Significance
Pseudomonas aeruginosa pulmonary infections cause prolonged and destructive inflammation for cystic fibrosis patients. Despite vigorous neutrophilic responses, P. aeruginosa persists in a chronic hyperinflammatory environment. We show that the P. aeruginosa virulence factor, cystic fibrosis transmembrane conductance regulator inhibitory factor (Cif), promotes sustained airway inflammation by reducing host pro-resolving lipid mediators. Cif hydrolyzes epithelial-derived 14,15-epoxyeicosatrienoic acid, disrupting transcellular production of the proresolving lipid 15-epi lipoxin A4 (15-epi LXA4) by neutrophils. Clinical data from cystic fibrosis patients revealed that Cif abundance correlated with increased inflammation, decreased 15-epi LXA4, and reduced pulmonary function. Our study and the recent identification of Cif homologs in Acinetobacter and Burkholderia species suggest that bacterial epoxide hydrolases represent a novel virulence strategy shared by multiple respiratory pathogens.
Keywords: Pseudomonas aeruginosa, epoxide hydrolase, inflammation, lipoxin, cystic fibrosis
Abstract
Recurrent Pseudomonas aeruginosa infections coupled with robust, damaging neutrophilic inflammation characterize the chronic lung disease cystic fibrosis (CF). The proresolving lipid mediator, 15-epi lipoxin A4 (15-epi LXA4), plays a critical role in limiting neutrophil activation and tissue inflammation, thus promoting the return to tissue homeostasis. Here, we show that a secreted P. aeruginosa epoxide hydrolase, cystic fibrosis transmembrane conductance regulator inhibitory factor (Cif), can disrupt 15-epi LXA4 transcellular biosynthesis and function. In the airway, 15-epi LXA4 production is stimulated by the epithelial-derived eicosanoid 14,15-epoxyeicosatrienoic acid (14,15-EET). Cif sabotages the production of 15-epi LXA4 by rapidly hydrolyzing 14,15-EET into its cognate diol, eliminating a proresolving signal that potently suppresses IL-8–driven neutrophil transepithelial migration in vitro. Retrospective analyses of samples from patients with CF supported the translational relevance of these preclinical findings. Elevated levels of Cif in bronchoalveolar lavage fluid were correlated with lower levels of 15-epi LXA4, increased IL-8 concentrations, and impaired lung function. Together, these findings provide structural, biochemical, and immunological evidence that the bacterial epoxide hydrolase Cif disrupts resolution pathways during bacterial lung infections. The data also suggest that Cif contributes to sustained pulmonary inflammation and associated loss of lung function in patients with CF.
Chronic pulmonary inflammation and persistent bacterial infections are pathological hallmarks of the genetic disease cystic fibrosis (CF) (1). CF is caused by mutations that impair the function of the cystic fibrosis transmembrane conductance regulator (CFTR), an ion channel that controls epithelial fluid and ion homeostasis. The resulting failure of mucociliary clearance in the CF lung allows microorganisms to repeatedly infect the respiratory tract (2). These bacterial infections incite robust inflammatory responses, dominated by elevated proinflammatory cytokines and continued accumulation of neutrophils in the CF airway (1). However, these responses are ineffective at clearing pathogenic microbes in the CF lung (3), instead creating a hyperinflammatory cycle that leads to host tissue damage, respiratory failure, transplant, or death.
Most airways of adult patients with CF are chronically infected by the opportunistic bacterial pathogen Pseudomonas aeruginosa, which is a major cause of morbidity and mortality. P. aeruginosa thrives in the hyperinflammatory CF lung, forming biofilms that are mechanically robust and resistant to clinically achievable levels of antibiotics (2). P. aeruginosa also persists in the airways by interfering with host defense via secreted bacterial virulence factors and small molecules (2). We recently showed that P. aeruginosa secretes the CFTR inhibitory factor (Cif), an epoxide hydrolase that triggers the degradation of ABC transporter family members, including CFTR (4–8). Cif transcripts have been observed in sputum from patients with CF, and longitudinal studies of clinical isolates from individual patients confirm that P. aeruginosa maintains Cif expression for up to 15 y (7, 9). Nonetheless, Cif’s role in CF pathogenesis and the identity of possible host epoxide substrates have remained unclear.
Following a pathogenic insult, the host rapidly releases polyunsaturated fatty acids from cell membranes and converts them into various lipid mediators that either stimulate or inhibit inflammation. The correct balance of these signals is required to optimize clearance while minimizing collateral damage to host tissues, and perturbations in either direction can be deleterious (10). Among these lipid mediators, arachidonic acid-derived eicosanoids, including epoxides, play important roles. Although many eicosanoids induce proinflammatory cascades, recent studies have also identified immunomodulatory and proresolving functions (11, 12). In particular, lipoxins decrease neutrophil extravasation and enhance macrophage efferocytosis, thus promoting the resolution of inflammation and a return to tissue homeostasis (13, 14). In the CF lung, the concentration of lipoxin A4 (LXA4) is significantly reduced, suggesting that a failure to activate proresolving mechanisms contributes to excessive inflammation in the airway (15). In this study, we show that Cif selectively converted an endogenous epoxide-containing eicosanoid 14,15-epoxyeicosatrienoic acid (14,15-EET) to its corresponding diol, destroying the signal that triggers increased biosynthesis of the specialized proresolving mediator 15-epi LXA4. The translational relevance of this unexpected biochemical virulence activity to clinical CF was investigated by retrospective analysis of bronchoalveolar lavage fluid (BALF) samples and suggested that Cif contributes to the hyperinflammatory environment of the chronically infected CF lung.
Results
Cif Specifically Hydrolyzed the Mammalian Epoxyeicosatrienoic Acid 14,15-EET.
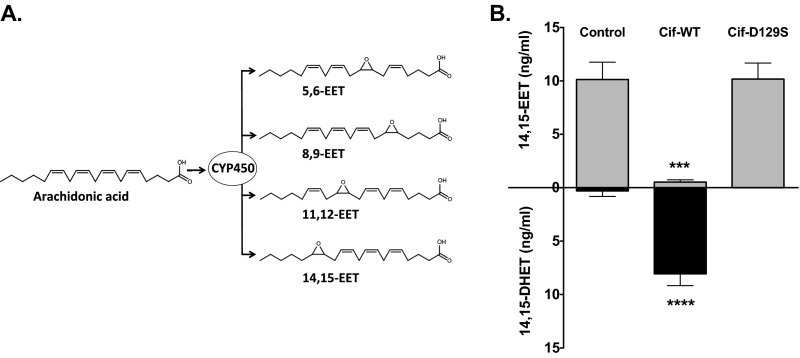
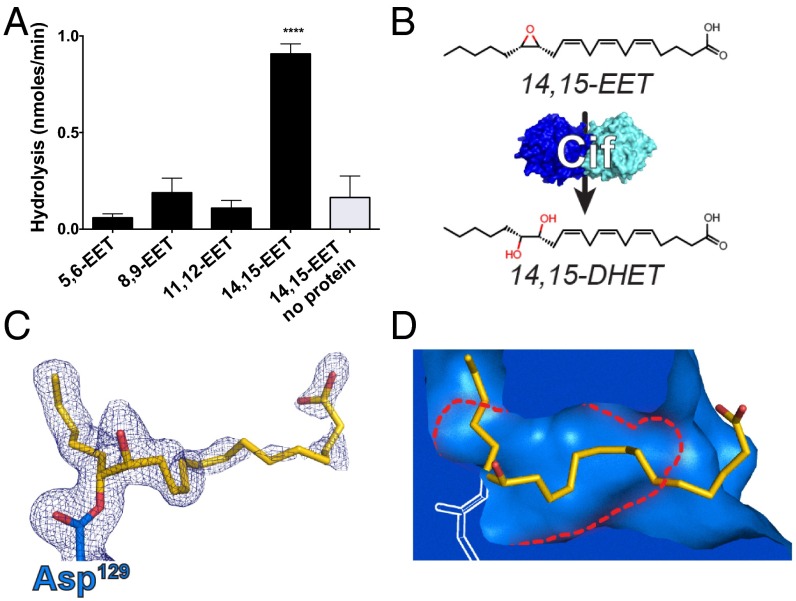
Airway epithelial cells (AECs) produce numerous lipid mediators. Via a major cytochrome P450 monooxygenase-catalyzed pathway, they convert arachidonic acid into epoxide-containing eicosanoids called EETs (Fig. S1A) (10). To assess the ability of Cif to hydrolyze these candidate epoxide substrates, we incubated the enzyme with each of the four mammalian EET regioisomers, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET, and assayed for production of a vicinal diol through a colorimetric assay (5). Cif hydrolyzed 14,15-EET, but did not exhibit detectable epoxide hydrolase activity for any of the other three regioisomers (Fig. 1A), suggesting selective conversion of 14,15-EET by Cif to the corresponding vicinal diol 14,15-dihydroxyeicosatrienoic acid (DHET) (Fig. 1B). An immunoassay selective for the diol product confirmed conversion of 14,15-EET to 14,15-DHET by Cif, but not by the structurally conserved mutant Cif-D129S (Fig. S1B), which is enzymatically inactive (16).
Fig. S1.
EET biosynthesis and hydrolysis. (A) Cellular cytochrome P450 enzymes can catalyze the epoxidation of arachidonic acid, a 20-carbon, ω-6, polyunsaturated fatty acid, resulting in the biosynthesis of four EET regioisomers. (B) The 14,15-EET is hydrolyzed by Cif-WT but not by catalytic mutant Cif-D129S. A total of 1 µM 14,15-EET was incubated with 1 µM recombinant Cif-WT or Cif-D129S (a catalytically inactive mutant) for 45 min. The reactions were stopped with ice-cold MeOH, extracted, and analyzed by ELISA for 14,15-EET (gray bars) and 14,15-DHET (black bars) concentrations. ***P < 0.001, ****P < 0.0001; one-way ANOVA Tukey’s post hoc test; n ≥ 3; mean ± SD.
Fig. 1.
The P. aeruginosa enzyme Cif hydrolyzes the epoxyeicosatrienoic acid 14,15-EET. (A) Incubation of recombinant Cif with each of the EET regioisomers demonstrates its ability to selectively hydrolyze 14,15-EET (black bars). ****P < 0.0001 for 14,15-EET compared with all other experimental conditions, one-way ANOVA with Tukey’s post hoc test; n ≥ 3; mean ± SD. (B) Cif hydrolyzes the epoxide moiety of 14,15-EET, converting it to the vicinal diol 14,15-DHET. (C) Experimental electron density (2mFO-DFC; blue mesh, contoured at 1σ) shows the refined position of the covalent enzyme–substrate intermediate formed by nucleophilic attack of Asp129 (D129; blue carbons; chain D) on 14,15-EET (yellow carbons). (D) In the covalently linked structure, several residues shift, opening a tunnel through the active site (blue surface area representation), allowing for accommodation of the 14,15-EET (yellow carbons) adduct. The active site of the apo enzyme (PDB ID 3KD2) does not contain this tunnel. Its boundaries are shown as red dotted lines. Noncarbon atoms are colored by atom type (N, blue; O, red).
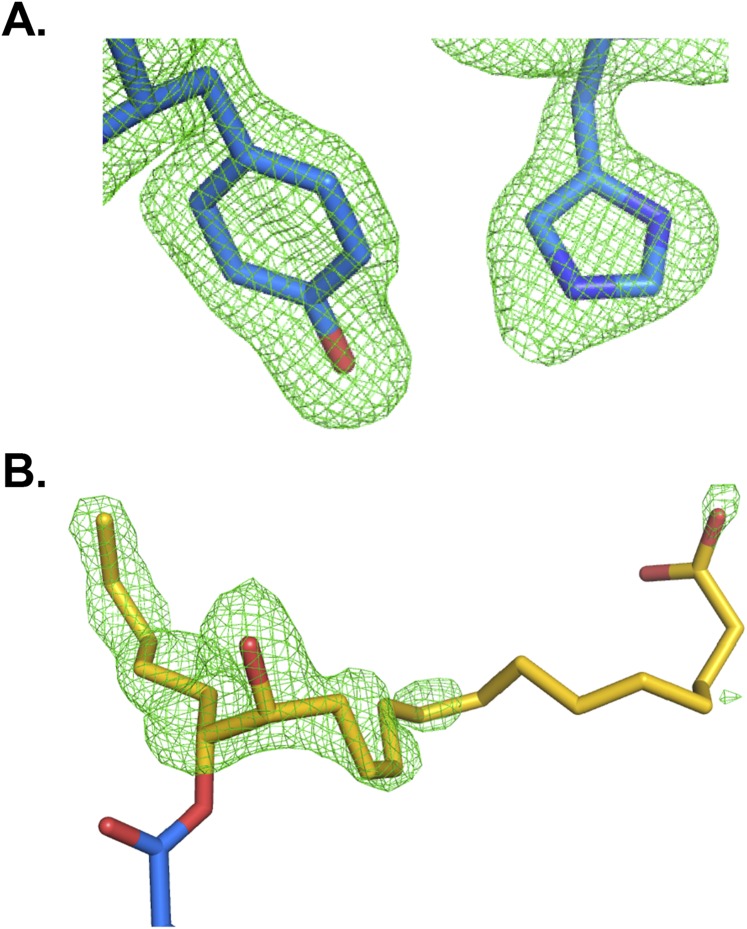
Previous crystal structures show a limited steric volume for the Cif active site (5), and almost all previously reported Cif substrates are terminal epoxides, which can enter deep into the active site. In contrast, lipid mediators typically carry epoxide groups in the middle of an extended carbon chain and were thus thought unlikely to serve as Cif substrates. To investigate how the enzyme catalyzes 14,15-EET hydrolysis, we incubated the compound with the Cif-E153Q mutant, which can attack substrates with its active-site nucleophile, but cannot catalyze the secondary attack required for product release (17). We then crystallized the resulting complex for X-ray diffraction analysis. The refined structure (Table S1) showed the expected adduct of the catalytic Asp129 (D129) nucleophile with the 20-carbon EET chain attached at the C15 moiety (Fig. 1C and Fig. S2). Unexpectedly, it also showed that the active site could expand at both ends, opening a tunnel through the enzyme to accommodate the extended eicosanoid substrate (Fig. 1D). The strong preference for 14,15-EET presumably reflects the shorter distance in the other regioisomers between the epoxide moiety and the terminal carboxylate, which would thus have to be unfavorably sequestered within the active site during hydrolysis.
Table S1.
X-ray data collection and refinement statistics
| Parameter | Cif-E153Q with 14,15-EET |
| Data collection | |
| Wavelength, Å | 0.9795 |
| Space group | C2 |
| Unit cell dimensions: | |
| a,b,c, Å | 168.9, 83.6, 89.2 |
| α,β,γ, ° | 90, 100.4, 90 |
| Resolution*, Å | 35.4–2.00 (2.05–2.00) |
| †, % | 11.4 (36.6) |
| I/σI | 14.27 (4.44) |
| Completeness, % | 99.9 (99.8) |
| Redundancy | 6.24 (6.16) |
| Refinement | |
| Total no. reflections | 82,560 |
| Reflections in the test set | 4,138 |
| ‡,§, % | 15.3/19.1 |
| No. atoms | |
| Protein | 9,577 |
| Solvent | 801 |
| Ligand | 92 |
| Ramachandran plot¶, % | 97.8/2.2/0.0 |
| Protein | 19.7 |
| Solvent | 27.9 |
| Ligand | 34.6 |
| Bond length rmsd, Å | 0.006 |
| Bond angle rmsd, ° | 0.829 |
| PDB ID | 5JYC |
Values in parentheses are for data in the highest-resolution shell.
Rmrgd-F is a robust indicator of the agreement of structure factors of symmetry-related reflections and is described in detail in Diederichs and Karplus (56).
Rwork = Σh |Fobs(h) – Fcalc(h)|/Σh Fobs(h), h ∈ {working set}.
Rfree = Σh |Fobs(h) – Fcalc(h)|/Σh Fobs(h), h ∈ {test set}.
Favored/allowed/outliers.
Fig. S2.
Electron density maps reveal the position of key side chains and of the adduct resulting from the nucleophilic attack of Cif-E153Q on the substrate 14,15-EET. (A) A simulated-annealing omit map was generated in phenix.refine by removing residues H177 and Y239 (depicted as sticks with carbons in blue) from chain D of the Cif asymmetric unit. The resulting mFo-DFc map shown in green mesh, contoured to 3σ. (B) mFo-DFc map (green mesh, contoured at 3σ) overlaid on a stick representation of D129 (blue carbons) covalently linked to 14,15-DHET (yellow carbons) from chain D of the Cif asymmetric unit. The mFo-DFc map was generated before the adduct was included in the model. Noncarbon atoms are colored by atom type (N, blue; O, red).
P. aeruginosa Can Trigger Hydrolysis of Epithelial 14,15-EET Produced in Response to Inflammation.
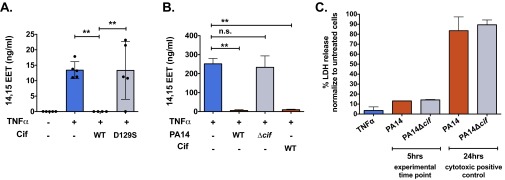
To determine whether Cif’s regioselectivity matches the biological epoxides present in the airway, we first tested whether polarized immortalized CF AECs (CFBE41o-, hereafter called CFBE cells) produce 14,15-EET in response to inflammatory signals. We treated CFBE cells with tumor necrosis factor-α (TNFα) (18) and performed lipid extractions on apical supernatants. Eicosanoid levels were determined in parallel by immunoassay (Fig. 2) and by mass spectrometry (Fig. S3A). Supernatants from CFBE cells exposed to TNFα showed a substantial increase in 14,15-EET levels, compared with untreated cells (Fig. 2A and Fig. S3A).
Fig. 2.
Cif hydrolyzes 14,15-EET derived from CF AECs. (A–C) Treatment of polarized CF AECs with TNFα (1 ng/mL) for 24 h stimulates increased apical secretion of 14,15-EET (blue bars or symbols) compared with untreated cells (black bars or symbols). (A) Recombinant Cif protein hydrolyzes 14,15-EET produced by TNFα-treated CFBE cells. CFBE cells were apically treated with either recombinant Cif-WT or Cif-D129S (1 µM) for 45 min (red or gray bars, respectively). Apical secretions were collected in cold MeOH, solid-phase lipid extracted, and analyzed for 14,15-EET concentrations by ELISA. ***P < 0.001; one-way ANOVA with Tukey’s post hoc test; n ≥ 3; mean ± SD. (B) Cif secreted by P. aeruginosa hydrolyzes 14,15-EET produced by TNFα-treated CFBE cells. CFBE cells were inoculated apically with either PA14 or deletion mutant PA14∆cif (MOI = 25) for 5 h (red bars and gray bars, respectively) and analyzed for 14,15-EET concentrations by ELISA. ***P < 0.001 and ****P < 0.0001; one-way ANOVA with Tukey’s post hoc test; n ≥ 3; mean ± SD. (C) P. aeruginosa hydrolyzes 14,15-EET produced by primary CF airway epithelial cells. Primary CF HBEs were apically exposed to either PA14 or deletion mutant PA14∆cif (MOI = 25) for 5 h (red and gray symbols). Apical secretions were collected and analyzed for 14,15-EET concentrations by ELISA. Each symbol represents one individual primary donor. *P < 0.05 and ***P < 0.001; one-way ANOVA with Tukey’s post hoc test; n ≥ 3; mean ± SD.
Fig. S3.
Cif hydrolyzes 14,15-EET derived from CF or non-CF AECs. (A) Cif-mediated hydrolysis of 14,15-EET derived from CF AECs was confirmed by LC/MS mass spectrometry. CFBE cells were treated with TNFα (1 ng/mL) for 24 h (blue bars) and then exposed to Cif-WT or Cif-D129S (red and gray bars, respectively) for 45 min. Apical supernatants were collected in methanol and lipid extracted, and 14,15-EET was measured by liquid chromatography-mass spectrometry. Each data point represents one replicate. **P < 0.01; one-way ANOVA with Tukey’s post hoc test; n ≥ 4; mean ± SD. (B) Cif hydrolyzes 14,15-EET produced by non-CF AECs. Polarized immortalized Calu3 cells were stimulated with TNFα (1 ng/mL) for 24 h to generate the apical secretion of 14,15-EET (blue bar). The Calu3 cells were subsequently apically treated with either PA14 (red bar) or PA14∆cif (gray bar) for 5 h or 1 µM Cif-WT (red bar) for 45 min. Apical secretions were collected in cold methanol, lipid extracted, and analyzed for 14,15-EET concentrations by ELISA. **P < 0.01; one-way ANOVA with Tukey’s post hoc test; data are represented as mean ± SD. (C) Cytotoxicity data are shown for polarized epithelial cells exposed either to 1 ng/mL of TNFα for 24 h or to PA14 or PA14∆cif applied apically for 5 h or 24 h. Cell supernatants and whole-cell lysates were collected and lysed, and lactate dehydrogenase (LDH) levels were measured. Data are represented as percentage of LDH release, compared with lysed untreated controls. n ≥ 2; mean ± SD.
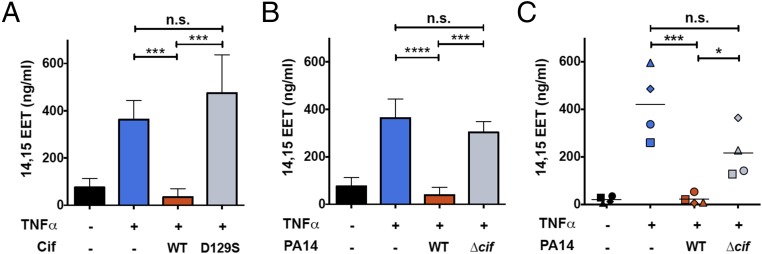
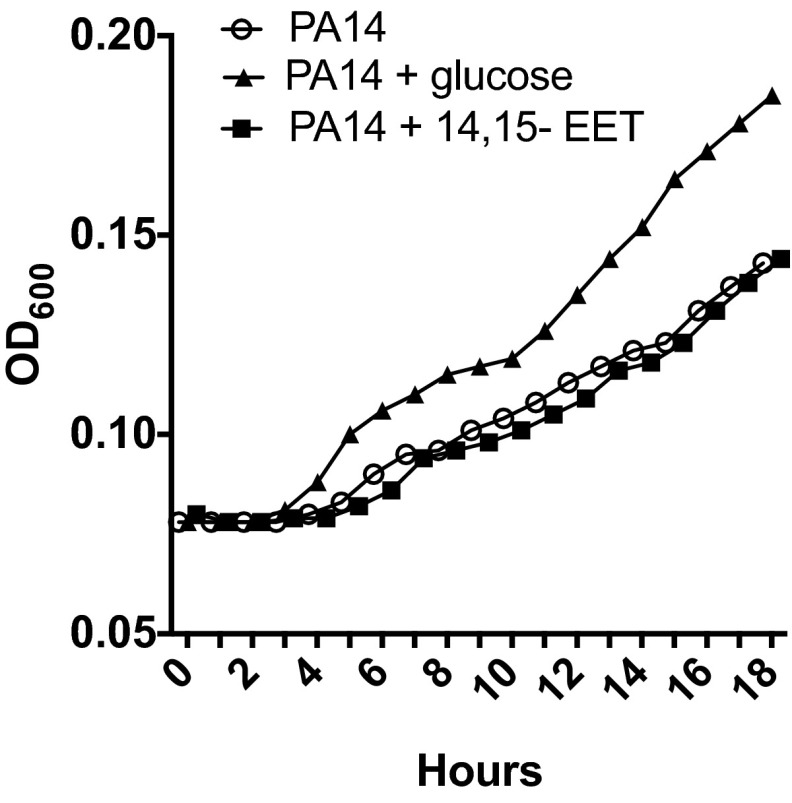
Furthermore, when CFBE cells were exposed to TNFα in the presence of purified Cif-WT protein, the apical level of 14,15-EET was reduced to that of unstimulated controls, whereas the catalytically inactive Cif-D129S protein had no significant impact on 14,15-EET levels (Fig. 2A). We next sought to determine whether P. aeruginosa could use Cif activity to hydrolyze CFBE-derived 14,15-EET. We first showed that presence of 14,15-EET did not affect the growth rate of P. aeruginosa strain PA14, which expresses Cif (Fig. S4). CFBE cells were treated with TNFα and exposed to strain PA14 or to the corresponding cif deletion strain PA14∆cif (7). Apical supernatant concentrations of 14,15-EET were significantly reduced in the presence of the Cif-producing strain PA14, compared with the TNFα-only control (Fig. 2B). Similar results were observed for well-differentiated primary CF human bronchial epithelial cells (CF HBEs) (Fig. 2C) and also for the non-CF Calu3 cell line (Fig. S3B). In each case, PA14∆cif had no statistically significant effect on the 14,15-EET levels. The Cif effect is not associated with differences in overall P. aeruginosa CF AEC cytotoxicity. Both PA14 and PA14∆cif showed minimal cytotoxicity at the 5-h time point used in our experiments (Fig. S3C). Interestingly, we detected comparable levels of 14,15-EET secreted by CF and non-CF AECs following TNFα treatment, suggesting that both CF and non-CF epithelial cells were similarly capable of secreting 14,15-EET in response to an inflammatory stimulus. Taken together, our data demonstrate that Cif enables P. aeruginosa to promote the hydrolysis of 14,15-EET secreted by AECs and that Cif is necessary for efficient hydrolysis.
Fig. S4.
P. aeruginosa grown in minimal media supplemented with 14,15-EET does not alter growth kinetics. An overnight culture of PA14 was subcultured in M63 minimal media without a carbon source at a starting OD600 of 0.08. The bacterial suspension was supplemented with 0.5% glucose (as a positive control for growth) or 14,15-EET (1 µM) and bacterial growth was measured at 1-h intervals for 18 h.
Cif-Mediated 14,15-EET Hydrolysis Reduced 15-epi LXA4 Production and Disinhibited Transepithelial Migration.
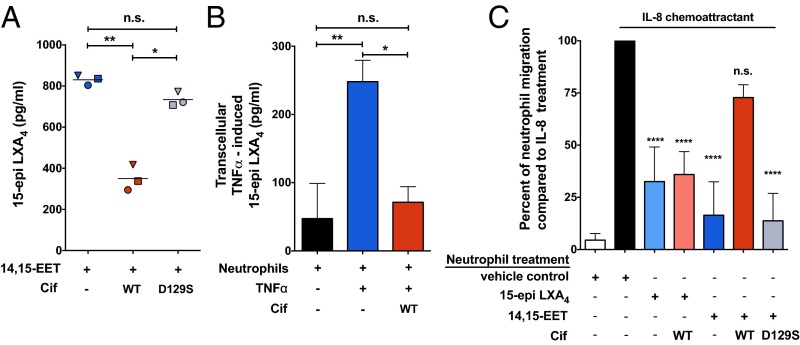
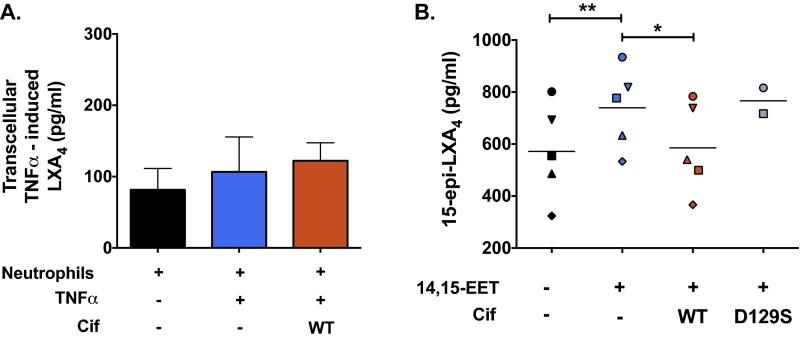
AEC-derived 14,15-EET acts as a transcellular signal and stimulates neutrophil generation of the proresolving mediator 15-epi LXA4, whereas other EET regioisomers do not (18, 19). Thus, we next examined whether Cif-mediated hydrolysis of 14,15-EET impacts the transcellular biosynthesis of 15-epi LXA4. Neutrophils obtained from healthy donors or subjects with CF were exposed to 14,15-EET alone or in combination with either Cif-WT or Cif-D129S. Following incubation, lipids were extracted from supernatants and quantified by ELISA for 15-epi LXA4. As expected, neutrophils generated 15-epi LXA4 when incubated with 14,15-EET, and 15-epi LXA4 levels were significantly reduced in the presence of Cif-WT (Fig. 3A). Similar results were observed for neutrophils from donors with CF (Fig. S5B). In a neutrophil–CFBE coculture model, CFBE cells were treated with TNFα to induce the production of 14,15-EET and then incubated with neutrophils. Apical supernatants from the neutrophil–CFBE coculture showed robust production of 15-epi LXA4 following TNFα stimulation. The addition of Cif to the neutrophil–CFBE coculture substantially reduced transcellular generation of 15-epi LXA4 to near baseline levels (Fig. 3B). The 15-epi LXA4 is the 15(R) epimer of LXA4, yet these epimers have distinct biosynthetic pathways (11). As a control, we also used the coculture system to assess transcellular generation of LXA4. As expected, Cif had no significant effect on LXA4 levels (Fig. S5A), but instead appeared to be specific for the 15-epi LXA4 biosynthetic pathway.
Fig. 3.
Cif-mediated 14,15-EET hydrolysis suppresses neutrophil-derived 15-epi LXA4 and restores neutrophil transepithelial migration. (A) Neutrophil generation of 15-epi LXA4 in the presence of 14,15-EET (blue symbols) is reduced in the presence of recombinant Cif-WT (red symbols) but not by the catalytic mutant Cif-D129S (gray symbols). Freshly isolated human neutrophils from healthy donors (106) were incubated with 14,15-EET (1 µM) in the presence of either Cif-WT (1 µM) or Cif-D129S (1 µM). The reactions were stopped with cold MeOH, lipid extracted, and analyzed for 15-epi LXA4 concentrations by ELISA. *P < 0.05, **P < 0.01; one-way ANOVA with Tukey’s post hoc test; n ≥ 3; mean. (B) Cif hydrolysis of 14,15-EET suppresses the production of 15-epi LXA4 in CFBE–neutrophil coculture. Polarized CFBEs stimulated with TNFα (1 ng/mL) for 24 h (blue bar) were treated apically with Cif-WT (1 µM) for 45 min (red bar), before the apical addition of 1.5 × 106 of freshly isolated neutrophils for an additional 15 min. The apical supernatants from the cocultures were collected and analyzed for 15-epi LXA4 by ELISA. Data are normalized to CFBEs treated with TNFα. *P < 0.05, **P < 0.01; one-way ANOVA with Tukey’s post hoc test; n ≥ 3; mean ± SD. (C) Cif hydrolysis of 14,15-EET abrogates the reduction of neutrophil transepithelial migration mediated by 15-epi LXA4. Freshly isolated human neutrophils (5 × 105) were calcein-AM loaded and treated either with 15-epi LXA4 alone (light blue bar) or in the presence of Cif-WT (light red bar) or with 14,15-EET alone (blue bar) or in the presence of Cif-WT (red bar) or Cif-D129S (gray bar) for 15–45 min, as noted in Materials and Methods. The treated neutrophils were applied to the basolateral side of polarized CFBEs following the apical addition of the chemoattractant IL-8 (black bar, IL-8 positive control; white bar, no IL-8 negative control). After 2 h, calcein-AM fluorescence was measured in the apical compartment to assess neutrophil transepithelial migration. Results are shown for the comparison of each experimental condition to the migration measured in the IL-8 positive control condition. ****P < 0.0001; n.s., not significant; one-way ANOVA with Tukey’s post hoc test; n ≥ 4; mean ± SD.
Fig. S5.
Cif hydrolysis of 14,15-EET mediates differential effects on lipoxin epimers. (A) TNFα does not stimulate, and Cif does not inhibit, baseline LXA4 production by neutrophils during coculture with AECs in vitro. Polarized CFBE cells were treated with TNFα (1 ng/mL) for 24 h (blue bar) and apically exposed to 1 µM Cif for 45 min (red bar) before the addition of 1.25 × 106 neutrophils from healthy donors for 15 min. The apical supernatants were collected, added to 3 vol of cold HPLC-grade methanol, and processed for quantification of LXA4 by ELISA. (B) Cif reduces 14,15-EET–stimulated biosynthesis of 15-epi LXA4 by CF neutrophils. Freshly isolated human neutrophils from patients with CF (106) were incubated with A23187 (5 µM) and 14,15-EET (1 µM) alone (blue symbols) or in the presence of Cif-WT (1 µM) (red symbols) or Cif-D129S (gray bars). The reactions were stopped with cold MeOH, lipid extracted, and analyzed for 15-epi LXA4 concentrations by ELISA. Each symbol represents a single patient with CF and the line indicates the mean of the experimental condition. *P < 0.05, **P < 0.01; one-way ANOVA with Tukey’s post hoc test.
The 15-epi LXA4 is a potent inhibitor of neutrophil transepithelial migration (14), so we next modified our coculture system to examine whether Cif affects neutrophil transepithelial migration. Polarized CFBE cell monolayers were grown on the bottom of Transwell permeable membrane supports to physiologically model neutrophil transepithelial migration in the basolateral-to-apical direction. The neutrophil chemoattractant IL-8 was added in the apical compartment to drive neutrophil migration (20). Exposure of neutrophils to 14,15-EET (100 nM, 45 min) or to 15-epi LXA4 (100 nM, 15 min) significantly decreased transepithelial migration by 74% and 84%, respectively (Fig. 3C). Cif-WT, but not Cif-D129S, significantly abrogated the inhibition of neutrophil transepithelial migration mediated by the addition of 14,15-EET (Fig. 3C). However, Cif-WT had no effect on migration when added in the presence of 15-epi LXA4, consistent with our proposal that its inhibitory effect on 15-epi LXA4 is not direct, but instead mediated by hydrolysis of 14,15-EET. These data demonstrate that Cif can disrupt 15-epi LXA4 regulation of neutrophil transepithelial migration in the presence of IL-8.
Cif Expression in Patients with CF Is Widespread and Correlates with Elevated IL-8, Loss of 15-epi LXA4, and Reduced Pulmonary Function.
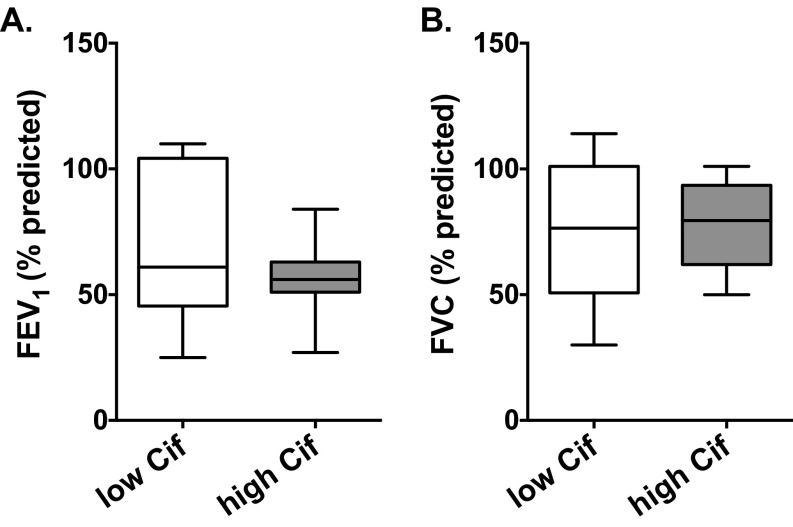
To assess the extent of patient exposure to Cif, we tested serum samples from a collection of adult donors with CF and donors without CF for the presence of Cif-specific antibodies by ELISA (Table S2 and Fig. 4A). Patients with CF uniformly exhibited high levels of α-Cif antibodies, suggesting widespread exposure to Cif protein during the natural history of disease. Based on our in vitro preclinical data (Figs. 1–3), we hypothesized that Cif levels in patients with CF would be positively correlated with lung inflammation and inversely correlated with BALF levels of 15-epi LXA4 and with several measures of lung function. To test these hypotheses, we obtained BALF from a random cross-sectional cohort of pediatric subjects with CF who had undergone bronchoscopy (Table S3). Using newly developed Cif-specific antisera (Fig. S6), we quantified Cif abundance in BALF samples that tested positive for the presence of P. aeruginosa lipopolysaccharide. We also quantified the levels of IL-8 and 15-epi LXA4 in these samples by ELISA. A Pearson cross-correlation matrix of available data demonstrated patterns clearly consistent with our predictions (Fig. 4B). Pulmonary function tests (Fig. 4B, solid outline) showed strong positive correlations with each other, consistent with CF airway disease. The 15-epi LXA4 levels positively correlated with lung function (Fig. 4B, dashed outline), consistent with the idea that proresolution signals are protective against inflammatory lung damage in CF. As predicted, Cif inversely correlated with both 15-epi LXA4 and pulmonary function measures (Fig. 4B, dotted outline), and IL-8 correlated inversely with 15-epi LXA4 and positively with Cif (Fig. 4B, red line). To investigate these patterns in more detail, we divided patients into “Cif low” and “Cif high” groups (Fig. 4C), using 10 ng Cif per milligram of total protein to delineate groups. Individual analyses confirmed significantly reduced levels of 15-epi LXA4 and poor FEV1/FVC, as well as elevated levels of IL-8, for patients with higher Cif (Fig. 4 D, F, and G). IL-8 and 15-epi LXA4 are strongly inversely correlated (Fig. 4E). Additionally, in the high-Cif patient cohort FEV1 values were reduced compared with the low-Cif group (Fig. S7A). Overall, among subjects with P. aeruginosa infections, higher Cif levels are associated with worse obstructive lung disease.
Table S2.
Demographics of the adult patients with CF and healthy patient cohorts tested for the presence of serum antibodies to Cif
| Patient | Age, y | Gender | P. aeruginosa culture positive at time of blood draw? |
| 1 | 28 | M | Y |
| 2 | 29 | M | Y |
| 3 | 28 | M | N |
| 4 | 27 | F | Y |
| 5 | 24 | M | Y |
| 6 | 26 | M | Y |
| 7 | 30 | M | N |
| 8 | 25 | M | N |
| 9 | 22 | M | NA |
| 10 | 33 | M | NA |
| 11 | 36 | M | NA |
| 12 | 26 | F | NA |
| 13 | 29 | M | NA |
| 14 | 23 | F | NA |
| 15 | 28 | M | NA |
| 16 | 24 | F | NA |
F, female; M, male; N, no; NA, not analyzed; Y, yes.
Fig. 4.
Elevated levels of Cif in CF patient bronchial lavage fluid result in lower 15-epi LXA4 and higher IL-8 concentrations and worsening pulmonary function. (A) Anti-Cif serum antibodies are generated in adult patients with CF. Sera collected from a CF and a non-CF cohort were probed for anti-Cif antibodies via ELISA. Patients with CF have significantly increased levels of anti-Cif serum antibodies compared with a non-CF cohort. *P ≤ 0.05, Wilcoxon rank-sum test. (B–G) BALF was obtained from pediatric patients with CF who received bronchoscopy. n = 17. (B) A visualization of a Pearson correlation matrix depicts positive correlations in blue and negative correlations in red. Color intensity and the size of the circle are proportional to the correlation coefficients. Below the correlogram, the scale shows the correlation coefficients and the corresponding colors. (C) Cif expression in BALF from patients with CF was determined by Western blot analysis and binned into “low” and “high” categories. (D) Concentrations of 15-epi LXA4 are significantly reduced in patients with CF with elevated levels of Cif. CF BALF samples were lipid extracted and analyzed for 15-epi LXA4 concentrations by ELISA. *P < 0.05, Wilcoxon rank-sum test. (E) Increased IL-8 concentrations strongly correlate with lower 15-epi LXA4 concentrations in BALF from patients with CF. P = 0.0016, Pearson correlation. (F) Patients with CF with elevated Cif have increased IL-8 concentrations in the airways. IL-8 concentrations in CF BALF were determined by sandwich ELISA and normalized to total protein in each sample. P = 0.055, Wilcoxon rank-sum test. (G) Patients with CF with high Cif have worse lung obstruction. Patient FEV1/FVC ratio was measured at time of bronchoscopy, stratified by high- or low-Cif groups. *P < 0.05, Wilcoxon rank-sum test.
Table S3.
Demographics of the patient cohort from which CF BALF was obtained
| Patient | Age, y | Gender | Binned Cif group |
| 1 | 13 | F | High |
| 2 | 16 | M | Low |
| 3 | 18 | F | Low |
| 4 | 17 | F | Low |
| 5 | 4 | M | High |
| 6 | 13 | F | Low |
| 7 | 11 | M | High |
| 8 | 17 | F | Low |
| 9 | 13 | F | High |
| 10 | 7 | F | High |
| 11 | 13 | M | Low |
| 12 | 12 | F | Low |
| 13 | 16 | F | Low |
| 14 | 14 | M | High |
| 15 | 16 | F | Low |
| 16 | 11 | F | High |
| 17 | 17 | F | Low |
Fig. S6.
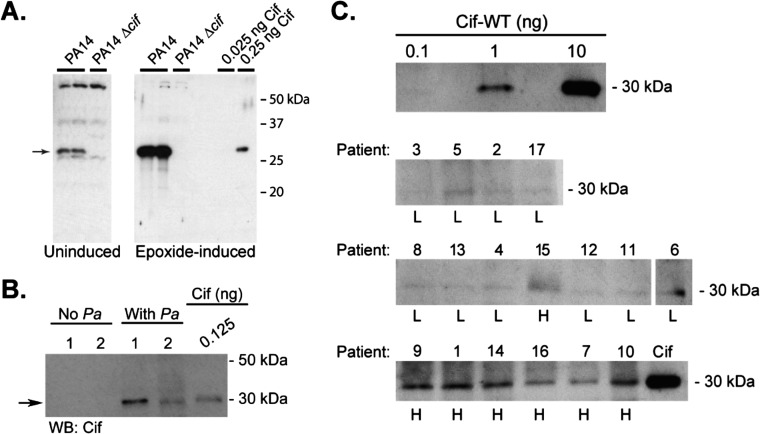
Cif is detected in BALF from patients with CF. (A) Cif antiserum displays low cross-reactivity to P. aeruginosa whole-cell lysate. P. aeruginosa was grown to stationary phase in the absence (A, Left; 10 μL of a 1:4 dilution) or presence (A, Right; 2.5 μL of a 1:20 dilution) of a known Cif inducer, styrene oxide. The samples were separated with 12% SDS/PAGE and Cif was detected using anti-Cif polyclonal rabbit serum. Recombinant Cif-WT protein is shown as a positive control. The arrow denotes the molecular mass of Cif. (B) Cif antiserum is specific for Cif protein in BALF from patients with CF. BALF from patients with CF (6 µg per lane) was separated by SDS/PAGE and anti-Cif polyclonal rabbit serum was used for detection by Western blot. Samples were tested for P. aeruginosa polysaccharide and were categorized as P. aeruginosa positive (With Pa) or negative (No Pa). Recombinant Cif-WT protein is shown as a positive control. Arrow denotes the position of Cif. (C) Cif protein is detected in BALF from patients with CF. A standard curve was generated using recombinant Cif-WT protein (C, Top). BALF for each study subject (6 µg/lane) was separated by SDS/PAGE and Cif protein was detected by Western blot. Cif was quantified in each BALF sample by densitometry, using a standard curve of known amounts of recombinant Cif protein. Cif protein concentrations are reported in Fig. 4C. Patient ID number and the associated classification as low Cif (L) or high Cif (H) are shown above and below each lane, respectively.
Fig. S7.
Reduced FEV1 pulmonary function is observed in the high-Cif CF subject cohort. Pulmonary function tests assessed by spirometry were performed on all subjects before bronchoscopy. (A) Forced expiratory volume in 1 s (FEV1) and (B) forced vital capacity (FVC) are reported in both the low-Cif and high-Cif groups. Although the difference is not statistically significant, there is a notable reduction in FEV1 in the high-Cif cohort.
Discussion
A major hallmark of the disease cystic fibrosis is vigorous and persistent pulmonary inflammation, which damages host lung tissue, eventually causing respiratory failure and death. Chronic P. aeruginosa infections often accompany robust airway inflammatory processes, provoking continuous immune cell infiltration into the lung. In the current study, we identified the epithelial-derived eicosanoid 14,15-EET as an endogenous substrate for the P. aeruginosa virulence factor Cif. We also showed that the Cif-mediated reduction of 14,15-EET disrupted paracrine signaling to neutrophils to produce 15-epi LXA4, blocking its proresolving functions (Fig. S8). Consistent with the Cif-mediated effect of blocking the generation of proresolving mediators, we demonstrated that higher Cif levels in the bronchoalveolar lavage fluid of patients with CF correlated with reduced 15-epi LXA4, increased inflammatory marker IL-8, and reduced pulmonary function. Taken together, our results provide evidence for an unanticipated role of the bacterial epoxide hydrolase Cif in obstructing normal resolution pathways in the airway and promoting pulmonary inflammation in patients with CF colonized with P. aeruginosa.
Fig. S8.
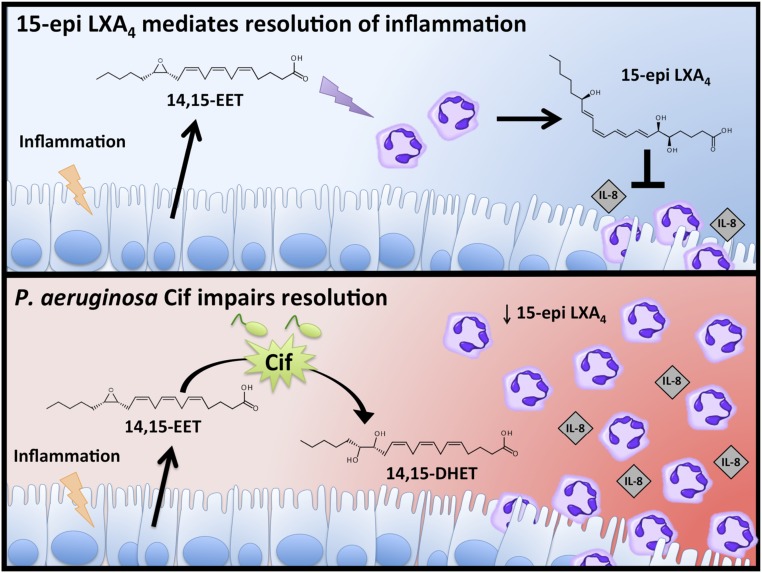
Cif restricts 15-epi LXA4 biosynthesis and promotes a hyperinflammatory environment in the CF airway. During the resolution of lung inflammation, airway epithelial cells produce the eicosanoid 14,15-EET, a paracrine signal that stimulates neutrophils to produce the proresolving lipid mediator 15-epi LXA4. Transcellular generation of 15-epi LXA4 reduces IL-8 release by the epithelium and neutrophil migration into the airway, initiating the return to tissue homeostasis (Top). However, during P. aeruginosa pulmonary infections, the secreted virulence factor Cif catalyzes the hydrolysis of 14,15-EET, thus impairing the production of 15-epi LXA4. Cif-mediated reduction of 15-epi LXA4 in the airway promotes elevated levels of IL-8 and continual neutrophil accumulation, contributing to worsening pulmonary function characteristically seen in CF (Bottom).
In contrast to the proinflammatory effects of Cif, several pathogens manipulate lipid mediators to suppress the immune response and evade detection. Mycobacterium tuberculosis and Toxoplasma gondii both enhance the generation of lipoxins (21, 22), whereas the fungal pathogen Candida albicans synthesizes resolvin E1 (23). M. tuberculosis can also inhibit the formation of the proinflammatory lipid LTB4, further shifting the host lipid environment toward an antiinflammatory state (24). P. aeruginosa produces the 15-lipoxygenase LoxA (25, 26), as well as the phospholipase ExoU (27), both of which can potentially generate antiinflammatory signals. However, unlike many airway pathogens, P. aeruginosa survives in a hyperinflammatory environment, particularly in the context of chronic lung disease (2). Consistent with the notion that P. aeruginosa actively manipulates the inflammatory environment in the airway, other studies have demonstrated that i.v. antibiotic treatments increase LXA4 levels, while decreasing proinflammatory chemokine concentrations in BALF of patients with CF (28). As a result, P. aeruginosa may be uniquely poised to exploit both pro- and antiinflammatory strategies to thwart host defense mechanisms.
It was not expected that 14,15-EET could serve as a host substrate for P. aeruginosa Cif. Previous crystallization studies identified Cif as a member of the α/β-hydrolase family of proteins, with epoxide hydrolase activity focused on small, monosubstituted substrates (29). Thus, it was surprising to discover that Cif could accommodate the extended carbon chain of an eicosanoid such as 14,15-EET. Our crystal structure revealed an unexpected rearrangement of specific residues that define the boundaries of the active site. Small-molecule inhibitors were recently reported to drive conformational changes in an epoxide hydrolase active site (30), but our study represents an example of a substrate-associated shift in this class of enzymes. Furthermore, whereas our work shows that Cif cannot hydrolyze the other EET regioisomers, its active-site flexibility may enable it to hydrolyze other members of the broad network of regulatory lipids (31).
Among its multiple antiinflammatory functions (32, 33), 14,15-EET plays a particularly critical role as a paracrine stimulus for the production of the proresolving lipid 15-epi LXA4 by neutrophils (19). In addition to enzymatically targeting 14,15-EET, our study also demonstrated that Cif indirectly reduced levels of the proresolving lipid mediator 15-epi LXA4. Previous studies have shown that CF airways exhibit substantially reduced levels of its epimer LXA4 (15), although the infection status of the patients was not reported. Our data provide evidence that the capacity to generate 15-epi LXA4 is fundamentally intact in the context of CF, yet susceptible to Cif-mediated reductions when P. aeruginosa is present in the CF airway. Specifically, the production of 14,15-EET and the associated modulation of neutrophil behavior were both observed with primary cells derived from patients with CF. Consistent with this proposal, elevated levels of Cif in BALF from patients with CF correlated with reduced concentrations of 15-epi LXA4, increased levels of the inflammatory cytokine IL-8, and worsened pulmonary function. Our study suggests that by inhibiting normal host resolution programs, the secreted P. aeruginosa virulence factor Cif contributes to the characteristic hyperinflammatory environment of the CF airway.
These results have important implications for our understanding of CF disease pathogenesis. The targeting of the proresolution 14,15-EET/15-epi LXA4 axis represents an unexpected bacterial virulence strategy. As is demonstrated by decades-long chronic infections in the inflamed lungs of patients with CF, P. aeruginosa thrives in a hyperinflammatory environment (34). Longitudinal clinical isolates of P. aeruginosa collected over a decade express cif (35) and our survey of serum titers from a group of adult subjects with CF shows that all had generated an immune response to Cif protein, suggesting that it is widely present in CF airway infections. Because our results show that Cif enables P. aeruginosa to manipulate the host inflammatory environment, it may play a significant role in defining the nature of these ongoing infections, which ultimately lead to lung tissue damage and respiratory failure. Cif homologs have been identified in other airway pathogens, including Acinetobacter nosocomialis and Burkholderia cepacia (4, 36). As a result, these bacterial epoxide hydrolases may represent a distinct class of therapeutic targets in CF and other airway diseases in which hyperinflammatory responses lead to accelerated tissue damage.
Our observations also suggest additional therapeutic approaches, as increased Cif levels correlated with more severe obstructive lung disease, as measured by FEV1/FVC ratios, in patients with CF. As increasing FEV1/FVC ratio remains a key therapeutic objective of clinical therapy, one strategy may be direct replacement of the absent proresolving lipid mediator. Multiple studies have demonstrated that exogenous administration of specialized proresolving mediators improves morbidity and mortality outcomes following infection (11). In particular, the administration of a LXA4 analog in mice challenged intratracheally with P. aeruginosa led to reduced neutrophil infiltration, weight loss, and bacterial burden, resulting in overall lessening of disease severity (15). An alternative strategy may be to inhibit Cif activity with a targeted small-molecule approach (16, 37). In addition to blocking the enzymatic degradation of lipid mediators, inhibitors would also block the ability of Cif to subvert rescue of CFTR by recently approved clinical correctors (38). Finally, because Cif represents a key link between chronic infections and the damaging, hyperinflammatory environment present in the CF airway, it may serve as a valuable biomarker of airway disease and treatment options in CF.
Materials and Methods
Protein Expression and in Vitro Hydrolysis Assay.
Wild-type Cif protein (Cif-WT) and Cif-D129S and Cif-E153Q mutants were expressed as described previously (16, 17, 29). Hydrolysis of the epoxyeicosatrienoic acids by Cif was measured using a modified adrenochrome assay (36, 39).
Crystallographic Structure of Cif with 14,15-EET.
Cif-E153Q protein incubated with 14,15-EET was crystallized, as described previously (17, 29). Oscillation data were collected and were processed and scaled with the XDS package (v. December 6, 2010). Phases were calculated by molecular replacement with the Cif-WT structure [Protein Data Bank (PDB) ID 3KD2] in the Phenix suite (v. 1.10.2055) (40–42). Standard iterative refinement was performed with Phenix and WinCoot (v. 0.7), yielding models for two dimers in the asymmetric unit. The ligand was added after the second round, before the addition of water. We deposited coordinates to the PDB (PDB ID 5JYC) and used Pymol (www.pymol.org) to render images of chain D.
Epithelial Cell Culture and Bacterial Strains.
The immortalized human CF bronchial epithelial cell line CFBE41o− (referred to here as CFBE cells) was seeded on 0.4-µM polyester Transwell filters (Corning) coated with collagen and fibronectin and grown at air–liquid interface for at least 7–10 d, as previously described (43). Primary human CF AECs (CF HBEs) acquired from the University of Pittsburgh Airway Cell and Tissue Core were cultured from explanted lungs of patients with CF, using an Institutional Review Board approved protocol at the University of Pittsburgh (IRB no. 11070367) (44). P. aeruginosa strains PA14 and PA14∆cif were gifts from George O’Toole, Geisel School of Medicine at Dartmouth (7, 45).
Quantification of 14,15-EET.
Polarized CFBE cells were treated with TNFα (R&D Systems) for 24 h prior to treatment with either Cif-WT or PA14 [multiplicity of infection (MOI) = 25]. Apical supernatants were collected in methanol, layered with N2, and solid-phase lipid extracted using C18 Sep-Pak cartridges (Waters) as previously described (19, 46). The 14,15-EET concentrations were quantified by ELISA (Detroit R&D) using prostaglandin B2 (100 ng) as an internal standard and monitored by HPLC to control for variances in extraction recovery. The 14,15-EET concentrations in apical supernatants were confirmed by liquid chromatography mass spectrometry, using previously described techniques (47, 48).
Neutrophil Isolation, 15-epi LXA4 Quantification, and Transepithelial Migration.
Neutrophils were obtained by venipuncture from volunteers who had given written informed consent according to a protocol approved by the University of Pittsburgh IRB Committee (IRB no. 14070447). Neutrophils isolated as previously described (49) were incubated with 14,15-EET (Cayman Chemical). In some conditions 14,15-EET was previously incubated with Cif-WT or Cif-D129S before neutrophil treatment. All reactions were collected in methanol and solid-phase lipid extracted, and 15-epi LXA4 concentrations were determined by ELISA (Neogen) (50). For neutrophil–AEC coculture experiments, CFBE cells were treated with TNFα for 24 h and exposed to Cif before the addition of neutrophils. For transepithelial neutrophil migration, CFBE cells were seeded on inverted 3-µM pore polycarbonate Transwell filters (51). Calcein AM (Life Technologies)-labeled neutrophils were treated with 15-epi LXA4 or 14,15-EET in the presence of Cif-WT or Cif-D129S and applied to the basolateral side of CFBE cells. IL-8 (100 pg/mL) applied to the apical compartment stimulated neutrophil transepithelial migration (20), which was measured by Calcein AM fluorescence following a 2-h incubation.
Antibody Detection in Human Sera.
Deidentified human serum samples were obtained from the Translational Research Core at Geisel School of Medicine, in accordance with the approved IRB protocol (IRB no. 22781). An indirect ELISA, with purified Cif-WT protein as bait, was developed to detect human antibodies against Cif from a 1:5,000 dilution of the serum.
Analysis of BALF from Subjects with CF.
BALF was collected prospectively over time in a pediatric population followed for management of CF at Children’s Hospital of Pittsburgh, in accordance with a protocol approved by the University of Pittsburgh IRB Committee (IRB no. 504067). ELISAs were used to determine 15-epi LXA4 (Neogen) and IL-8 (R&D Systems) concentrations in BALF of patients with CF. Cif protein quantification was determined by Western blot analysis detected by Cif-specific rabbit antisera (Cocalico Biologicals). Cif and IL-8 values were normalized and expressed per milligram of total BALF protein. Pulmonary function testing (PFT) was performed on all patients as part of their CF management and the most recent PFT data in relation to the bronchoscopy were collected.
Statistics.
Experimental differences for in vitro assays were evaluated for statistical significance by one-way analysis of variance (ANOVA) with a Tukey’s post hoc test (GraphPad 6.0). For the human studies, a Pearson correlation matrix was created using the cor package in R from measurements of Cif, 15-epi LXA4, IL8, and spirometry values for the 16 patients for whom a complete set of values was available. The corrplot package in R was used to visualize the results. Significance was determined using the nonparametric Wilcoxon rank-sum test (GraphPad 6.0) for serum antibody titers and differences between high and low Cif cohorts.
Additional methods can be found in SI Materials and Methods.
SI Materials and Methods
Protein Expression and Purification.
WT Cif protein (Cif-WT) and the Cif-D129S and Cif-E153Q mutants were expressed as described previously (18, 19, 31). Briefly, 6-His–tagged Cif, Cif-D129S, and Cif-E153Q were expressed from an arabinose-inducible vector in TOP10 Escherichia coli cells. The proteins were isolated using nickel-affinity purification, concentrated, and dialyzed into PBS.
In Vitro Hydrolysis Assay.
Hydrolysis of the epoxyeicosatrienoic acids by Cif was measured using an adrenochrome reporter assay (38, 41). Briefly, 40 μM Cif protein was incubated separately with 1 mM of each EET regioisomer (Cayman Chemical) in 2% DMSO in PBS at 37 °C for 60 min. The reactions were quenched with NaIO4 in 90% acetonitrile to a final concentration equimolar to initial EET concentrations and incubated at room temperature for 30 min. Epinephrine was added in excess to react with the residual NaIO4. A490 values were measured and compared with a standard curve generated with 14,15-DHET.
Crystallographic Structure of Cif with 14,15-EET.
Cif-E153Q:14,15-EET cocrystals were obtained by vapor diffusion against 400 µL of reservoir solution in a 4-µL hanging drop at 291 K (19, 31). A total of 5 mg/mL Cif-E153Q protein in PBS was incubated with neat 14,15-EET at 277 K overnight. The mixture was added in a 1:1 ratio with reservoir solution consisting of 12% (wt/vol) polyethylene glycol 8,000, 200 mM CaCl2, and 100 mM sodium acetate (pH 5). Before data collection, crystals were washed in cryoprotectant solution consisting of 12% (wt/vol) polyethylene glycol 8,000, 200 mM CaCl2, 100 mM sodium acetate (pH 5), and 20% (wt/vol) glycerol and flash cooled by plunging into a liquid nitrogen bath. Oscillation data were collected at 100 K at the X6A beamline of the National Synchrotron Light Source at Brookhaven National Laboratory. Diffraction images were processed and scaled with the XDS package (v. December 6, 2010) (52). Molecular replacement with the Phenix suite (v. 1.10.2055) used the apo-Cif-WT structure (PDB ID 3KD2) as the search model and revealed two dimers in the asymmetric unit (43–45). Iterative rounds of automated and manual refinement were carried out with Phenix and WinCoot (v. 0.7), respectively. The adducted ligand was included in the model after two rounds of automated and manual refinement and before the placement of waters. Final coordinates were deposited in the PDB (PDB ID 5JYC). Pymol (www.pymol.org) was used to render structure images of the final model of chain D, in which the active-site structures are best defined.
Epithelial Cell Culture.
The immortalized human CF bronchial epithelial cell line CFBE41o− (referred to here as CFBE cells) was a gift from J. P. Clancy (University of Cincinnati). CFBE cells were seeded on 0.4-µM polyester Transwell filters (Corning) and coated with collagen and fibronectin and grown at air–liquid interface for at least 7–10 d, as previously described (46). Primary human CF AECs (CF HBEs) acquired from the University of Pittsburgh Airway Cell and Tissue Core were cultured from explanted lungs from patients with CF, using an Institutional Review Board approved protocol at the University of Pittsburgh (IRB no. PRO11070367), described in ref. 47. CF HBEs were cultured on collagen-coated Transwell filters (Corning) for 4–6 wk at air–liquid interface (ALI) before use in experiments.
Bacterial Strains and Culture Conditions.
P. aeruginosa strains PA14 and PA14∆cif (gifts from George O’Toole, Geisel School of Medicine at Dartmouth) (7, 48) were used in this study. Overnight cultures grown in LB were washed and diluted in MEM (Gibco) supplemented with 2 mM glutamine to an OD600 of 0.5. Epithelial cells were inoculated with ∼7 × 106 bacteria, corresponding to a MOI of 25.
Quantification of 14,15-EET from Airway Epithelial Cell Secretions.
Polarized epithelial cells were exposed to 1 ng/mL of TNFα (R&D Systems) in MEM (Gibco) supplemented with 2 mM glutamine for 24 h before apical treatment with either 1 µM of purified Cif protein for 45 min or 7 × 106 bacteria for 5 h at 37 °C with 5% CO2. The apical supernatants were collected and immediately added to 3 vol of cold HPLC-grade methanol (Fisher Chemical), layered with N2, and stored at −80 °C. To identify 14,15-EET concentrations in the apical secretions, the samples were acidified to a pH of 4 and solid-phase lipid extracted using C18 Sep-Pak cartridges (Waters) as previously described (21, 49, 50, 53). The methyl formate (Sigma) fraction was collected, brought to dryness under a gentle stream of N2, and resuspended in 1 mL of methanol before quantification by ELISA (Detroit R&D). Prostaglandin B2 (100 ng) was added to each sample as an internal standard and monitored by HPLC to control for variances in extraction recovery. Additionally, 14,15-EET concentrations in apical supernatants were confirmed by liquid chromatography mass spectrometry, using previously described techniques (51, 52).
Neutrophil Isolation.
Neutrophils (polymorphonuclear leukocytes or neutrophils) were obtained by venipuncture from volunteers who had given written informed consent to a protocol approved by the University of Pittsburgh IRB Committee (IRB no. PRO14070447). Neutrophils were isolated from heparinized peripheral blood, by dextran (Sigma) sedimentation followed by a histopaque (Sigma-Aldrich) gradient (53). Following RBC hypotonic water lysis neutrophils were washed, counted, and resuspended to 2 × 107 cells/mL in PBS without calcium and magnesium (referred to here as PBS−/−). Only neutrophil preparations that exceeded 90% viability were used in experiments.
Quantification of Neutrophil Generation of 15-epi LXA4.
A total of 1 × 106 neutrophils were resuspended in 0.15 mL PBS with 1 mM calcium chloride and 0.5 mM magnesium chloride (PBS+/+) (Gibco) and incubated for 15 min at 37 °C with 1 µM 14,15-EET (Cayman Chemical) in the presence of 5 µM A23187 (Sigma). In some conditions 14,15-EET was previously incubated with 1 µM Cif-WT or Cif-D129S at 37 °C for 30 min before neutrophil treatment. The reactions were stopped with 3 vol of ice-cold methanol, layered with N2, and stored at −80 °C until solid-phase extraction and quantification of 15-epi LXA4 by ELISA (Neogen) as previously described (54). Prostaglandin B2 (100 ng) was added to each sample as an internal standard and monitored by HPLC to control for variances in extraction recovery. For neutrophil–airway epithelial cell coculture experiments, 2.5 × 105 polarized CFBE cells were treated with TNFα (1 ng/mL) for 24 h and apically exposed to 1 µM Cif for 45 min before the addition of 1.25 × 106 neutrophils for 15 min. The apical supernatants were collected, added to 3 vol of cold HPLC grade methanol, layered with N2, and stored at −80 °C until further processed for quantification of 15-epi LXA4 by ELISA. Similar methodology was used for quantification of LXA4 by ELISA (Neogen).
Neutrophil Transepithelial Migration.
Polycarbonate Transwell filters with 3-µm pores were inverted, coated with collagen and fibronectin, seeded with 1 × 105 CF AECs, and allowed to adhere overnight at 37 °C (55). The following day the filters were moved to a sterile 24-well tissue culture plate with 0.5 mL of medium added to the apical chamber and 0.1 mL to the basolateral compartment. After allowing the cells to grow to confluency for 3 d, apical medium was removed and the cells were grown at air–liquid interface for 7 d. CF AECs were washed with PBS+/+ and allowed to calibrate for 1 h before the application of neutrophils on the basolateral surface. Transepithelial electrical resistance (TEER) measurements were conducted to confirm polarization of monolayers.
Isolated neutrophils were labeled with 3 µM Calcein-AM (Life Technologies) in PBS without calcium and magnesium (PBS −/−) (Gibco) for 30 min at 37 °C in 5% CO2. The labeled neutrophils were washed twice and resuspended in PBS+/+ before incubation in the presence or absence of 14,15-EET (100 nM), Cif-WT (100 nM), or Cif-D129S (100 nM) for 45 min at 37 °C with 5% CO2. In some conditions neutrophils were incubated in parallel with 15-epi LXA4 (100 nM) for 15 min at 37 °C with 5% CO2. IL-8 (100 ng/mL) in PBS+/+ was applied to the apical chamber 10 min before the addition of 5 × 105 of the treated neutrophils to the basolateral compartment (22). After incubating for 2 h at 37 °C the filters were removed and the plate was gently spun at 300 × g for 5 min. Neutrophils that had migrated to the apical chamber were measured by Calcein-AM fluorescence detected by a SpectraMax M2 plate reader.
Analysis of BALF from Subjects with CF.
BALF was collected prospectively over time in a pediatric population followed for management of CF at Children’s Hospital of Pittsburgh, in accordance with a protocol approved by the University of Pittsburgh IRB Committee (IRB no. 504067). Documentation of a CF diagnosis was evidenced by one or more clinical features consistent with the CF phenotype and one or more of the following criteria: sweat chloride equal to or greater than 60 mEq/L by quantitative pilocarpine iontophoresis test or two well-characterized mutations in the CFTR gene. The 17 samples analyzed in the current study tested positive for P. aeruginosa infection by dot blot, using an anti-Pseudomonas antibody (Pierce). Patient BALF was methanol trapped and lipid extracted before quantification of 15-epi LXA4 by ELISA (Neogen). IL-8 concentrations were determined using sandwich ELISA (R&D Systems). Cif protein quantification was determined by Western blot analysis. BALF samples were separated by SDS/PAGE, transferred to PVDF membrane, and detected by Cif-specific rabbit antisera (Fig. S7). Cif and IL-8 values were normalized and expressed per milligram of total BALF protein. As a result, overall changes in total protein concentration were factored out, providing a more stringent threshold for detection of differences. PFT was performed on all patients as part of their CF management and the most recent PFT data in relation to the bronchoscopy were collected.
Cif Antisera.
Cocalico Biologicals provided test bleed sera for initial screening of rabbits as a host species for the development of a Cif-specific polyclonal antibody. We selected two rabbits with the lowest background sera reaction as determined by Western blot against multiple P. aeruginosa strains. Purified protein (500 μL at 4.3 mg/mL) was submitted to Cocalico Biologicals. Animals were initially inoculated at day 0 and boosted on days 14, 21, 49, 97, 140, and 170. Test bleeds were taken on days 35, 56, 111, and 154. The desired level of Cif detection was reached at day 154. Sera were harvested via two production bleeds (days 177 and 184) followed by exsanguination on day 191. Sera were aliquoted and stored at −80 °C.
Detection of Cif Antibody in Human Sera.
Deidentified human serum samples were obtained from the Translational Research Core at Geisel School of Medicine, in accordance with the approved IRB protocol (IRB no. 22781). Purified Cif protein was adsorbed to 96-well flat-bottom plates (IMMULON-4-HBX). After blocking, adsorbed wells were incubated with 1:5,000 dilution of human serum in PBS. The plate was washed and blocked, and adherent antibodies were detected with goat anti-human Ig(MGA-HL) biotin (Southern Biotech) and Streptavidin-conjugated horseradish peroxidase (Southern Biotech). The samples were developed with the TBM Microwell Peroxidase Substrate System (KPL) and quenched with 0.2 N sulfuric acid, and an A450 value was read.
Statistics.
Experimental differences for in vitro assays were evaluated for statistical significance by one-way ANOVA with a Tukey’s post hoc test (GraphPad 6.0 software). All in vitro data are shown as mean ± SD. P < 0.05 was considered statistically significant. For the human studies, a Pearson correlation matrix was created using the cor package in R from measurements of Cif, 15-epi LXA4, IL-8, and spirometry values for the 16 patients for whom a complete set of values was available. The corrplot package in R was used to visualize the results. For statistical analyses pertaining to serum antibody titers and the differences between high- and low-Cif cohorts, significance was determined using the nonparametric Wilcoxon rank-sum test (GraphPad 6.0 software).
Acknowledgments
We thank Jessica St. Laurent and Christopher Pennil (Dartmouth) and Stefanie Brown, Dr. Joseph Pilewski, and Dr. Michael Myerburg (University of Pittsburgh) for technical assistance; Dr. Vivian Stojanoff, Dr. Jean Jakoncic, and Edwin Lazo (NSLS/Brookhaven) for assistance with data collection; and Emily Dolben, Drs. Deborah Hogan, George O’Toole, Sven Wilger, and Alix Ashare and the Lung Biology Translational Research Core (Dartmouth) and Dr. Jeffrey Melvin (University of Pittsburgh) for samples, intellectual support, and helpful discussions. This work was supported by the National Institutes of Health Grants [K99/R00HL098342 and P30DK072506 (to J.M.B.), T32AI060525 (to B.A.F.), P01GM095467 and U24AI118656 (to B.D.L.), U24DK097154 (to B.D.H.), R01AI091699, P20GM113132, and P30GM106394 (to D.R.M.), T32GM008704 (to K.L.H.), and GM-0800 (beamline access)], a Gilead Sciences Research Scholars in Cystic Fibrosis Award (to J.M.B.), Cystic Fibrosis Foundation Grants MADDEN08G0 and STANTO19R0 (to D.R.M.), and a Munck-Pfefferkorn Award (to D.R.M.).
Footnotes
Conflict of interest statement: C.D.B., C.M., B.D.H., and D.R.M. are coinventors of patent-pending Cif inhibitor compounds. B.D.L. is an inventor on patents (resolvins) licensed by Brigham and Women’s Hospital to Resolvyx Pharmaceuticals, a company that seeks to develop Resolvin therapeutics for inflammatory diseases. B.D.L. also owns equity in the company. B.D.L.’s interests were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies.
This article is a PNAS Direct Submission. C.L.K. is a Guest Editor invited by the Editorial Board.
Data deposition: For the crystallographic structure reported in this paper, atomic coordinates and structure factors have been deposited in the Protein Data Bank (PDB), www.pdb.org (PDB ID code 5JYC).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1610242114/-/DCSupplemental.
References
- 1.Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol. 2015;50(Suppl 40):S39–S56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 2.Cohen TS, Prince A. Cystic fibrosis: A mucosal immunodeficiency syndrome. Nat Med. 2012;18(4):509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gifford AM, Chalmers JD. The role of neutrophils in cystic fibrosis. Curr Opin Hematol. 2014;21(1):16–22. doi: 10.1097/MOH.0000000000000009. [DOI] [PubMed] [Google Scholar]
- 4.Bahl CD, Madden DR. Pseudomonas aeruginosa Cif defines a distinct class of α/β epoxide hydrolases utilizing a His/Tyr ring-opening pair. Protein Pept Lett. 2012;19(2):186–193. doi: 10.2174/092986612799080392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahl CD, et al. Crystal structure of the cystic fibrosis transmembrane conductance regulator inhibitory factor Cif reveals novel active-site features of an epoxide hydrolase virulence factor. J Bacteriol. 2010;192(7):1785–1795. doi: 10.1128/JB.01348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bomberger JM, et al. Pseudomonas aeruginosa Cif protein enhances the ubiquitination and proteasomal degradation of the transporter associated with antigen processing (TAP) and reduces major histocompatibility complex (MHC) class I antigen presentation. J Biol Chem. 2014;289(1):152–162. doi: 10.1074/jbc.M113.459271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacEachran DP, et al. The Pseudomonas aeruginosa secreted protein PA2934 decreases apical membrane expression of the cystic fibrosis transmembrane conductance regulator. Infect Immun. 2007;75(8):3902–3912. doi: 10.1128/IAI.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye S, MacEachran DP, Hamilton JW, O’Toole GA, Stanton BA. Chemotoxicity of doxorubicin and surface expression of P-glycoprotein (MDR1) is regulated by the Pseudomonas aeruginosa toxin Cif. Am J Physiol Cell Physiol. 2008;295(3):C807–C818. doi: 10.1152/ajpcell.00234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballok AE, Filkins LM, Bomberger JM, Stanton BA, O’Toole GA. Epoxide-mediated differential packaging of Cif and other virulence factors into outer membrane vesicles. J Bacteriol. 2014;196(20):3633–3642. doi: 10.1128/JB.01760-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dennis EA, Norris PC. Eicosanoid storm in infection and inflammation. Nat Rev Immunol. 2015;15(8):511–523. doi: 10.1038/nri3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Basil MC, Levy BD. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16(1):51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godson C, et al. Cutting edge: Lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol. 2000;164(4):1663–1667. doi: 10.4049/jimmunol.164.4.1663. [DOI] [PubMed] [Google Scholar]
- 14.Fierro IM, et al. Lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 inhibit human neutrophil migration: Comparisons between synthetic 15 epimers in chemotaxis and transmigration with microvessel endothelial cells and epithelial cells. J Immunol. 2003;170(5):2688–2694. doi: 10.4049/jimmunol.170.5.2688. [DOI] [PubMed] [Google Scholar]
- 15.Karp CL, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004;5(4):388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 16.Bahl CD, et al. Inhibiting an epoxide hydrolase virulence factor from Pseudomonas aeruginosa protects CFTR. Angew Chem Int Ed Engl. 2015;54(34):9881–9885. doi: 10.1002/anie.201503983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahl CD, Hvorecny KL, Morisseau C, Gerber SA, Madden DR. Visualizing the mechanism of epoxide hydrolysis by the bacterial virulence enzyme Cif. Biochemistry. 2016;55(5):788–797. doi: 10.1021/acs.biochem.5b01229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Planagumà A, et al. Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal Immunol. 2010;3(3):270–279. doi: 10.1038/mi.2009.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ono E, et al. National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190(8):886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu M, Miller EJ, Lin X, Simms HH. Transmigration across a lung epithelial monolayer delays apoptosis of polymorphonuclear leukocytes. Surgery. 2004;135(1):87–98. doi: 10.1016/s0039-6060(03)00347-7. [DOI] [PubMed] [Google Scholar]
- 21.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: A mechanism for regulation of microbial immunity. Nat Immunol. 2002;3(1):76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 22.Bafica A, et al. Host control of Mycobacterium tuberculosis is regulated by 5-lipoxygenase-dependent lipoxin production. J Clin Invest. 2005;115(6):1601–1606. doi: 10.1172/JCI23949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas-Stapleton EJ, et al. Candida albicans modulates host defense by biosynthesizing the pro-resolving mediator resolvin E1. PLoS One. 2007;2(12):e1316. doi: 10.1371/journal.pone.0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tobin DM, Roca FJ, Ray JP, Ko DC, Ramakrishnan L. An enzyme that inactivates the inflammatory mediator leukotriene b4 restricts mycobacterial infection. PLoS One. 2013;8(7):e67828. doi: 10.1371/journal.pone.0067828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vance RE, Hong S, Gronert K, Serhan CN, Mekalanos JJ. The opportunistic pathogen Pseudomonas aeruginosa carries a secretable arachidonate 15-lipoxygenase. Proc Natl Acad Sci USA. 2004;101(7):2135–2139. doi: 10.1073/pnas.0307308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deschamps JD, et al. Biochemical and cellular characterization and inhibitor discovery of Pseudomonas aeruginosa 15-lipoxygenase. Biochemistry. 2016;55(23):3329–3340. doi: 10.1021/acs.biochem.6b00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saliba AM, et al. Eicosanoid-mediated proinflammatory activity of Pseudomonas aeruginosa ExoU. Cell Microbiol. 2005;7(12):1811–1822. doi: 10.1111/j.1462-5822.2005.00635.x. [DOI] [PubMed] [Google Scholar]
- 28.Chiron R, Grumbach YY, Quynh NV, Verriere V, Urbach V. Lipoxin A(4) and interleukin-8 levels in cystic fibrosis sputum after antibiotherapy. J Cyst Fibros. 2008;7(6):463–468. doi: 10.1016/j.jcf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Bahl CD, MacEachran DP, O’Toole GA, Madden DR. Purification, crystallization and preliminary X-ray diffraction analysis of Cif, a virulence factor secreted by Pseudomonas aeruginosa. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66(Pt 1):26–28. doi: 10.1107/S1744309109047599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Y, et al. Fragment screening of soluble epoxide hydrolase for lead generation-structure-based hit evaluation and chemistry exploration. ChemMedChem. 2016;11(5):497–508. doi: 10.1002/cmdc.201500575. [DOI] [PubMed] [Google Scholar]
- 31.Serhan CN, Chiang N, Dalli J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol. 2015;27(3):200–215. doi: 10.1016/j.smim.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morin C, Sirois M, Echave V, Gomes MM, Rousseau E. EET displays anti-inflammatory effects in TNF-alpha stimulated human bronchi: Putative role of CPI-17. Am J Respir Cell Mol Biol. 2008;38(2):192–201. doi: 10.1165/rcmb.2007-0232OC. [DOI] [PubMed] [Google Scholar]
- 33.Thomson SJ, Askari A, Bishop-Bailey D. Anti-inflammatory effects of epoxyeicosatrienoic acids. Int J Vasc Med. 2012;2012:605101. doi: 10.1155/2012/605101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao J, et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc Natl Acad Sci USA. 2012;109(15):5809–5814. doi: 10.1073/pnas.1120577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ballok AE, et al. Epoxide-mediated CifR repression of cif gene expression utilizes two binding sites in Pseudomonas aeruginosa. J Bacteriol. 2012;194(19):5315–5324. doi: 10.1128/JB.00984-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bahl CD, et al. Signature motifs identify an Acinetobacter Cif virulence factor with epoxide hydrolase activity. J Biol Chem. 2014;289(11):7460–7469. doi: 10.1074/jbc.M113.518092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitamura S, et al. Rational design of potent and selective inhibitors of an epoxide hydrolase virulence factor from Pseudomonas aeruginosa. J Med Chem. 2016;59(10):4790–4799. doi: 10.1021/acs.jmedchem.6b00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanton BA, Coutermarsh B, Barnaby R, Hogan D. Pseudomonas aeruginosa reduces VX-809 stimulated F508del-CFTR chloride secretion by airway epithelial cells. PLoS One. 2015;10(5):e0127742. doi: 10.1371/journal.pone.0127742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cedrone F, Bhatnagar T, Baratti JC. Colorimetric assays for quantitative analysis and screening of epoxide hydrolase activity. Biotechnol Lett. 2005;27(23-24):1921–1927. doi: 10.1007/s10529-005-3904-1. [DOI] [PubMed] [Google Scholar]
- 40.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66(Pt 2):213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 42.DeLano WL. The PyMOL Molecular Graphics System. 2008 (DeLano Scientific LLC, Palo Alto, CA). Available at www.pymol.org.
- 43.Bomberger JM, et al. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog. 2009;5(4):e1000382. doi: 10.1371/journal.ppat.1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zemke AC, et al. Nitrite modulates bacterial antibiotic susceptibility and biofilm formation in association with airway epithelial cells. Free Radic Biol Med. 2014;77:307–316. doi: 10.1016/j.freeradbiomed.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomberger JM, Barnaby RL, Stanton BA. The deubiquitinating enzyme USP10 regulates the post-endocytic sorting of cystic fibrosis transmembrane conductance regulator in airway epithelial cells. J Biol Chem. 2009;284(28):18778–18789. doi: 10.1074/jbc.M109.001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powell WS. Extraction of eicosanoids from biological fluids, cells, and tissues. Methods Mol Biol. 1999;120:11–24. doi: 10.1385/1-59259-263-5:11. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Eiserich JP, Cross CE, Morrissey BM, Hammock BD. Metabolomic profiling of regulatory lipid mediators in sputum from adult cystic fibrosis patients. Free Radic Biol Med. 2012;53(1):160–171. doi: 10.1016/j.freeradbiomed.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- 50.Chiang N, et al. Aspirin-triggered 15-epi-lipoxin A4 (ATL) generation by human leukocytes and murine peritonitis exudates: Development of a specific 15-epi-LXA4 ELISA. J Pharmacol Exp Ther. 1998;287(2):779–790. [PubMed] [Google Scholar]
- 51.Kusek ME, Pazos MA, Pirzai W, Hurley BP. In vitro coculture assay to assess pathogen induced neutrophil trans-epithelial migration. J Vis Exp. 2014;(83):e50823. doi: 10.3791/50823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krauth-Siegel RL, et al. Crystallization and preliminary crystallographic analysis of trypanothione reductase from Trypanosoma cruzi, the causative agent of Chagas' disease. FEBS Lett. 1993;317(1-2):105–108. doi: 10.1016/0014-5793(93)81501-p. [DOI] [PubMed] [Google Scholar]
- 53.Levy BD, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172(7):824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]