Abstract
Objective
We aimed to evaluate the value of novel high-resolution impedance manometry (HRIM) metrics, bolus flow time (BFT) and esophagogastric junction (EGJ) contractile integral (CI), as well as EGJ pressure (EGJP) and the integrated relaxation pressure (IRP), as indicators of treatment response in achalasia.
Design
We prospectively evaluated 75 patients (ages 19–81, 32 female) with achalasia during follow-up after pneumatic dilation or myotomy with Eckardt score (ES), timed-barium esophagram (TBE), and HRIM. Receiver-operating characteristic (ROC) curves for good symptomatic outcome (ES ≤ 3) and good radiographic outcome (TBE column height at 5 minutes < 5 cm) were generated for each potential predictor of treatment response (EGJP, IRP, BFT, and EGJ-CI).
Results
Follow-up occurred at a median (range) 12 (3 – 291) months following treatment. 49 patients had good symptomatic outcome and 46 had good radiographic outcome. The area-under-the-curves (AUCs) on the ROC curve for symptomatic outcome were 0.55 (EGJP), 0.62 (IRP), 0.77 (BFT) and 0.56 (EGJ-CI). The AUCs for radiographic outcome were 0.64 (EGJP), 0.48 (IRP), 0.73 (BFT), and 0.65 (EGJ-CI). Optimal cut-points were determined as 11 mmHg (EGJP) 12 mmHg (IRP), zero seconds (BFT), and 30 mmHg•cm (EGJ-CI) that provided sensitivities/specificities of 57%/46% (EGJP), 65%/58% (IRP), 78%/77% (BFT), and 53%/62% (EGJ-CI) to predict symptomatic outcome and 57%/66% (EGJP), 57%/41% (IRP), 76%/69% (BFT), and 57%/66% (EGJ-CI) to predict radiographic outcome.
Conclusions
BFT, a novel HRIM metric, provided an improved functional assessment over manometric measures of EGJP, IRP, and EGJ-CI at follow-up after achalasia treatment and may help direct clinical management.
Keywords: achalasia, high-resolution manometry, impedance, esophagram
INTRODUCTION
Achalasia is a primary esophageal motor disorder that typically presents with symptoms of dysphagia, regurgitation, and/or chest pain. Achalasia is characterized and diagnosed by manometry demonstrating impaired deglutitive lower esophageal sphincter (LES) relaxation and absent peristalsis.(1) As the symptoms of achalasia involve esophagogastric junction (EGJ) outflow obstruction and subsequent esophageal bolus retention and stasis, the management of achalasia targets relieving the obstruction at the EGJ by pneumatic dilation (PD), laparoscopic Heller myotomy (LHM), or more recently, per-oral endoscopic myotomy (POEM).(2–4) While treatment is often successful in improving both symptoms and esophageal emptying, follow-up of patients is essential to confirm sustained treatment response, to assess the need for further treatment, and hopefully to prevent progression to advanced disease (e.g. dilated, sigmoid esophagus).(2–4)
Although manometry is considered the optimal test to diagnose achalasia, follow-up after treatment relies heavily upon symptom assessment. However, symptom severity can correlate poorly with objective testing (esophagram and/or manometry) and previous studies have shown that esophageal stasis as measured with timed-barium esophagram (TBE) is a better predictor of treatment failure than symptom severity.(5–7) Current recommendations for post-treatment follow-up evaluation in patients with achalasia involves intermittent assessment with TBE and/or manometry.(2) Studies utilizing the conventional manometric measurement of LES pressures have demonstrated an association with improved long-term clinical outcomes after treatment,(8–10) However, reports conflict on the utility of conventional manometry to evaluate clinical outcomes. Though high-resolution manometry (HRM) facilitated improved diagnosis of achalasia utilizing the 4-second integrated relaxation pressure (IRP) compared to conventional manometric measures, the association of IRP measurement with achalasia treatment outcomes has yielded varied results.(7, 11, 12)
High-resolution impedance manometry (HRIM) incorporates multichannel, intraluminal impedance sensors onto the HRM catheters and allows for the simultaneous assessment of intraluminal pressures, bolus presence, and bolus flow.(13, 14) A study using concurrent HRIM and esophageal intraluminal ultrasound demonstrated that impedance measurement can assess EGJ opening and esophageal emptying.(13) Based on this concept, we developed and validated a novel HRIM metric to specifically assess flow across the EGJ: the bolus flow time (BFT).(15) Utilizing simultaneous videofluoroscopy with HRIM, bolus flow across the EGJ was observed on fluoroscopy when two criteria were met: 1) bolus was present, which was associated with a decrease in impedance and 2) a preferential pressure gradient existed across the EGJ such that pressure in the distal esophagus was greater than at the crural diaphragm. Both of these criteria were incorporated into a computer-based algorithm for automated calculation of the BFT. We recently reported that the BFT was a useful measure in patients with suspected achalasia and borderline IRP measures and that BFT had a better symptom-association than basal EGJ pressure (EGJP) or IRP in patients with achalasia prior to intervention.(16)
Another novel HRM metric of basal EGJ pressure is the EGJ contractile integral (EGJ-CI), which incorporates an intragastric pressure reference and the respiratory cycle to assess the barrier function of the EGJ.(17) Reduced EGJ-CI was initially reported to be associated with gastroesophageal reflux disease.(17, 18) Recently, a greater-than-normal EGJ-CI was reported in patients with newly-diagnosed achalasia and a reduction in EGJ-CI was observed following Heller myotomy.(19)
Therefore, we hypothesized BFT and EGJ-CI would be well suited to assess patients with achalasia during follow-up after treatment. We aimed to evaluate the value of BFT and EGJ-CI in addition to the established HRM metrics of EGJP and IRP, in assessing clinical outcomes in patients with achalasia during follow-up after treatment with PD or myotomy.
METHODS
Subjects
We prospectively recruited and evaluated patients with achalasia and previous treatment with PD, LHM (often with Dor or Toupet fundoplasty), and/or POEM returning for follow-up or referred from elsewhere. 75 consecutive patients (without hiatal hernia > 2 cm) evaluated between April 2013 and December 2015 that completed HRIM, Eckhart score, and TBE were included in analysis, noting the time interval from most recent PD or myotomy. When available, HRM performed prior to intervention was evaluated according to the Chicago Classification.(1) The study protocol was approved by the Northwestern University Institutional Review Board.
Study protocol
After a minimum 6-hour fast, HRIM studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals and 18 impedance segments at 2-cm intervals (Medtronic Inc, Shoreview, MN). The HRIM assembly was placed transnasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. The HRIM protocol included a 5-minute baseline recording, 10 5-ml swallows in a supine position, and five 5-ml swallows in the upright position using 50% saline for test swallows at 20–30 second intervals.
Data analysis
Manometry studies were analyzed using ManoView version 3.0 analysis software to measure EGJP, IRP, distal contractile integral (DCI), and distal latency. The EGJP was measured at end-expiration using the isobaric contour tool with pressure referenced to gastric pressure during the supine, baseline recording over a period of easy breathing without swallows. The IRP was the mean pressure of four contiguous or non-contiguous seconds of maximal relaxation during the 10-second deglutitive period as referenced to gastric pressure; the median IRP of 10 supine swallows was used for each patient. Esophageal motility diagnoses were in accordance with the Chicago Classification v3.0, using a median IRP of > 15 mmHg as the upper-limit of normal.(1) Although the Chicago Classification was designed and intended for patients without previous surgery, we utilized the classification scheme in our post-treatment cohort to objectively describe the motility patterns observed on follow-up.
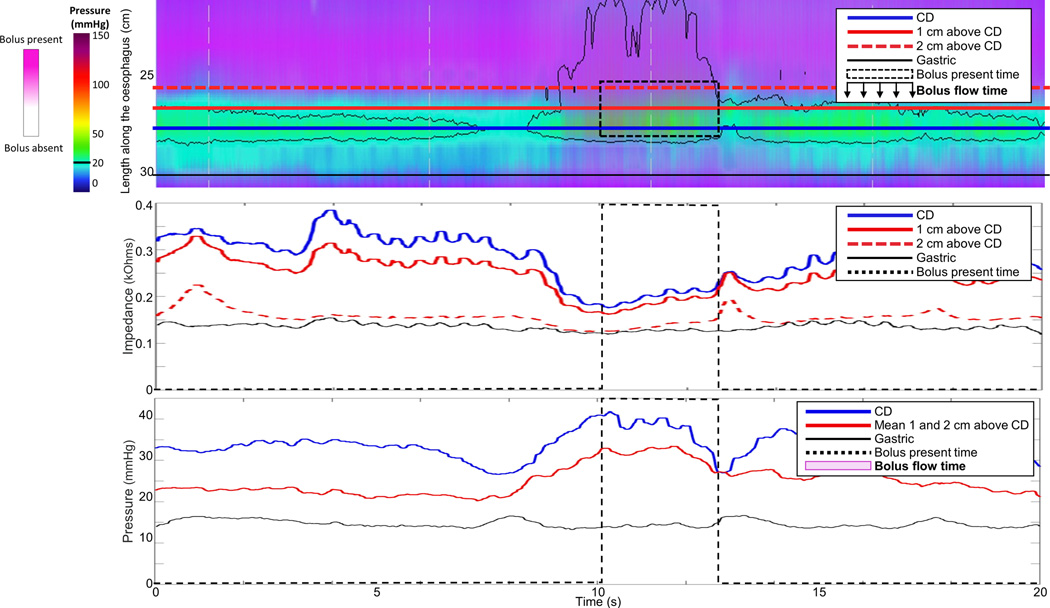
The HRIM data for each subject were exported to MATLAB™ (The MathWorks Inc., Natick, MA, U.S.A.) to apply to a customized program to calculate the BFT. To measure the BFT, three impedance and three manometry signals were placed through the EGJ at 1-cm intervals (thus, impedance and pressure signals were interpolated by the analysis software). The distal impedance and manometry signal was positioned within the hiatus as identified by crural contractions.(15, 16) Example swallows are displayed in Figure 1. Using the impedance signals, the duration of bolus presence was determined: The onset of bolus presence was defined as the point at which the impedance dropped to 90% of the nadir; the offset of bolus presence was defined as the return to 50% of the impedance baseline. Using the three manometry signals, periods of a trans-EGJ flow-permissive pressure gradient (i.e. when the esophageal pressure was greater than both the crural and intra-gastric pressure signals) were determined. The BFT was then derived as the sum of all periods meeting the criteria of both bolus presence and a flow-permissive pressure gradient time. If the impedance drop was not greater than 50% at each axial location and/or a flow-permissive pressure gradient was not achieved (as in Figure 1B), the BFT was considered to be zero. The median BFT value of the five upright swallows was utilized for each patient. Previous study of asymptomatic volunteers demonstrated a median (interquartile range, IQR) BFT of 3.2s (2.3 – 3.9 s) for upright swallows; lower BFT values indicate reduced esophageal emptying.(16)
Figure 1. Measurement of bolus flow time (BFT).
Examples from a single swallow from two patients 10 months after pneumatic dilation are displayed. At the time of follow-up, patient A had an Eckardt score (ES) of 1 and a 5-minute timed-barium esophagram column height (TBE5) of 0-cm. Patient B had an ES of 10 and TBE5 of 10-cm. The top panel is the esophageal pressure topography of the distal esophagus; the overlaid horizontal lines represent the placement of the esophagogastric junction (EGJ) and gastric impedance and manometry signals. The middle panel represents the impedance signals which were used to determine the time of bolus presence. The bottom panel represents the pressure signals used to determine periods of a flow-permissive pressure gradient, i.e. when the esophageal pressure, red line, was greater than both the hiatal (crural diaphragm, CD) and intra-gastric pressure signals. The BFT was then derived as the time when both criteria (1. bolus presence and 2. trans-EGJ flow permissive pressure gradient) were met. The BFT in A was 1.25s. In B, although the impedance drop represented bolus presence, because a flow-permissive pressure gradient was not achieved, the BFT was considered to be zero. Figure used with permission from the Esophageal Center at Northwestern.
The EGJ-CI was measured during a similar study period as EGJP by positioning the DCI-tool domain to contain the proximal and distal borders of the EGJ and over a duration of exactly three respiratory cycles.(17) The isobaric contour was set to 2-mmHg above the mean gastric pressure. The EGJ DCI value (mmHg•s•cm) was then divided by the duration (seconds) of the total measurement domain to generate the EGJ-CI (mmHg•cm). A previous study including asymptomatic volunteers reported a median (IQR) EGJ-CI of 39 mmHg•cm (25 – 55).(17)
Symptom assessment
Symptoms were assessed with the Eckardt score (ES).(8) The ES (range 0 – 12) was generated by the sum of scores for dysphagia, chest pain, and regurgitation based on the frequency of each symptom (0: never, 1: occasional, 2: daily, 3: with each meal) plus a score based on the degree of weight loss since the last therapeutic intervention (0: none, 1: < 10 lbs, 2: 10–20 lbs, 3: > 20 lbs). Patients reporting an ES ≤ 3 were considered as a good symptomatic outcome; those with ES > 3, a poor symptomatic outcome.
Timed barium esophagram
Timed barium esophagrams were performed in the upright position with x-ray images of the esophagus obtained at one, two, and five minutes after ingestion of 200-ml of low-density (45% weight to volume) barium sulfate. The height of the barium column was measured vertically from the EGJ. A column height < 5 cm at five minutes was considered a good radiographic outcome while a column height ≥ 5 cm was considered a poor radiographic outcome.(7, 20)
A combined (symptomatic and radiographic) outcome assessment was performed by considering a good outcome for patients with ES ≤ 3 and TBE 5-minute column height < 5 cm and a poor outcome in patients with ES > 3 or TBE 5-minute column height ≥ 5 cm.
Statistical analysis
Descriptive statistics for all continuous and ordinal measures were presented as median and IQR, unless otherwise stated. Outcome groups were compared using the Mann-Whitney U test for continuous variables) and Χ2 or Fischer’s Exact tests for dichotomous and categorical variables. For primary symptom and radiographic outcomes (good ES and good TBE, as defined above), a series of logistic regression models were employed and corresponding receiver operating characteristic (ROC) curves were generated by plotting the sensitivity by false positive rate (1 − specificity) for incremental value increases of each HRIM metric. The optimal threshold value for each metric was chosen as the closest value to 100% sensitivity and 0% false positive rate (the 0,1 point on the ROC curve). Analyses assumed a 5% level of statistical significance, and no adjustments were made for multiple hypothesis tests.
RESULTS
Patient characteristics
Table 1 summarizes demographic and clinical characteristics of the 75 included patients (ages 19 – 81; 32 female). Follow-up HRIM studies were obtained at a median (range) of 12 (3 – 291) months after patients’ most recent PD, LHM, or POEM. Most recent treatment modality is included in Table 1; 61 patients had their most recent treatment at Northwestern, while 14 were referred for evaluation after treatment (two PD, 12 LHM) outside our institution. Nine patients had undergone more than one previous intervention; two patients had received esophageal botulinum toxin injection in the interval since a previous intervention (LHMs) which occurred 6-months and 2-years prior to follow-up. Table 1 also includes achalasia subtypes based on HRM prior to intervention; 18 patients had remote previous treatment or manometry at outside institutions which prohibited determination of pre-treatment HRM-achalasia subtypes.
Table 1.
Patient characteristics.
| Outcome group | All patients | Symptomatic | Radiographic | Combined | ||||
|---|---|---|---|---|---|---|---|---|
| Good | Poor | Good | Poor | Good | Poor | |||
| N | 75 | 49 | 26 | 46 | 29 | 36 | 39 | |
|
Age (years), mean (SD) |
50 (17) | 53 (17) | 46 (16) | 51 (18) | 50 (15) | 53 (19) | 48 (16) | |
| Gender (female/male) | 32/43 | 16/33 | 16/101 | 20/26 | 12/17 | 13/23 | 19/20 | |
|
Intervention, n (%)1, 2, 3 |
||||||||
| Pneumatic dilation | 10 (13) | 6 (60) | 4 (40) | 4 (40) | 6 (60) | 4 (40) | 6 (60) | |
| LHM | 28 (37) | 10 (36) | 18 (64) | 14 (50) | 14 (50) | 7 (25) | 21 (75) | |
| POEM | 37 (49) | 33 (89) | 4 (11) | 28 (76) | 9 (24) | 25 (68) | 12 (32) | |
|
Follow-up interval, (months) |
12 (8–38) | 10 (7 – 16) | 47 (23 – 130)1 | 11 (8 – 18) | 30 (10 – 72)2 | 10 (7 – 15) | 31 (9 – 76)3 | |
|
Achalasia subtype*, n (%)1, 2, 3 |
||||||||
| Type I | 19 (25) | 13 (68) | 6 (32) | 13 (68) | 6 (32) | 10 (53) | 9 (47) | |
| Type II | 31 (41) | 28 (90) | 3 (10) | 22 (71) | 9 (29) | 19 (61) | 12 (39) | |
| Type III | 7 (9) | 6 (86) | 1 (14) | 7 (100) | 0 (0) | 6 (86) | 1 (14) | |
| Unknown | 18 (24) | 2 (11) | 16 (89) | 4 (22) | 14 (78) | 1 (6) | 17 (94) | |
| Eckardt score | 2 (1 – 4) | 2 (1–2) | 5 (4–7)1 | 2 (1 – 3) | 4 (2 – 7)2 | 1.5 (1 – 2) | 4 (2 – 6)3 | |
|
TBE 5-minute column height (cm) |
3.3 (0 – 8.0) | 1.0 (0 – 5.0) |
6.7 (3.3 – 10.8)1 |
0 (0 – 3.0) |
10 (6.9 – 13.1)2 |
0 (0 – 2.4) |
7.8 (4.0 – 10.8)3 |
|
Values are displayed as median (interquartile range) unless otherwise specified.
Achalasia subtype indicates high-resolution manometry results prior to intervention.
P-value < 0.05 comparing good with poor outcome groups among 1symptomatic, 2radiographic, or 3combined outcomes.
SD – standard deviation.
Patient characteristics by outcome groups are also displayed in Table 1. There were 36 patients with both good symptomatic and radiographic outcomes and 16 patients that had both poor symptomatic and radiographic outcomes; the remaining 23 had discordant symptomatic and radiographic outcomes. Agreement on outcome between ES and TBE was fair with a kappa statistic of 0.341 (95% confidence interval 0.12 – 0.56). Outcome groups differed by proportion of intervention (generally with a greater proportion of patients treated with POEM in good than poor outcome groups), follow-up interval (shorter interval duration in good than in poor outcomes), and pre-treatment achalasia subtype (greater proportion of type II and III patients in the good than poor outcomes). Follow-up interval also differed across intervention (p < 0.001) such that POEM (median, IQR 8, 7 – 13 months) had earlier follow-up than PD (22, 10 – 66 months; p = 0.17) and LHM (36, 11 – 121 months; p < 0.001); follow-up interval was similar between PD and LHM (p = 0.272).
Manometry characteristics
Esophageal motility patterns at the follow-up HRIM included 10 patients with type I achalasia, five with type II achalasia, two with type III achalasia, four with EGJ outflow obstruction, 31 with absent contractility, six with distal esophageal spasm, and 17 with ineffective esophageal motility; none of the patients met criteria for normal motility. Thus, an achalasia pattern was not present (resolved achalasia pattern) in 58 (77%) and there was some evidence of peristalsis in 21 (28%) patients.
Patients with a resolved achalasia pattern more frequently had a good (42/58, 72%) than poor (16/58, 28%) symptomatic outcome (p = 0.023). However, the proportion of patients with a resolved achalasia pattern was not significantly different between those with a good (37/58, 64%) vs poor (21/58, 36%; p = 0.572) radiographic outcome or with a good (30/58, 52%) vs poor (28/58, 48%; p = 0.278) combined outcome. The presence of peristalsis in the post-treatment HRIM study was associated with outcome such that a greater proportion of patients with peristalsis demonstrated good outcomes by symptomatic [20/21 (95%) vs poor: 1/21 (5%); p < 0.001], radiographic [18/21 (86%) vs poor: 3/21 (14%); p = 0.008], and combined [17/21 (81%) vs poor: 4/21 (19%); p = 0.001) criteria.
High resolution impedance manometry parameters
Among all patients, the median (IQR) EGJP was 10 mmHg (6 – 14), IRP was 12 mmHg (9 – 15), BFT was 0.61 seconds (0 – 1.1), and EGJ-CI was 27.4 mmHg•cm (21.4 – 46.9). Table 2 segregates HRIM metrics by treatment outcomes. There was no significant difference in EGJP or EGJ-CI between patients by symptomatic (EGJP: p = 0.451; EGJ-CI: p = 0.376) or combined (EGJP: p = 0.290; EGJ-CI: p = 0.099) outcome. Patients with a good radiographic outcome tended to have higher EGJP and higher EGJ-CI than those with a poor outcome (EGJP: p = 0.037; EGJ-CI: p = 0.025). IRP did not significantly differ between symptomatic (p = 0.094), radiographic (p = 0.739), or combined (p = 0.674) outcome groups. Median BFT was greater in good outcome than poor outcome by symptomatic (BFT: p < 0.001), radiographic (BFT: p = 0.001), and combined (BFT: p < 0.001) outcomes.
Table 2.
Comparison of high-resolution impedance manometry parameters by achalasia outcomes.
| Outcome group | Symptomatic | Radiographic | Combined | |||
|---|---|---|---|---|---|---|
| Good | Poor | Good | Poor | Good | Poor | |
| EGJP (mmHg) | 10 (5 – 15) | 11 (7 – 14) | 12 (7 – 17) | 8 (5 – 13)2 | 12 (6 – 18) | 10 (5 – 13) |
| IRP (mmHg) | 12 (9 – 14) | 14 (9 – 20) | 12 (9 – 17) | 12 (9 – 15) | 12 (9 – 15) | 12 (10 – 18) |
| BFT (seconds) | 0.8 (0.4 – 1.2) | 0 (0 – 0)1 | 0.7 (0.2 – 1.2) | 0 (0 – 0.8)2 | 0.8 (0.6 – 1.3) | 0 (0 – 0.7)3 |
| EGJ-CI | 31 (18 – 53) | 26 (22 – 46) | 40 (23 – 51) | 25 (13 – 37)2 | 36 (22 – 54) | 26 (15 – 46) |
Values represent median (interquartile range).
P-value < 0.05 comparing good with poor outcome groups among 1symptomatic, 2radiographic, or 3combined outcomes.
Figure 2 shows the ROC curves for EGJP, IRP, BFT and EGJ-CI to predict symptomatic and radiographic outcomes. The areas-under-the-curve (AUCs) for prediction of symptomatic outcome (good ES, Figure 2A), were 0.55 (95% confidence interval 0.42 – 0.69) for EGJP, 0.62 (0.48 – 0.76) for IRP, 0.77 (0.67 – 0.88) for BFT, and 0.56 (0.43 – 0.70) for EGJ-CI. The AUCs for prediction of radiographic outcome (good TBE, Figure 2B), were 0.64 (0.52 – 0.77) for EGJP, 0.48 (0.34 – 0.62) for IRP, 0.73 (0.61 – 0.84) for BFT, and 0.65 (0.52 – 0.78) for EGJ-CI. The AUCs for prediction of combined outcome (ROC curve not displayed) were 0.57 (0.44 – 0.70) for EGJP, 0.53 (0.40 – 0.66) for IRP, 0.76 (0.66 – 0.87) for BFT, and 0.61 (0.48 – 0.74) for EGJ-CI. When controlling for potential manometric co-variables of resolved achalasia pattern and presence of peristalsis, these results did not substantially change. Based on the comprehensive assessment of the ROC curves, an optimal cut-point to discriminate by outcomes was determined to be 11 mmHg for EGJP, 12 mmHg for IRP, zero seconds for BFT, and 30 mmHg•cm for EGJ-CI. Table 3 reports the sensitivities and specificities to predict each outcome using these cut-points.
Figure 2. Receiver operating characteristic (ROC) curves for high-resolution impedance manometry parameters to predict symptomatic (2A) and radiographic (2B) outcomes.
A good symptomatic outcome was defined as an Eckardt score ≤ 3. A good radiographic outcome was defined as a barium column height < 5 cm at 5 minutes during a timed barium esophagram. Figure used with permission from the Esophageal Center at Northwestern.
Table 3.
Sensitivities and specificities to predict good clinical outcomes based on optimal threshold values.
| Outcome | Symptomatic | Radiographic | Combined | ||||
|---|---|---|---|---|---|---|---|
| Measure | Cut-point | Sensitivity (%) |
Specificity (%) |
Sensitivity (%) |
Specificity (%) |
Sensitivity (%) |
Specificity (%) |
| EGJP | 11 mmHg | 57 | 46 | 57 | 66 | 56 | 59 |
| IRP | 12 mmHg | 65 | 58 | 57 | 41 | 61 | 46 |
| BFT | 0 seconds | 78 | 77 | 76 | 69 | 86 | 67 |
| EGJ-CI | 30 mmHg•cm | 53 | 62 | 57 | 66 | 56 | 59 |
It is worth noting that the values reported for EGJP and EGJ-CI reflect measures greater than the optimal cut-point.
Discussion
The major finding of this this study was that the novel HRIM parameter, the BFT, better discriminated patients with achalasia by clinical outcomes after treatment compared to another novel HRM metric, the EGJ-CI, and established HRM metrics of EGJP and IRP. As manometry is often considered the standard to assess LES function, our findings suggest that utilization of EGJP, IRP, and EGJ-CI at follow-up after achalasia intervention are of limited utility and incorporation of BFT may be a better tool to assess treatment effectiveness.
Previous studies of post-treatment manometry in achalasia demonstrated that achievement of an EGJP less than 10 mmHg is predictive of long-term outcome in patients with achalasia following PD.(8, 9) However, those studies analyzed conventional manometry studies obtained four weeks after intervention, as opposed to at the time of follow-up (either routine or symptom prompted) and symptom assessment as in the present study. Additionally, because objective evidence of esophageal dysfunction (e.g. barium retention) can be incongruent with symptoms, we utilized both symptoms and TBE to assess outcomes in our study.(5, 6) As HRM is thought to provide a more accurate assessment of LES pressures for diagnosis of achalasia, HRM measurement of EGJP and IRP could provide enhanced assessment in achalasia following treatment.(7, 11) Comparable to previous studies utilizing conventional manometry, we identified an optimal cut-point of 11 mmHg for post-treatment EGJP; however, this measure demonstrated poor specificity for poor clinical outcomes in our study. Although we previously reported that an IRP < 15 mmHg was associated with a good clinical outcome, that association was not demonstrated in the current study that included three-times as many patients, none of whom were included in the previous report.(7) Another study assessing HRM six and 12 months following POEM demonstrated predominantly good symptomatic outcomes (assessed via ES) at both time points, although mean IRP values remained close to 15 mmHg.(12)
The use of the EGJ-CI in achalasia was reported in a recent study evaluating 21 patients before and after Heller myotomy.(19) They reported that EGJ-CI in patients with achalasia (median, IQR: 67.1 mmHg•cm, 37.3 – 113.5) was greater than asymptomatic volunteers (34.7 mmHg•cm, 26.2 – 58.3) prior to intervention and that a reduction in EGJ-CI (to 27 mmHg•cm, 11.3 – 43, similar to our study) was observed following myotomy. However, in that study HRM was typically obtained following Heller myotomy for evaluation of both dysphagia and reflux symptoms and no association with clinical outcomes was reported.
As the primary role of the esophagus is to clear bolus, measurement of pressures at the EGJ (EGJP, IRP, or EGJ-CI) may not completely reflect esophageal function in patients with achalasia following treatment. Thus, treatment of achalasia to a target IRP < 15 mmHg (or EGJP < 10 mmHg) may provide false assurance of treatment success. Accepting that different target thresholds may be appropriate following treatment (as opposed to use for diagnosis), we used an ROC analysis to optimize these measures. Even so, EGJP, IRP, and EGJ-CI demonstrated limited sensitivity, and more so, specificity, to predict clinical outcomes. Furthermore, the ROC sensitivity and specificity results of EGJP and EGJ-CI pressure actually reflect the counter-intuitive finding of higher values associated with good radiographic outcomes.
We again found that the presence of peristalsis on follow-up HRM was associated with a good clinical outcome.(7) This likely reflects the capacity to generate sufficient intra-esophageal pressures to establish a flow permissive gradient across the EGJ, evident by the BFT (19/21 patients with peristalsis had a BFT > 0 seconds). For comparison, 10/21 patients with peristalsis had an EGJP < 11 mmHg, 9/21 had an IRP < 12 mmHg, and 7/21 had an EGJ-CI < 30 mmHg•cm. However, when we controlled our ROC curve for the presence of post-treatment peristalsis, the results did not change substantially, suggesting that the BFT may account for additional complexity to esophageal emptying beyond simply the return of peristalsis.
Although measurement of LES pressures (EGJP, IRP, or EGJ-CI) may be partially indicative of an adequate LES-targeted intervention, symptom persistence or recurrence from impaired esophageal emptying is better reflected with the functional assessment provided by the BFT. The BFT incorporates measures of EGJ opening and flow-permissive pressure gradient required for trans-EGJ bolus flow (and thus esophageal emptying) providing a more comprehensive evaluation of esophageal function than the EGJP, IRP, or EGJ-CI. We recently demonstrated that the BFT was well correlated with dysphagia in achalasia patients prior to treatment and now also demonstrate that BFT measurement can discriminate patient groups by both symptomatic and radiographic outcomes at follow-up after treatment.(16)
Given its ability to discriminate patients by clinical outcome measures, the BFT may improve the clinical utility of HRIM in achalasia management. Recommendations for further intervention, including repeat PD, LES myotomy, or even esophagectomy, are complex decisions for both providers and patients. Thus, a multi-modal evaluation of esophageal function is essential to help direct management decisions with each modality contributing to the global assessment of clinical outcome. Our study does not intend to suggest that HRIM replace the symptomatic or radiographic outcome assessment in achalasia, but instead that an improved manometric evaluation would better aid in achalasia management decisions, particularly given the frequent discordance between symptomatic and objective measures of esophageal function.(5) Impaired esophageal emptying demonstrated by a BFT of zero may support the need for re-intervention, even if EGJP, IRP, and EGJ-CI are low. Further, if a patient with minimal or no symptoms demonstrated a BFT > 0, even with elevated EGJP, IRP, and EGJ-CI (such as the patient displayed in Figure 1A with good symptomatic and radiographic outcomes 10 months after PD), it indicates sufficient esophageal function to facilitate emptying, and supports postponing any further therapy. EGJ pressure measures, particularly the IRP, appear to carry their primary value in the initial diagnostic evaluation of achalasia.(11) Given the variable association of these measures with clinical outcomes, reliance on EGJP, IRP, or EGJ-CI to direct clinical decisions following therapeutic intervention in achalasia should be cautiously applied. Though greater clinical experience and longitudinal patient follow-up will be necessary to support practical application of the BFT, it appears promising as a measure to help direct management decisions in patients with achalasia.
Limitations of our study include the restricted availability of BFT, as it involves the use of both HRM with impedance and a customized MATLAB program. While this currently limits the generalizability of the study, future incorporation of this metric into HRM-analytic software could offer broader utilization of the BFT. Additionally, although commonly used, the ES remains a non-validated symptom assessment tool with its own inherent limitations and dichotomizing both outcome measures may have inaccurately categorized some patients. Furthermore, although the association of clinical factors, such as achalasia subtype or treatment modality, with treatment outcome could be inferred from our results (as in Table 1), our study did not intend to assess these associations. Thus, related conclusions are limited by potential biases (such as referral bias and patient motivation for follow-up HRIM resulting in a selection bias) and other uncontrolled factors (e.g. follow-up interval).
In conclusion, the novel HRIM metric, BFT, outperformed the EGJ-CI and traditional HRM metrics of EGJP and IRP to differentiate between achalasia outcome groups defined by standardized symptomatic (ES) and radiographic (TBE) methods. While continued longitudinal study of our cohort is required to fully define the utility of these measures, it appears that application of the BFT during the follow-up evaluation may add further insight into esophageal function to help guide patient management in achalasia.
STUDY HIGHLIGHTS.
What is current knowledge?
A major criterion for the diagnosis of achalasia is the finding of an elevated integrated relaxation pressure (IRP) and therapeutic interventions in achalasia target the lower esophageal sphincter (LES) aiming to reduce LES pressure.
Post-treatment symptoms and objective studies (e.g. timed barium esophagram) are often incongruent in achalasia patients, making management decisions, particularly the need for repeat intervention, challenging.
Novel high-resolution impedance manometry metrics, bolus flow time (BFT) and esophagogastric junction (EGJ) contractile integral (CI), are useful in evaluating untreated achalasia and reflux disease, respectively, and thus may be useful in evaluating patients with achalasia during the post-treatment evaluation.
What is new here?
The BFT distinguished patients with good versus poor symptomatic and radiographic outcomes with associated sensitivities > 75% and specificities > 65%.
Measures of EGJ pressure, including the IRP, basal EGJ pressure, and EGJ-CI were inconsistently associated with symptomatic and radiographic outcomes in achalasia.
Incorporation of high-resolution impedance manometry using the BFT may complement the evaluation of esophageal function during treatment follow-up in achalasia to help direct clinical management decisions.
Acknowledgments
None
Grant support: This work was supported by T32 DK101363 (JEP) and R01 DK079902 (JEP) from the Public Health service.
John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
Footnotes
Disclosures:
Dustin A. Carlson, Zhiyue Lin, Peter J. Kahrilas, Joel Sternbach, Eric S. Hungness, Nathaniel J. Soper, Michelle Balla, Zoe Listernick, Michael Tye, Katherine Ritter, Jenna Craft, Jody D. Ciolino: None
Author contributions: DAC contributed to study concept and design, data analysis, data interpretation, drafting of the manuscript, and approval of the final version. ZL contributed to data analysis, data interpretation, manuscript revision, and approval of the final version. PJK contributed to the study design, revising the manuscript critically, and approval of the final version. JS, ESH, NJS, MB contributed to organization of data, recruitment of patients, and approval of the final version. ZL, MT, KR, JC contributed to data acquisition, data analysis and approval of the final version. JDC contributed to data analysis, manuscript revision, and approval of the final version. JEP contributed to study concept and design, analysis and interpretation of the data, revising the manuscript critically, and approval of the final version.
References
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108(8):1238–1249. doi: 10.1038/ajg.2013.196. quiz 50. [DOI] [PubMed] [Google Scholar]
- 3.Boeckxstaens GE, Annese V, des Varannes SB, et al. Pneumatic dilation versus laparoscopic Heller's myotomy for idiopathic achalasia. N Engl J Med. 2011;364(19):1807–1816. doi: 10.1056/NEJMoa1010502. [DOI] [PubMed] [Google Scholar]
- 4.Inoue H, Sato H, Ikeda H, et al. Per-Oral Endoscopic Myotomy: A Series of 500 Patients. J Am Coll Surg. 2015;221(2):256–264. doi: 10.1016/j.jamcollsurg.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 5.Vaezi MF, Baker ME, Achkar E, et al. Timed barium oesophagram: better predictor of long term success after pneumatic dilation in achalasia than symptom assessment. Gut. 2002;50(6):765–770. doi: 10.1136/gut.50.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaezi MF, Baker ME, Richter JE. Assessment of esophageal emptying post-pneumatic dilation: use of the timed barium esophagram. Am J Gastroenterol. 1999;94(7):1802–1807. doi: 10.1111/j.1572-0241.1999.01209.x. [DOI] [PubMed] [Google Scholar]
- 7.Nicodeme F, de Ruigh A, Xiao Y, et al. A comparison of symptom severity and bolus retention with Chicago classification esophageal pressure topography metrics in patients with achalasia. Clin Gastroenterol Hepatol. 2013;11(2):131–137. doi: 10.1016/j.cgh.2012.10.015. quiz e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckardt VF, Aignherr C, Bernhard G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology. 1992;103(6):1732–1738. doi: 10.1016/0016-5085(92)91428-7. [DOI] [PubMed] [Google Scholar]
- 9.Ponce J, Garrigues V, Pertejo V, et al. Individual prediction of response to pneumatic dilation in patients with achalasia. Dig Dis Sci. 1996;41(11):2135–2141. doi: 10.1007/BF02071392. [DOI] [PubMed] [Google Scholar]
- 10.Vaezi MF. Quantitative methods to determine efficacy of treatment in achalasia. Gastrointest Endosc Clin N Am. 2001;11(2):409–424. viii–ix. [PubMed] [Google Scholar]
- 11.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293(4):G878–G885. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 12.Ju H, Ma Y, Liang K, et al. Function of high-resolution manometry in the analysis of peroral endoscopic myotomy for achalasia. Surg Endosc. 2015 doi: 10.1007/s00464-015-4304-9. [DOI] [PubMed] [Google Scholar]
- 13.Hong SJ, Bhargava V, Jiang Y, et al. A unique esophageal motor pattern that involves longitudinal muscles is responsible for emptying in achalasia esophagus. Gastroenterology. 2010;139(1):102–111. doi: 10.1053/j.gastro.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rommel N, Van Oudenhove L, Tack J, et al. Automated impedance manometry analysis as a method to assess esophageal function. Neurogastroenterol Motil. 2014;26(5):636–645. doi: 10.1111/nmo.12308. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Imam H, Nicodeme F, et al. Flow time through esophagogastric junction derived during high-resolution impedance-manometry studies: a novel parameter for assessing esophageal bolus transit. Am J Physiol Gastrointest Liver Physiol. 2014;307(2):G158–G163. doi: 10.1152/ajpgi.00119.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin Z, Carlson DA, Dykstra K, et al. High-resolution impedance manometry measurement of bolus flow time in achalasia and its correlation with dysphagia. Neurogastroenterol Motil. 2015;27(9):1232–1238. doi: 10.1111/nmo.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicodeme F, Pipa-Muniz M, Khanna K, et al. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26(3):353–360. doi: 10.1111/nmo.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tolone S, De Bortoli N, Marabotto E, et al. Esophagogastric junction contractility for clinical assessment in patients with GERD: a real added value? Neurogastroenterol Motil. 2015;27(10):1423–1431. doi: 10.1111/nmo.12638. [DOI] [PubMed] [Google Scholar]
- 19.Wang D, Patel A, Mello M, et al. Esophagogastric junction contractile integral (EGJ-CI) quantifies changes in EGJ barrier function with surgical intervention. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohof WO, Lei A, Boeckxstaens GE. Esophageal stasis on a timed barium esophagogram predicts recurrent symptoms in patients with long-standing achalasia. Am J Gastroenterol. 2013;108(1):49–55. doi: 10.1038/ajg.2012.318. [DOI] [PubMed] [Google Scholar]