The levels of 12 microRNAs on 10 cases of OTSCC and paired normal tissues.
Supplementary table S2. The levels of 12 microRNAs on 15 paired OTSCC and normal tissues (TCGA dataset).
Footnotes
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
PEER REVIEW: Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1018 words, excluding any confidential comments to the academic editor.
FUNDING: This research was made possible, in part, by NIH PHS grants (CA139596 and CA171436) and the Lilly USA Research Award in Cancer Prevention and Early Detection funded by Lilly USA, LLC and awarded by Prevent Cancer Foundation to XZ. YJ is supported by a T32 training grant (DE018381) from NIH/NIDCR. IM is supported by a scholarship under the International Research Support Initiative Program from Higher Education Commission of Pakistan. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
Author Contributions
Conceived and designed the experiments: RJC, YJ, XL, YD, XZ. Performed experiments: ZC, YJ, IM. Analyzed the data: TY, LH, YD, XZ. Wrote the first draft of the manuscript: TY, RJC, YD, XZ. Contributed to the writing of the manuscript: ZC, YJ, IM, XL, LH. Agree with manuscript results and conclusions: ZC, TY, RJC, YJ, IM, XL, LH, YD, XZ. Made critical revisions and approved final version: TY, RJC, YD. All authors reviewed and approved of the final manuscript.
REFERENCES
- 1.Boring CC, Squires TS, Tong T. Cancer statistics, 1991. Bol Asoc Med P R. 1991;83:225–242. [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi D, Gupta R, Spiro RH, Shah JP. Improved survival in the treatment of squamous carcinoma of the oral tongue. Am J Surg. 1993;166:360–365. doi: 10.1016/s0002-9610(05)80333-2. [DOI] [PubMed] [Google Scholar]
- 4.Byers RM, Weber RS, Andrews T, McGill D, Kare R, Wolf P. Frequency and therapeutic implications of “skip metastases” in the neck from squamous carcinoma of the oral tongue. Head Neck. 1997;19:14–19. doi: 10.1002/(sici)1097-0347(199701)19:1<14::aid-hed3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg. 2002;128:751–758. doi: 10.1001/archotol.128.7.751. [DOI] [PubMed] [Google Scholar]
- 6.Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989;158:309–313. doi: 10.1016/0002-9610(89)90122-0. [DOI] [PubMed] [Google Scholar]
- 7.Wong TS, Liu XB, Wong BY, Ng RW, Yuen AP, Wei WI. Mature miR-184 as potential oncogenic microRNA of squamous cell carcinoma of tongue. Clin Cancer Res. 2008;14:2588–2592. doi: 10.1158/1078-0432.CCR-07-0666. [DOI] [PubMed] [Google Scholar]
- 8.Kozaki K, Imoto I, Mogi S, Omura K, Inazawa J. Exploration of tumor-suppressive microRNAs silenced by DNA hypermethylation in oral cancer. Cancer Res. 2008;68:2094–2105. doi: 10.1158/0008-5472.CAN-07-5194. [DOI] [PubMed] [Google Scholar]
- 9.Tran N, McLean T, Zhang X, et al. MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun. 2007;358:12–17. doi: 10.1016/j.bbrc.2007.03.201. [DOI] [PubMed] [Google Scholar]
- 10.Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer. 2007;6:5. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Z, Jin Y, Yu D, et al. Down-regulation of the microRNA-99 family members in head and neck squamous cell carcinoma. Oral Oncol. 2012;48:686–691. doi: 10.1016/j.oraloncology.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esquela-Kerscher A, Slack FJ. Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 13.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.Nair VS, Maeda LS, Ioannidis JP. Clinical outcome prediction by microRNAs in human cancer: a systematic review. J Natl Cancer Inst. 2012;104:528–540. doi: 10.1093/jnci/djs027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou X, Jordan RCK, Li Y, Huang BL, Wong DT. Frequent allelic imbalance at 8p and 11q22 in oral cavity and oropharyngeal epithelial dysplastic lesions. Cancer Genet Cytogenet. 2005;161:86–89. doi: 10.1016/j.cancergencyto.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Lugli G, Kataria Y, Richards Z, Gann P, Zhou X, Nonn L. Laser-capture microdissection of human prostatic epithelium for RNA analysis. J Vis Exp. 2015;105:e53405. doi: 10.3791/53405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nonn L, Vaishnav A, Gallagher L, Gann PH. mRNA and micro-RNA expression analysis in laser-capture microdissected prostate biopsies: valuable tool for risk assessment and prevention trials. Exp Mol Pathol. 2010;88:45–51. doi: 10.1016/j.yexmp.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kolokythas A, Schwartz JL, Pytynia KB, et al. Analysis of RNA from brush cytology detects changes in B2M, CYP1B1 and KRT17 levels with OSCC in tobacco users. Oral Oncol. 2011;47:532–536. doi: 10.1016/j.oraloncology.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Kayala MA, Baldi P. Cyber-T web server: differential analysis of high-throughput data. Nucleic Acids Res. 2012;40:W553–W559. doi: 10.1093/nar/gks420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baldi P, Long AD. A Bayesian framework for the analysis of microarray expression data: regularized t-test and statistical inferences of gene changes. Bioinformatics. 2001;17:509–519. doi: 10.1093/bioinformatics/17.6.509. [DOI] [PubMed] [Google Scholar]
- 23.Metsalu T, Vilo J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015;43:W566–W570. doi: 10.1093/nar/gkv468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elkayam E, Kuhn CD, Tocilj A, et al. The structure of human argonaute-2 in complex with miR-20a. Cell. 2012;150:100–110. doi: 10.1016/j.cell.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gyongyosi A, Docs O, Czimmerer Z, et al. Measuring expression levels of small regulatory RNA molecules from body fluids and formalin-fixed, paraffin-embedded samples. Methods Mol Biol. 2014;1182:105–119. doi: 10.1007/978-1-4939-1062-5_10. [DOI] [PubMed] [Google Scholar]
- 26.Tanic M, Yanowski K, Gomez-Lopez G, et al. MicroRNA expression signatures for the prediction of BRCA1/2 mutation-associated hereditary breast cancer in paraffin-embedded formalin-fixed breast tumors. Int J Cancer. 2014;136(3):593–602. doi: 10.1002/ijc.29021. [DOI] [PubMed] [Google Scholar]
- 27.Meng W, McElroy JP, Volinia S, et al. Comparison of microRNA deep sequencing of matched formalin-fixed paraffin-embedded and fresh frozen cancer tissues. PLoS One. 2013;8:e64393. doi: 10.1371/journal.pone.0064393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall JS, Taylor J, Valentine HR, et al. Enhanced stability of microRNA expression facilitates classification of FFPE tumour samples exhibiting near total mRNA degradation. Br J Cancer. 2012;107:684–694. doi: 10.1038/bjc.2012.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng L, Wu X, Gao H, et al. MicroRNA profiling of clear cell renal cell carcinoma by whole-genome small RNA deep sequencing of paired frozen and formalin-fixed, paraffin-embedded tissue specimens. J Pathol. 2010;222:41–51. doi: 10.1002/path.2736. [DOI] [PubMed] [Google Scholar]
- 30.Giangreco AA, Vaishnav A, Wagner D, et al. Tumor suppressor microRNAs, miR-100 and -125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev Res (Phila) 2013;6:483–494. doi: 10.1158/1940-6207.CAPR-12-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan X, Qin W, Zhang L, et al. A 5-microRNA signature for lung squamous cell carcinoma diagnosis and hsa-miR-31 for prognosis. Clin Cancer Res. 2011;17:6802–6811. doi: 10.1158/1078-0432.CCR-11-0419. [DOI] [PubMed] [Google Scholar]
- 32.Navon R, Wang H, Steinfeld I, Tsalenko A, Ben-Dor A, Yakhini Z. Novel rank-based statistical methods reveal microRNAs with differential expression in multiple cancer types. PLoS One. 2009;4:e8003. doi: 10.1371/journal.pone.0008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ragusa M, Majorana A, Statello L, et al. Specific alterations of microRNA transcriptome and global network structure in colorectal carcinoma after cetuximab treatment. Mol Cancer Ther. 2010;9:3396–3409. doi: 10.1158/1535-7163.MCT-10-0137. [DOI] [PubMed] [Google Scholar]
- 34.Oh HK, Tan AL, Das K, et al. Genomic loss of miR-486 regulates tumor progression and the OLFM4 antiapoptotic factor in gastric cancer. Clin Cancer Res. 2011;17:2657–2667. doi: 10.1158/1078-0432.CCR-10-3152. [DOI] [PubMed] [Google Scholar]
- 35.Peng Y, Dai Y, Hitchcock C, et al. Insulin growth factor signaling is regulated by microRNA-486, an underexpressed microRNA in lung cancer. Proc Natl Acad Sci U S A. 2013;110:15043–15048. doi: 10.1073/pnas.1307107110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tahiri A, Leivonen SK, Luders T, et al. Deregulation of cancer-related miRNAs is a common event in both benign and malignant human breast tumors. Carcinogenesis. 2014;35:76–85. doi: 10.1093/carcin/bgt333. [DOI] [PubMed] [Google Scholar]
- 37.Sun H, Cui C, Xiao F, et al. miR-486 regulates metastasis and chemosensitivity in hepatocellular carcinoma by targeting CLDN10 and CITRON. Hepatol Res. 2015;45:1312–1322. doi: 10.1111/hepr.12500. [DOI] [PubMed] [Google Scholar]
- 38.Shen J, Liu Z, Todd NW, et al. Diagnosis of lung cancer in individuals with solitary pulmonary nodules by plasma microRNA biomarkers. BMC Cancer. 2011;11:374. doi: 10.1186/1471-2407-11-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren C, Chen H, Han C, et al. miR-486-5p expression pattern in esophageal squamous cell carcinoma, gastric cancer and its prognostic value. Oncotarget. 2016;7(13):15840–15853. doi: 10.18632/oncotarget.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krichevsky AM, Gabriely G. miR-21: a small multi-faceted RNA. J Cell Mol Med. 2009;13:39–53. doi: 10.1111/j.1582-4934.2008.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selcuklu SD, Donoghue MT, Spillane C. miR-21 as a key regulator of oncogenic processes. Biochem Soc Trans. 2009;37:918–925. doi: 10.1042/BST0370918. [DOI] [PubMed] [Google Scholar]
- 42.Jamali Z, Asl Aminabadi N, Attaran R, Pournagiazar F, Ghertasi Oskouei S, Ahmadpour F. MicroRNAs as prognostic molecular signatures in human head and neck squamous cell carcinoma: a systematic review and meta-analysis. Oral Oncol. 2015;51:321–331. doi: 10.1016/j.oraloncology.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 43.Ko YH, Won HS, Sun DS, et al. Human papillomavirus-stratified analysis of the prognostic role of miR-21 in oral cavity and oropharyngeal squamous cell carcinoma. Pathol Int. 2014;64:499–507. doi: 10.1111/pin.12201. [DOI] [PubMed] [Google Scholar]
- 44.Ren W, Qiang C, Gao L, et al. Circulating microRNA-21 (MIR-21) and phosphatase and tensin homolog (PTEN) are promising novel biomarkers for detection of oral squamous cell carcinoma. Biomarkers. 2014;19:590–596. doi: 10.3109/1354750X.2014.955059. [DOI] [PubMed] [Google Scholar]
- 45.Hedback N, Jensen DH, Specht L, et al. MiR-21 expression in the tumor stroma of oral squamous cell carcinoma: an independent biomarker of disease free survival. PLoS One. 2014;9:e95193. doi: 10.1371/journal.pone.0095193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li J, Huang H, Sun L, et al. MiR-21 indicates poor prognosis in tongue squamous cell carcinomas as an apoptosis inhibitor. Clin Cancer Res. 2009;15:3998–4008. doi: 10.1158/1078-0432.CCR-08-3053. [DOI] [PubMed] [Google Scholar]
- 47.Nair J, Jain P, Chandola U, et al. Gene and miRNA expression changes in squamous cell carcinoma of larynx and hypopharynx. Genes Cancer. 2015;6:328–340. doi: 10.18632/genesandcancer.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duz MB, Karatas OF, Guzel E, et al. Identification of miR-139-5p as a saliva biomarker for tongue squamous cell carcinoma: a pilot study. Cell Oncol (Dordr) 2015;39(2):187–193. doi: 10.1007/s13402-015-0259-z. [DOI] [PubMed] [Google Scholar]
- 49.Lee Y, Yang X, Huang Y, et al. Network modeling identifies molecular functions targeted by miR-204 to suppress head and neck tumor metastasis. PLoS Comput Biol. 2010;6:e1000730. doi: 10.1371/journal.pcbi.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X, Li F, Zhou X. miR-204-5p regulates cell proliferation and metastasis through inhibiting CXCR4 expression in OSCC. Biomed Pharmacother. 2016;82:202–207. doi: 10.1016/j.biopha.2016.04.060. [DOI] [PubMed] [Google Scholar]
- 51.Yu CC, Chen PN, Peng CY, Yu CH, Chou MY. Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis. Oncotarget. 2016;7:20180–20192. doi: 10.18632/oncotarget.7745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roldo C, Missiaglia E, Hagan JP, et al. MicroRNA expression abnormalities in pancreatic endocrine and acinar tumors are associated with distinctive pathologic features and clinical behavior. J Clin Oncol. 2006;24:4677–4684. doi: 10.1200/JCO.2005.05.5194. [DOI] [PubMed] [Google Scholar]
- 53.Zanette DL, Rivadavia F, Molfetta GA, et al. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007;40:1435–1440. doi: 10.1590/s0100-879x2007001100003. [DOI] [PubMed] [Google Scholar]
- 54.Xia Y, Zhu Y, Ma T, et al. miR-204 functions as a tumor suppressor by regulating SIX1 in NSCLC. FEBS Lett. 2014;588:3703–3712. doi: 10.1016/j.febslet.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Lam EK, Wang X, Shin VY, et al. A microRNA contribution to aberrant Ras activation in gastric cancer. Am J Transl Res. 2011;3:209–218. [PMC free article] [PubMed] [Google Scholar]
- 56.Xiong F, Liu K, Zhang F, et al. MiR-204 inhibits the proliferation and invasion of renal cell carcinoma by inhibiting RAB22A expression. Oncol Rep. 2016;35:3000–3008. doi: 10.3892/or.2016.4624. [DOI] [PubMed] [Google Scholar]
- 57.De Sarkar N, Roy R, Mitra JK, et al. A quest for miRNA biomarker: a track back approach from gingivo buccal cancer to two different types of precancers. PLoS One. 2014;9:e104839. doi: 10.1371/journal.pone.0104839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Findlay VJ, Turner DP, Moussa O, Watson DK. MicroRNA-mediated inhibition of prostate-derived Ets factor messenger RNA translation affects prostate-derived Ets factor regulatory networks in human breast cancer. Cancer Res. 2008;68:8499–8506. doi: 10.1158/0008-5472.CAN-08-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imam JS, Plyler JR, Bansal H, et al. Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS One. 2012;7:e52397. doi: 10.1371/journal.pone.0052397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7:3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 61.Turner DP, Findlay VJ, Moussa O, et al. Mechanisms and functional consequences of PDEF protein expression loss during prostate cancer progression. Prostate. 2011;71:1723–1735. doi: 10.1002/pros.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ding M, Lin B, Li T, et al. A dual yet opposite growth-regulating function of miR-204 and its target XRN1 in prostate adenocarcinoma cells and neuroendocrine-like prostate cancer cells. Oncotarget. 2015;6:7686–7700. doi: 10.18632/oncotarget.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scapoli L, Palmieri A, Lo Muzio L, et al. MicroRNA expression profiling of oral carcinoma identifies new markers of tumor progression. Int J Immunopathol Pharmacol. 2010;23:1229–1234. doi: 10.1177/039463201002300427. [DOI] [PubMed] [Google Scholar]
- 64.Kikkawa N, Hanazawa T, Fujimura L, et al. miR-489 is a tumour-suppressive miRNA target PTPN11 in hypopharyngeal squamous cell carcinoma (HSCC) Br J Cancer. 2010;103:877–884. doi: 10.1038/sj.bjc.6605811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen D, Cabay RJ, Jin Y, et al. MicroRNA deregulations in head and neck squamous cell carcinomas. J Oral Maxillofac Res. 2013;4:e2. doi: 10.5037/jomr.2013.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manikandan M, Deva Magendhra Rao AK, Arunkumar G, et al. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol Cancer. 2016;15:28. doi: 10.1186/s12943-016-0512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tachibana H, Sho R, Takeda Y, et al. Circulating miR-223 in oral cancer: its potential as a novel diagnostic biomarker and therapeutic target. PLoS One. 2016;11:e0159693. doi: 10.1371/journal.pone.0159693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu YC, Chang JT, Liao CT, et al. OncomiR-196 promotes an invasive phenotype in oral cancer through the NME4-JNK-TIMP1-MMP signaling pathway. Mol Cancer. 2014;13:218. doi: 10.1186/1476-4598-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou YY, You JJ, Yang CM, et al. Aberrant DNA hypomethylation of miR-196b contributes to migration and invasion of oral cancer. Oncol Lett. 2016;11:4013–4021. doi: 10.3892/ol.2016.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lu YC, Chang JT, Huang YC, et al. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin Biochem. 2015;48:115–121. doi: 10.1016/j.clinbiochem.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 71.Liu CJ, Tsai MM, Tu HF, Lui MT, Cheng HW, Lin SC. miR-196a overexpression and miR-196a2 gene polymorphism are prognostic predictors of oral carcinomas. Ann Surg Oncol. 2013;20(suppl 3):S406–S414. doi: 10.1245/s10434-012-2618-6. [DOI] [PubMed] [Google Scholar]
- 72.Valastyan S, Reinhardt F, Benaich N, et al. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–35302. doi: 10.1074/jbc.M110.160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu CJ, Kao SY, Tu HF, Tsai MM, Chang KW, Lin SC. Increase of microRNA miR-31 level in plasma could be a potential marker of oral cancer. Oral Dis. 2010;16:360–364. doi: 10.1111/j.1601-0825.2009.01646.x. [DOI] [PubMed] [Google Scholar]
- 75.Hung KF, Liu CJ, Chiu PC, et al. MicroRNA-31 upregulation predicts increased risk of progression of oral potentially malignant disorder. Oral Oncol. 2016;53:42–47. doi: 10.1016/j.oraloncology.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 76.Siow MY, Ng LP, Vincent-Chong VK, et al. Dysregulation of miR-31 and miR-375 expression is associated with clinical outcomes in oral carcinoma. Oral Dis. 2014;20:345–351. doi: 10.1111/odi.12118. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J, Yang Y, Yang T, et al. Double-negative feedback loop between microRNA-422a and forkhead box (FOX)G1/Q1/E1 regulates hepatocellular carcinoma tumor growth and metastasis. Hepatology. 2015;61:561–573. doi: 10.1002/hep.27491. [DOI] [PubMed] [Google Scholar]
- 78.Liang H, Wang R, Jin Y, Li J, Zhang S. MiR-422a acts as a tumor suppressor in glioblastoma by targeting PIK3CA. Am J Cancer Res. 2016;6:1695–1707. [PMC free article] [PubMed] [Google Scholar]
- 79.Liu M, Xiusheng H, Xiao X, Wang Y. Overexpression of miR-422a inhibits cell proliferation and invasion, and enhances chemosensitivity in osteosarcoma cells. Oncol Rep. 2016 doi: 10.3892/or.2016.5182. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 80.Bonnin N, Armandy E, Carras J, et al. MiR-422a promotes loco-regional recurrence by targeting NT5E/CD73 in head and neck squamous cell carcinoma. Oncotarget. 2016;7(28):44023–44038. doi: 10.18632/oncotarget.9829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng G, Du L, Yang X, et al. Serum microRNA panel as biomarkers for early diagnosis of colorectal adenocarcinoma. Br J Cancer. 2014;111:1985–1992. doi: 10.1038/bjc.2014.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin YZ, Xie XC, Liu HZ, Lai H, Qiu H, Ge LY. Screening and preliminary validation of miRNAs with the regulation of hTERT in colorectal cancer. Oncol Rep. 2015;33:2728–2736. doi: 10.3892/or.2015.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bahador R, Taheriazam A, Mirghasemi A, et al. Tissue expression levels of miR-29b and miR-422a in children, adolescents, and young adults’ age groups and their association with prediction of poor prognosis in human osteosarcoma. Tumour Biol. 2016;37:3091–3095. doi: 10.1007/s13277-015-4140-5. [DOI] [PubMed] [Google Scholar]
- 84.Zheng GX, Qu AL, Yang YM, Zhang X, Zhang SC, Wang CX. miR-422a is an independent prognostic factor and functions as a potential tumor suppressor in colorectal cancer. World J Gastroenterol. 2016;22:5589–5597. doi: 10.3748/wjg.v22.i24.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yuan J, Zheng Z, Zheng Y, Lu X, Xu L, Lin L. microRNA-328 is a favorable prognostic marker in human glioma via suppressing invasive and proliferative phenotypes of malignant cells. Int J Neurosci. 2016;126:145–153. doi: 10.3109/00207454.2014.1002610. [DOI] [PubMed] [Google Scholar]
- 86.Ulivi P, Foschi G, Mengozzi M, et al. Peripheral blood miR-328 expression as a potential biomarker for the early diagnosis of NSCLC. Int J Mol Sci. 2013;14:10332–10342. doi: 10.3390/ijms140510332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Z, Sun L, Wang H, et al. MiR-328 expression is decreased in high-grade gliomas and is associated with worse survival in primary glioblastoma. PLoS One. 2012;7:e47270. doi: 10.1371/journal.pone.0047270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keutgen XM, Filicori F, Crowley MJ, et al. A panel of four miRNAs accurately differentiates malignant from benign indeterminate thyroid lesions on fine needle aspiration. Clin Cancer Res. 2012;18:2032–2038. doi: 10.1158/1078-0432.CCR-11-2487. [DOI] [PubMed] [Google Scholar]
- 89.Arora S, Ranade AR, Tran NL, et al. MicroRNA-328 is associated with (non-small) cell lung cancer (NSCLC) brain metastasis and mediates NSCLC migration. Int J Cancer. 2011;129:2621–2631. doi: 10.1002/ijc.25939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li Y, Wang Y, Yu L, et al. miR-146b-5p inhibits glioma migration and invasion by targeting MMP16. Cancer Lett. 2013;339:260–269. doi: 10.1016/j.canlet.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 91.Chou CK, Chen RF, Chou FF, et al. miR-146b is highly expressed in adult papillary thyroid carcinomas with high risk features including extrathyroidal invasion and the BRAF(V600E) mutation. Thyroid. 2010;20:489–494. doi: 10.1089/thy.2009.0027. [DOI] [PubMed] [Google Scholar]
- 92.Patnaik SK, Kannisto E, Mallick R, Yendamuri S. Overexpression of the lung cancer-prognostic miR-146b microRNAs has a minimal and negative effect on the malignant phenotype of A549 lung cancer cells. PLoS One. 2011;6:e22379. doi: 10.1371/journal.pone.0022379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu YC, Chen YJ, Wang HM, et al. Oncogenic function and early detection potential of miRNA-10b in oral cancer as identified by microRNA profiling. Cancer Prev Res (Phila) 2012;5:665–674. doi: 10.1158/1940-6207.CAPR-11-0358. [DOI] [PubMed] [Google Scholar]
- 94.Brito JA, Gomes CC, Guimaraes AL, Campos K, Gomez RS. Relationship between microRNA expression levels and histopathological features of dysplasia in oral leukoplakia. J Oral Pathol Med. 2014;43:211–216. doi: 10.1111/jop.12112. [DOI] [PubMed] [Google Scholar]
- 95.Tsay JC, Li Z, Yie TA, et al. Molecular characterization of the peripheral airway field of cancerization in lung adenocarcinoma. PLoS One. 2015;10:e0118132. doi: 10.1371/journal.pone.0118132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Boldrup L, Coates PJ, Laurell G, Wilms T, Fahraeus R, Nylander K. Downregulation of miRNA-424: a sign of field cancerisation in clinically normal tongue adjacent to squamous cell carcinoma. Br J Cancer. 2015;112:1760–1765. doi: 10.1038/bjc.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wali RK, Hensing TA, Ray DW, et al. Buccal microRNA dysregulation in lung field carcinogenesis: gender-specific implications. Int J Oncol. 2014;45:1209–1215. doi: 10.3892/ijo.2014.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shi LJ, Zhang CY, Zhou ZT, et al. MicroRNA-155 in oral squamous cell carcinoma: overexpression, localization, and prognostic potential. Head Neck. 2015;37:970–976. doi: 10.1002/hed.23700. [DOI] [PubMed] [Google Scholar]
- 99.Nuovo GJ. In situ detection of microRNAs in paraffin embedded, formalin fixed tissues and the co-localization of their putative targets. Methods. 2010;52:307–315. doi: 10.1016/j.ymeth.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 100.Hiyoshi Y, Kamohara H, Karashima R, et al. MicroRNA-21 regulates the proliferation and invasion in esophageal squamous cell carcinoma. Clin Cancer Res. 2009;15:1915–1922. doi: 10.1158/1078-0432.CCR-08-2545. [DOI] [PubMed] [Google Scholar]
- 101.Nelson PT, Baldwin DA, Kloosterman WP, Kauppinen S, Plasterk RH, Mourelatos Z. RAKE and LNA-ISH reveal microRNA expression and localization in archival human brain. RNA. 2006;12:187–191. doi: 10.1261/rna.2258506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table S1. The levels of 12 microRNAs on 10 cases of OTSCC and paired normal tissues.
Supplementary table S2. The levels of 12 microRNAs on 15 paired OTSCC and normal tissues (TCGA dataset).