Hormesis has inspired fierce debates ranging from questioning its very existence to controversies about its practical implications for human health. Conceptually, it suffered from the unfortunate proximity of some of its earliest protagonists with homeopathy, while the experimental determination of mechanisms has been hampered by the apparent complexity underlying this phenomenon.
The field was inaugurated by the end of the 19th century, when Hugo Schulz realized that small doses of toxic compounds could enhance yeast growth.1 The toxin would draw a biphasic response: at high doses it would be increasingly deleterious, whereas at low doses the adaptive response to the compound would not only counteract the harm inflicted by the treatment but would also improve the organism's growth by augmenting stress resistance (Fig. 1).
Figure 1.
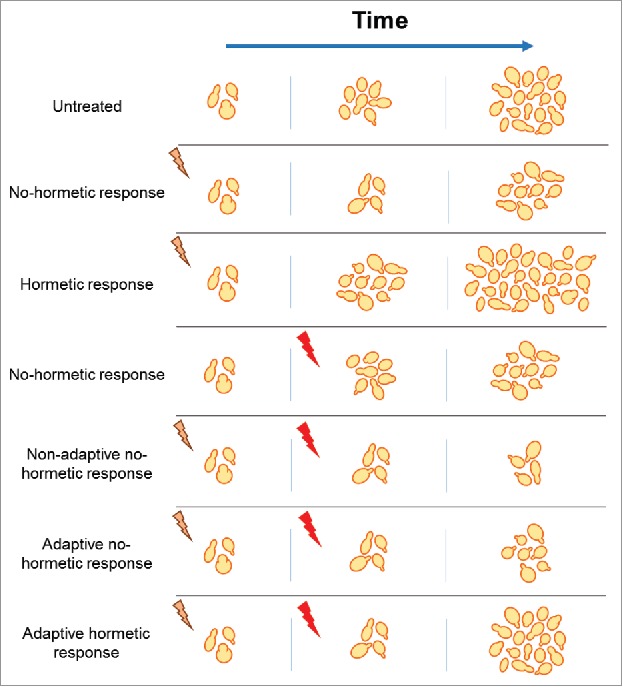
Conceptual possibilities of distinct consequences of adaptive and hormetic responses to toxic insults on fitness as measured by proliferation of yeast cells. Mild stress (yellow) such as low genotoxic treatments might trigger adaptive responses that under some circumstances could protect from severe stress (red). Markiewicz-Potoczny & Lydall observed adaptive but no hormetic responses in yeast cells with compromised telomere function or cell cycle regulation.
In recent years the interest in hormesis has been boosted through numerous observations of beneficial effects of low dose toxic treatments that have been systematically reviewed by Edward Calabrese who has concluded that hormesis might actually be a widespread phenomenon.2 Even though there are cases that have shown negative results, a range of studies on low dose of irradiation in humans have supported possible benefits to health suggesting a hormetic response to radiation.3
Other studies have contributed to the hormesis field after identifying mechanisms that could be underlying the positive effects upon specific stress agents. Genotoxic stress has been tested in cell cultures and Caenorhabditis elegans showing that the adaptive response to the damage can have positive effects. For instance, transcription-blocking DNA lesions led to the attenuation of the insulin-like growth factor and growth hormone signaling pathways –a phenomenon that is associated with extended longevity– in mouse and human cells, which increased the resistance of these cells upon posterior oxidative stress exposure.4 In C. elegans, DNA damage in the germ cells has been shown to induce a systemic stress response that allows somatic tissues to resist other stress sources.5 In these cases, the DNA damage response activates a stress response program that not only adapts the cells and organism upon further stress, but confers an exceptional resistance to stress that outperforms controls unexposed to the first insult. Similarly, in a progeria mouse model for trichothiodystrophy, carrying a genetic defect in the transcription factor II H (TFIIH) subunit XPD that is required for nucleotide excision repair, showed some specific benefits associated with a calorie restriction response including reduced tumors, lower inflammation and less cataracts besides strong segmental progeroid pathologies affecting a range of tissues.6
Markiewicz-Potoczny and Lydall investigated two yeast models with chronic stress associated with DNA damage and an impaired cell cycle progression. Yeast has been broadly used in search for hormetic effects and presents a simple model with a well-known response to stress, making it an optimal model for this purpose. By using temperature sensitive mutants, the authors could adjust the stress levels and evaluate if at mild stress infliction yeast showed an improved growth (Fig. 1). Of particular interest is the use of the temperature sensitive cdc13-1 allele that is defective in telomere capping leading to a DNA damage response. However, no hormetic effect was found and yeast with telomere defects never grew better than controls without telomere defects. Furthermore, cdc15-2 temperature sensitive mutants with a hampered mitotic progression also showed a similar behavior, with no improvement upon mild stress. In both sets of experiments, yeast that had been exposed to low level stress were able to respond better to high level stress, but this adaptive response was not sufficient to exceed the performance of unstressed cells (controls without mutations).7
The lack of hormetic effects in this strictly controlled experimental system suggests that hormesis could be a less common consequence of adaptive responses than previously thought. It remains certainly a possibility that the adaptive responses occurring upon DNA damage of cell cycle perturbations might result in hormesis under different stress conditions or environmental constraints. Studies on adaptive responses in simple model systems such as yeast or nematodes will help to discern the key factors in stress responses that are required to produce hormetic effects, and therefore, establish the bases for designing treatments that could harness this response with the minimal (if any) damage induction.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Schulz H. Ueber Hefegifte. Pflugers Arch Gesamte Physiol 1888; 42:517-41; http://dx.doi.org/ 10.1007/BF01669373 [DOI] [Google Scholar]
- [2].Calabrese EJ, Blain R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: An overview. Toxicol Appl Pharm 2005; 202:289-301; http://dx.doi.org/ 10.1016/j.taap.2004.06.023 [DOI] [PubMed] [Google Scholar]
- [3].Vaiserman AM. Radiation hormesis: Historical perspective and implications for low-dose cancer risk assessment. Dose Response 2010; 8:172-191; PMID:20585444; http://dx.doi.org/ 10.2203/dose-response.09-037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Garinis GA, et al.. Persistent transcription-blocking DNA lesions trigger somatic growth attenuation associated with longevity. Nat Cell Bio 2009; 11:604-15; http://dx.doi.org/ 10.1038/ncb1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ermolaeva MA, Segref A, Dakhovnik A, Ou HL, Schneider JI, Utermöhlen O, Hoppe T, Schumacher B . DNA damage in germ cells induces an innate immune response that triggers systemic stress resistance. Nature 2013; 501:416-20; PMID:23975097; http://dx.doi.org/ 10.1038/nature12452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wijnhoven SW, Beems RB, Roodbergen M, van den Berg J, Lohman PH, Diderich K, van der Horst GT, Vijg J, Hoejimakers JH, van Steeg H. Accelerated aging pathology in ad libitum fed Xpd(TTD) mice is accompanied by features suggestive of caloric restriction. DNA Repair (Amst) 2005; 4:1314-24; PMID:16115803; http://dx.doi.org/ 10.1016/j.dnarep.2005.07.002 [DOI] [PubMed] [Google Scholar]
- [7].Markiewicz-Potoczny M, Lydall D. Costs, benefits and redundant mechanisms of adaptation to chronic low-dose stress in yeast. Cell Cycle 2016;15(20):2732–41; PMID: 27628486; http://dx.doi.org/ 10.1080/15384101.2016.1218104 [DOI] [PMC free article] [PubMed] [Google Scholar]


