Abstract
Purpose of review
The past 20 years have seen the glutamatergic hypothesis go from theory to phase III trials of novel mechanism antipsychotics.
Recent findings
We review the recent literature on glutamatergic theory, covering assessment and genetic studies, as well as drug development in animals and humans.
Summary
Although evidence continues to accumulate in support of glutamate hypotheses, further research continues to be required and interactions with other key systems need to be explored.
Keywords: glutamate, N-methyl-D-aspartate, schizophrenia, sensory processing, treatment
INTRODUCTION
Over the past 20 years, attention has turned increasingly to dysfunction of the brain glutamate system as a fundamental underlying pathophysiology in schizophrenia. Attention first turned to glutamatergic systems with the observation that phencyclidine (PCP) and similarly acting psychotomimetic compounds induced their unique behavioral effects by blocking neurotransmission at N-methyl-D-aspartate (NMDA) type glutamate receptors. The glutamatergic hypothesis has since been expanded to include a potential role for dysfunction at other subtypes of glutamatergic receptors, as well as linkages between schizophrenia and glutamate receptor genes. Moreover, accumulating evidence supports a link between the NMDA system, disordered cognition, and sensory (auditory and visual) processing.
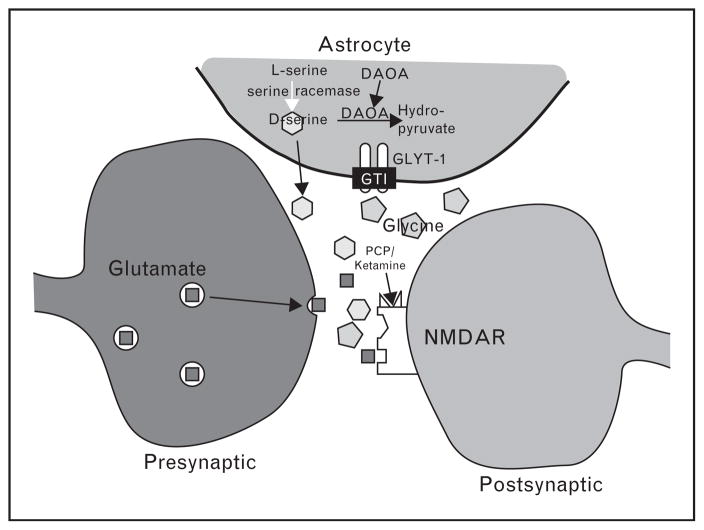
Perhaps most importantly, glutamatergic receptors include multiple modulatory sites that are actively being studied as potential treatment targets. Receptors for glutamate are divided into two broad families, ionotropic and metabotropic. Ionotropic receptors are differentiated based upon sensitivity to the synthetic glutamate derivatives NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), and kainate. In particular, NMDA receptors contain several allosteric modulatory sites that are treatment targets, including a site for the endogenous brain amino acids glycine and D-serine (Fig. 1) and a redox site that is sensitive to glutathione (GSH). Metabotropic receptors, which are G protein coupled and mediate longer-term neuromodulatory effects of glutamate, are divided into groups on the basis of effector coupling and ligand sensitivity.
FIGURE 1.
Schematic model of the N-methyl-D-aspartate (NMDA) receptor complex and synaptic glutamate (
 ), D-serine (
), D-serine (
 ), and glycine (
), and glycine (
 ) regulation and metabolism. The nonphysiologic NMDA antagonist PCP and ketamine site is also shown. DAOA, D-amino acid oxidase activator; PCP, phencyclidine. Reproduced from [2].
) regulation and metabolism. The nonphysiologic NMDA antagonist PCP and ketamine site is also shown. DAOA, D-amino acid oxidase activator; PCP, phencyclidine. Reproduced from [2].
In the following, we review key articles published since 2010, highlighting findings from basic research to phase III trials. Further background details regarding glutamatergic models are available as well in several recent reviews [1–6].
METHODS
In keeping with the mission of the journal, the goal of this review is to highlight findings of glutamatergic theories of schizophrenia of particular relevance and impact. A base search was conducted on MEDLINE and PSYCINFO using the keywords ‘NMDA and schizophrenia’ for all English-language articles published in 2010 through August 2011. Additional relevant articles not noted by this search were also included.
BIOMARKERS
Several recent studies have investigated whether plasma glutamatergic amino acid levels or NMDA-related genes are diagnostic, therapeutic, or symptomatic biomarkers. First, a comprehensive literature review [7▪▪] suggests that serum D-serine, glycine, glutathione, and alanine could be useful biomarkers. These findings were further supported by a case–control study by Hons et al. [8] that included 50 nonacute-schizophrenia patients and 50 age-matched and sex-matched controls. Glycine serum levels were measured, and the Positive and Negative Symptom Scale (PANSS) and the Scale for the Assessment of Negative Symptoms (SANS) were used to assess relationship of glycine levels with negative symptoms. As predicted, mean glycine serum levels were significantly lower in patients than in controls. Low levels of plasma glycine were associated with higher levels of negative symptoms assessed by the PANSS negative subscale and the SANS total scores in the patients.
Finally, two studies by a Japanese group have examined the association of NMDA-related genes in schizophrenia. Prior studies in Caucasians have found associations with glutathione-synthesis-related genes, but an attempt [9] to replicate this finding in the Japanese population found no significant associations with schizophrenia. In another study [10] by the same group, associations with D-amino acid-oxidase-related genes were examined in 1656 Japanese schizophrenia patients and 1842 matched controls. Again, no significant associations were seen. As prior studies in other groups were strongly positive, the authors concluded that ethnic differences might have led to the negative result.
SENSORY PROCESSING AND N-METHYL-D-ASPARTATE
In the auditory system, one of the strongest indices of sensory dysfunction is impaired generation of an event related potential (ERP) component termed mismatch negativity (MMN). MMN generation is impaired in schizophrenia and has been strongly linked to the NMDA system. The roles of the dopaminergic and the serotonergic systems in MMN generation were further explored in an investigation [11] of the effects of acutely depleting dopamine and serotonin [5-hydroxytryptamine or 5-hydroxytryptophan (5-HT)] in 16 healthy participants. A double-blind, placebo-controlled, crossover design was used, and individuals were tested in MMN under four acute-treatment conditions separated by a 5-day washout period: balanced amino acid control (no depletion), tyrosine/phenylalanine depletion (to reduce dopamine neurotransmission), tryptophan depletion (to reduce 5-HT neurotransmission), and combined tryptophan/tyrosine/phenylalanine depletion. There was no main effect of amino acid depletion on MMN amplitudes or latencies, suggesting that acute modulation of the dopaminergic and serotonergic systems does not lead to changes in MMN.
The relationship of sensory processing and NMDA was also evaluated in a double-blind, randomized ketamine challenge experiment [12▪]. Eight healthy controls were tested on a self-monitoring task in which auditory feedback was experimentally modified during two functional MRI (fMRI) sessions. The auditory feedback was modified by a decrease in pitch of either the individual’s own or someone else’s voice. This experiment was intended to study higher level cognitive processing, for example, discerning self versus other in the context of auditory hallucinations, and the primary hypothesis was that ketamine would lead to increased misattribution of voice source, and increased lateral temporal activation. Individuals made more external misattribution errors during the ketamine infusion than placebo, and moreover, as hypothesized by the investigators, ketamine-induced misattributions were associated with relative increases in left superior temporal cortex and Heschel’s gyrus. Of note, the superior temporal gyrus is also important in basic sensory (pitch) processing, and, in addition to deficits in higher order cognitive processing, this study also supports the importance of sensory regions in schizophrenia.
There is accumulating evidence for sensory processing deficits in the visual system, which can also be measured by ERP. P300, traditionally a measure of higher order cognitive processing, was recently shown [13] to be also strongly related to early sensory processing, as measured by MMN. In a double-blind, randomized ketamine challenge experiment [14], a visual oddball task known to generate P300 was tested. Ketamine led to a reduction in P300 amplitude, which was associated with a reduced fMRI response that was primarily seen in visual sensory areas. Similarly to the auditory experiment, differences in the frontal cortex were much less pronounced.
Finally, in a preclinical study, early postnatal PCP treatment of rats induced impairments in preattentive processing [15], indicated by a reduction in prepulse inhibition (PPI), although this study had the unexpected finding of a lack of effect on P1 or N1.
GENETICS
Dystrobrevin-binding protein-1 (dysbindin or DTNBP1) has been proposed as an NMDA-related schizophrenia susceptibility gene. Dysbindin has also been associated with cognitive impairments, especially poor working memory. This preclinical study [16] investigated the neurophysiology and behavioral effects in dysbindin mutant mice. The principal findings were decreased NMDA evoked currents in pyramidal neurons and decreases in NR1 (an NMDA subunit) expression. Decreases in NR1 expression correlated with deficits in spatial working memory performance, providing a link between structural changes in the NMDA receptor and cognitive changes previously associated with dysbindin expression.
Two post-mortem studies [17▪,18] focused on the expression of dopamine- and cyclic AMP-regulated phosphoprotein (DARPP)-32, a critical regulator of D1 dopamine receptor and NMDA receptor activity. Relative to age-matched controls, DARPP-32 levels were found to be reduced in both the superior temporal gyrus (STG) and the rostral agranular insular cortex (RIAC). Whereas the RIAC findings were hypothesized to at least partially explain decreased pain thresholds in schizophrenia, the author’s conclusions on the STG bear further discussion in the context of the relationship of sensory processing and schizophrenia. First, DARPP-32-related pathogenesis in schizophrenia appeared to be more severe in the STG than previously reported for the prefrontal cortex. Moreover, the STG is relatively understudied in schizophrenia, but given the region’s importance in MMN generation, and the strong relationship between MMN and NMDA, these findings add to the evidence for sensory-level dysfunction in schizophrenia.
Other genetic studies of interest include
a preliminary association [19] between habituation in PPI and a polymorphism on the NMDA receptor 2B subunit gene in schizophrenia patients;
demonstrations that NMDA antagonists can lead to aberrant neuronal migration and a decrease in the expression of disrupted-in-schizophrenia1 [20] and neuregulin 1 and its receptor ErbB4 [21] in rats; and
an association [22] between expression of a NR2B-associated trafficking complex and altered NR2B function in a subset of cortical neurons in schizophrenia.
CLINICAL DRUG DEVELOPMENTS
Although genetic and assessment studies build the foundation, the end goal is safer and more effective treatments. Recently, the two largest and most comprehensive meta-analytic reviews [23▪▪,24▪▪] of NMDA-based treatments were published. Although differing methodology and inclusion criteria were used across studies, both reviews support small-to-medium effect size improvements for total and negative symptoms when used in combination with nonclozapine antipsychotics in chronic schizophrenia, whereas significant effects for positive symptoms were only found by Tsai and Lin [23▪▪]. D-Serine, glycine, sarcosine, and N-acetylcysteine were all significantly effective in subgroup analysis of individual treatments, whereas D-cycloserine was not, in line with earlier meta-analysis.
The review by Singh and Singh [24▪▪] is the largest published to date, and is notable for being authored by an independent team with no previous involvement with NMDA theory. It includes 1253 cases from 29 trials, and differs from earlier meta-analysis by the decision to use a broader definition of NMDA-based treatments. This decision appears to have reduced the overall effect size, as this review included CX516, an AMPA receptor modulator, and memantine, a weak nonselective NMDA receptor antagonist, which were largely ineffective as adjuncts to nonclozapine antipsychotics. Unlike traditional NMDA-based treatments, however, subgroup analysis for CX516 and memantine suggests efficacy when adjunctive to clozapine in treatment-resistant schizophrenia. Neither meta-analysis included metabotropic glutamate receptor (mGlu2/3 agonist) treatments.
Results from four treatment trials not covered in these meta-analyses have been recently published. Prior to this, the majority of glutamatergic treatment trials have used naturally occurring agents, including the glycine transport inhibitor sarcosine. Glycine transport inhibitors, in general, increase brain glycine levels by blocking its removal from the synaptic space, although studies with sarcosine have not yet been performed. Sarcosine was shown to be more efficacious than D-serine in a recent head-to-head trial [25], but, because of safety concerns [26], is not available for use in the USA.
Recently, however, a novel glycine transport inhibitor, RG1678, showed significant improvement in negative symptoms as an adjunctive treatment [27,28▪▪] in a large phase II study. This represents the first positive phase II data for an NMDA-based treatment. As results have been primarily published as an abstract for the American College of Neuropsychopharmacology annual meeting [27], limited information is publicly available. Three doses of RG1678 were tested, with efficacy clearly better for the lower doses (10–30 mg) as opposed to 60 mg. Three hundred and twenty-three patients were randomized, and the intent-to-treat (ITT) population included 312 patients and the ‘per protocol’ population (patients who completed 8 weeks of treatment without any major protocol violations) included 231 patients. Negative symptoms showed a significantly greater decrease from baseline in the 10–30 mg dose groups (P <0.05) as compared with placebo in the per protocol group, although negative symptom reductions in the ITT population approached significance in both dose groups (P <0.09). The percentage of responders in the per protocol population was significantly higher in the 10 mg dose group than in the placebo group (65 vs. 43%; P = 0.013). These promising results are being followed up in phase III studies.
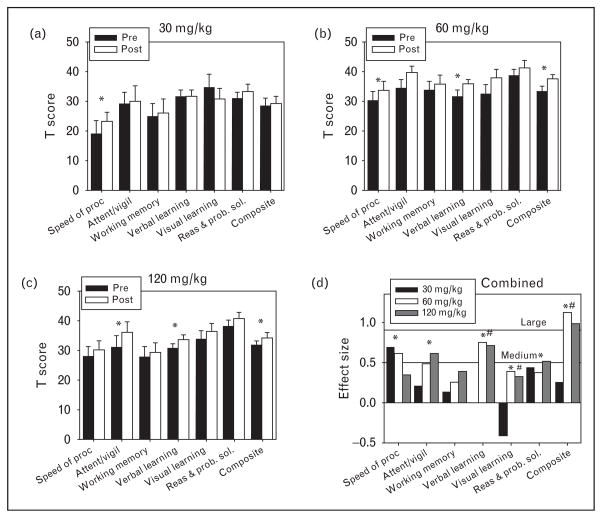
As shown by both meta-analyses, D-serine has shown promise at a dose of 30 mg/kg (~2 g per day). However, formal dose finding studies for D-serine were not performed prior to selection of this dose. Moreover, animal models suggest that higher doses of D-serine may be ideal. A 2010 study reported the first evaluation of D-serine at doses more than 30 mg/kg per day: a 4-week, open-label trial of adjunctive D-serine (30, 60 or 120 mg/kg per day) [29▪]. Significant improvement was noted across doses, including total symptoms and individual PANSS subscales (positive, negative, and general). Moreover, a significant dose-by-time effect was found for a measure of general cognitive functioning (Fig. 2). Whereas only nonsignificant improvement was noted at 30 mg/kg, a highly significant, large effect size improvement was seen for overall cognition for doses at least 60 mg/kg, leading to a significant dose-by-time interaction (P <0.01). No significant safety issues were noted in any patient taking less than 120 mg/kg. Pharmacokinetic analyses found significant dose-dependent increases in plasma D-serine levels. Furthermore, consistently with prior biomarker studies, lower baseline D-serine levels were significantly correlated with the higher baseline negative symptoms. Peak D-serine at both study initiation and study end correlated with the magnitude of improvement in negative and positive symptoms independently. Composite final Measurement and Treatment Research to Improve Cognition in Schizophrenia score also correlated with peak D-serine levels. These data suggest that 60 mg/kg may be more efficacious than the 30 mg/kg that was used in previous clinical trials with D-serine, and that it maintains the safety profile seen at lower doses. A follow-up double-blind study is ongoing.
FIGURE 2.
Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) outcomes for high-dose D-serine. Baseline (filled bars) and final (open bars) normalized MATRICS domains and overall mean composite (mean T-score of six tested domains) for (a) 30, (b) 60, and (c) 120 mg/kg. (d) Within group effect sizes for each dose. *P <0.05 in a paired t-test between baseline and final (a–c) or in a paired t-test for doses more than 60 mg/kg (d). #Significant (P <0.05) dose by time interaction for 30 vs. 60 mg/kg or more. Reproduced from [29▪].
Although D-cycloserine does not appear to be effective as an adjunctive treatment in schizophrenia, it has been successfully used to enhance learning and behavioral therapies in anxiety disorders [30]. Combining NMDA-based medication with a cognitive training program is supported by the strong relationship of NMDA receptors and learning; especially because NMDA dysfunction in schizophrenia is relative, rather than absolute, enhanced practice might be able to overcome reduced plasticity, as has recently been reported for sensory-based remediation training [31]. In a proof of concept study [32], an NMDA agonist dose of D-cycloserine was combined with a cognitive behavioral therapy (CBT) targeting delusions. A crossover design was used, so all individuals received a single dose of either D-cycloserine 50 mg or placebo in a randomized double-blind counterbalanced order over two consecutive weeks. Subsequent to study drug dose, individuals received a CBT intervention involving training in the generation of alternative beliefs. The main outcome was not significant, as there was no treatment effect on delusional distress or severity. There was, however, a significant order effect, as individuals who received D-cycloserine first had significantly reduced delusional severity, distress, and belief conviction as compared with individuals who received placebo first. This was interpreted by the authors as consistent with animal models in which D-cycloserine enhances learning only when accompanying the first exposure to training.
Finally, in an inconclusive phase II follow-up [33▪] to a positive proof of concept for the mGlu2/3 receptor agonist LY2140023 monohydrate, none of the tested arms separated from placebo, including the active olanzapine arm. This trial had a large placebo effect (14 points on total PANSS). As olanzapine did not separate from placebo, it is reasonable to present this trial as an inconclusive trial, rather than a negative trial. Follow-up phase III trials are ongoing.
PRECLINICAL DRUG DEVELOPMENT
In addition to ongoing clinical development of glycine-site agonists and glycine transport inhibitors, several novel mechanisms for enhancement of NMDA function are currently under preclinical development.
mGlu5
Several preclinical trials have evaluated the positive allosteric mGlu5 receptor modulator 3-cyano-N-(1,3-diphenyl-1H-pyrazol-5-yl) benzamide (CDPPB). In one study [34], CDPPB (10–30 mg/kg) was assessed alone and in combination with the NMDA receptor antagonist Dizocilpine (MK-801) on a task measuring cognitive set-shifting ability (the ability to switch between rules in response to feedback). Such tasks have been shown to be NMDA dependent in prior studies. Although CDPPB did not lead to any cognitive improvements on its own, it attenuated MK-801 (0.1 mg/kg)-induced impairments in a dose-dependent manner, with the 30 mg/kg dose having a greater effect than the 10 mg/kg dose across all trials. CDPPB appeared to produce its effects through a reduction in MK-801-induced perseverative responding. Another study [35] tested repeated, as opposed to acute, dosing of CDPPB, and did not find any evidence for the development of tolerance to the behavioral effects, providing proof of concept for ongoing treatment. Moreover, repeated dosing did not appear to induce tolerance to the effect on mGlu5 receptor density or phosphorylation of NMDA, NR1 (‘GluN1’) and NR2B (‘GluN2b’) receptor subunits in striatum, although tolerance was noted in the frontal cortex and in effects on sleep architecture. Finally, in a PET study in baboons [36▪], N-acetylcysteine treatment led to decreased binding of a radiotracer for the mGlu5 receptor, suggesting that N-acetylcysteine may lead to increased glutamate levels at the mGlu5 receptor and the NMDA receptor, possibly by exchange through the cystine-glutamate antiporter.
mGlu2 positive allosteric modulators
As reviewed in the clinical treatment section, mGlu2/3 agonists are a promising treatment for schizophrenia, but mGlu2 positive allosteric modulators may produce equivalent efficacy with less susceptibility to downregulation. In this study [37], pretreatment with biphenyl indanone-A suppressed the amplitude of the blood oxygen level-dependent response to PCP in the prefrontal cortex, caudate–putamen, nucleus accumbens, and mediodorsal thalamus. This was consistent with the a-priori hypothesis, and suggests that selective mGlu2 agonism may be efficacious on its own, as opposed to the more widely studied nonselective mGlu2/3 agents.
Neboglamine
Neboglamine is a glutamic acid derivative that appears to facilitate the effect of glycine on NMDA receptors, possibly at a separate allosteric site. In a recent preclinical study [38], neboglamine appeared to promote neuronal growth as measured by expression of Fos-like immunoreactivity, particularly in the prefrontal cortex, nucleus accumbens, and lateral septal nucleus. The pattern was most similar to effects produced by D-serine and, to a lesser extent, clozapine. Behaviorally, neboglamine also appeared to at least partially attenuate the effects of the NMDA receptor antagonist PCP on hyperlocomotion and rearing behavior. The authors also reported that phase I trials have been successfully completed in humans and that they planned to move forward with proof of concept trials in schizophrenia patients.
D-Serine and related enzymes
Several studies have evaluated the effects of D-serine itself and enzymes related to its metabolism. D-Amino acid oxidase (DAAO) inhibitors would inhibit the metabolism of D-serine and would raise the levels of D-serine in a manner similar to the mechanism of RG1478 on glycine. DAAO inhibitors were the subject of two recent ‘position papers’ [39,40] that outlined the rationale for potential use in schizophrenia, and reviewed the thus far inconsistent results. As reviewed, at least one DAAO inhibitor has entered phase I trials, albeit with a neuropathic pain focus. A recently published preclinical study [41] added to the inconsistent findings, finding that, although DAAO inhibition did not cause measurable increases in D-serine in forebrain, it did affect hippocampal and cortical activity, as measured by electrophysiology. In a separate study [42], however, knockout of serine racemase, an enzyme responsible for conversion of L-serine to D-serine, significantly disrupted representation of order memory, although novel object detection, relational memory and normal sociability, and preference for social novelty remained intact.
D-Serine has also been tested in preclinical models of cognition. D-Cycloserine has been extensively tested as an adjunct to cognitive remediation, but D-serine, as a full agonist, is hypothesized to be more effective at enhancing NMDA function and cognitive remediation. This hypothesis was supported by a preclinical study [43▪] in which D-serine enhanced recognition and working memory in mice. These improvements were associated with measurable increases in hippocampal D-serine. Additionally, D-serine reversed the effects of an NMDA antagonist. Of particular interest, this was the first preclinical demonstration of clinically relevant doses (50 vs. >600 mg/kg) being effective, and, moreover, demonstrated that, as opposed to D-cycloserine, D-serine may have continued effectiveness during repeated administration.
Finally, in a preclinical evaluation of the effect of NMDA medications on social behavior, D-serine, sarcosine, and clozapine all significantly enhanced social memory in rats, whereas haloperidol was ineffective [44]. Moreover, both clozapine and sarcosine significantly attenuated MK-801-disrupted cognition in the social recognition test.
CONCLUSION
Since the formulation of the alternative NMDA theory of schizophrenia, the goal has always been to develop novel, well tolerated and effective treatments. The past 20 years have seen the glutamatergic hypothesis go from theory to phase III trials of novel mechanism antipsychotics. Future directions include continued phase III trials and further assessments of the role of sensory processing and individual biomarkers. Although evidence continues to accumulate in support of glutamate hypotheses, further research continues to be required and interactions with other key systems need to be explored.
Acknowledgments
Preparation of this article was supported in part by the 2011 Irving Institute/Clinical Trials Office (CTO) Pilot Award and the Dr Joseph E. And Lillian Pisetsky Young Investigator Award For Clinical Research In Serious Mental Illness to J.T.K. and USPHS grants R01 DA03383, P50 MH086385 and R37 MH49334 to D.C.J.
Footnotes
Conflicts of interest
D.C.J. holds intellectual property rights for use of glycine, D-serine and glycine transport inhibitors in treatment of schizophrenia.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 158–159).
- 1.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantrowitz JT, Javitt DC. Thinking glutamatergically: changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Relat Psychoses. 2010;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicoletti F, Bockaert J, Collingridge GL, et al. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krystal JH, Mathew SJ, D’Souza DC, et al. Potential psychiatric applications of metabotropic glutamate receptor agonists and antagonists. CNS Drugs. 2011;24:669–693. doi: 10.2165/11533230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilmour G, Dix S, Fellini L, et al. NMDA receptors, cognition and schizophrenia: testing the validity of the NMDA receptor hypofunction hypothesis. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.03.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7▪▪.Ohnuma T, Arai H. Significance of NMDA receptor-related glutamatergic amino acid levels in peripheral blood of patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:29–39. doi: 10.1016/j.pnpbp.2010.08.027. This is a comprehensive review of serum measurement of glutamatergic amino acids. [DOI] [PubMed] [Google Scholar]
- 8.Hons J, Zirko R, Ulrychova M, et al. Glycine serum level in schizophrenia: relation to negative symptoms. Psychiatry Res. 2010;176:103–108. doi: 10.1016/j.psychres.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Hanzawa R, Ohnuma T, Nagai Y, et al. No association between glutathione-synthesis-related genes and Japanese schizophrenia. Psychiatry Clin Neurosci. 2011;65:39–46. doi: 10.1111/j.1440-1819.2010.02157.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohnuma T, Shibata N, Baba H, et al. No association between DAO and schizophrenia in a Japanese patient population: a multicenter replication study. Schizophr Res. 2010;118:300–302. doi: 10.1016/j.schres.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 11.Leung S, Croft RJ, Guille V, et al. Acute dopamine and/or serotonin depletion does not modulate mismatch negativity (MMN) in healthy human participants. Psychopharmacology (Berl) 2010;208:233–244. doi: 10.1007/s00213-009-1723-0. [DOI] [PubMed] [Google Scholar]
- 12▪.Stone JM, Abel KM, Allin MP, et al. Ketamine-induced disruption of verbal self monitoring linked to superior temporal activation. Pharmacopsychiatry. 2011;44:33–48. doi: 10.1055/s-0030-1267942. Further implication of sensory level dysfunction in schizophrenia. [DOI] [PubMed] [Google Scholar]
- 13.Leitman DI, Sehatpour P, Higgins BA, et al. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- 14.Musso F, Brinkmeyer J, Ecker D, et al. Ketamine effects on brain function: simultaneous fMRI/EEG during a visual oddball task. Neuroimage. 2011;58:508–525. doi: 10.1016/j.neuroimage.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 15.Broberg BV, Oranje B, Glenthoj BY, et al. Assessment of auditory sensory processing in a neurodevelopmental animal model of schizophrenia: gating of auditory-evoked potentials and prepulse inhibition. Behav Brain Res. 2010;213:142–147. doi: 10.1016/j.bbr.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Karlsgodt KH, Robleto K, Trantham-Davidson H, et al. Reduced dysbindin expression mediates N-methyl-D-aspartate receptor hypofunction and impaired working memory performance. Biol Psychiatry. 2011;69:28–34. doi: 10.1016/j.biopsych.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪.Kunii Y, Yabe H, Wada A, et al. Altered DARPP-32 expression in the superior temporal gyrus in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1139–1143. doi: 10.1016/j.pnpbp.2011.03.016. The demonstration of impairment in NMDA-related proteins in sensory areas. [DOI] [PubMed] [Google Scholar]
- 18.Nishiura K, Kunii Y, Wada A, et al. Profiles of DARPP-32 in the insular cortex with schizophrenia: a postmortem brain study. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1901–1907. doi: 10.1016/j.pnpbp.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Hokyo A, Kanazawa T, Uenishi H, et al. Habituation in prepulse inhibition is affected by a polymorphism on the NMDA receptor 2B subunit gene (GRIN2B) Psychiatr Genet. 2010;20:191–198. doi: 10.1097/YPG.0b013e32833a201d. [DOI] [PubMed] [Google Scholar]
- 20.Namba T, Ming GL, Song H, et al. NMDA receptor regulates migration of newly generated neurons in the adult hippocampus via disrupted-in-schizophrenia 1 (DISC1) J Neurochem. 2011;118:34–44. doi: 10.1111/j.1471-4159.2011.07282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng Y, Wang XD, Guo CM, et al. Expressions of neuregulin 1beta and ErbB4 in prefrontal cortex and hippocampus of a rat schizophrenia model induced by chronic MK-801 administration. J Biomed Biotechnol. 2010;2010:859516. doi: 10.1155/2010/859516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kristiansen LV, Bakir B, Haroutunian V, Meador-Woodruff JH. Expression of the NR2B-NMDA receptor trafficking complex in prefrontal cortex from a group of elderly patients with schizophrenia. Schizophr Res. 2010;119:198–209. doi: 10.1016/j.schres.2010.02.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23▪▪.Tsai GE, Lin PY. Strategies to enhance N-methyl-D-aspartate receptor mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr Pharm Des. 2010;16:522–537. doi: 10.2174/138161210790361452. This is a large meta-analysis of NMDA-based treatments. [DOI] [PubMed] [Google Scholar]
- 24▪▪.Singh SP, Singh V. Meta-analysis of the efficacy of adjunctive NMDA receptor modulators in chronic schizophrenia. CNS Drugs. 2011;25:859–868. doi: 10.2165/11586650-000000000-00000. This is a large meta-analysis of glutamategic treatments. [DOI] [PubMed] [Google Scholar]
- 25.Lane HY, Lin CH, Huang YJ, et al. A randomized, double-blind, placebo-controlled comparison study of sarcosine (N-methylglycine) and D-serine add-on treatment for schizophrenia. Int J Neuropsychopharmacol. 2010;13:451–460. doi: 10.1017/S1461145709990939. [DOI] [PubMed] [Google Scholar]
- 26.Sreekumar A, Poisson LM, Rajendiran TM, et al. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 27.Umbricht D, Yoo K, Youssef E, et al. Glycine transporter type 1 (GLYT1) inhibitor RG1678: positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropharmacology. 2010;35:S320–S321. [Google Scholar]
- 28▪▪.Pinard E, Alanine A, Alberati D, et al. Selective GlyT1 inhibitors: discovery of [4-(3-fluoro-5-trifluoromethylpyridin-2-yl)piperazin-1-yl][5-methanesulfonyl-2-((S)-2,2,2-trifluoro-1-methylethoxy)phenyl]methanone (RG1678), a promising novel medicine to treat schizophrenia. J Med Chem. 2010;53:4603–4614. doi: 10.1021/jm100210p. This is a review of RG1478, first glycine transport inhibitor to demonstrate efficacy in phase II trials. [DOI] [PubMed] [Google Scholar]
- 29▪.Kantrowitz JT, Malhotra AK, Cornblatt B, et al. High dose D-serine in the treatment of schizophrenia. Schizophr Res. 2010;121:125–130. doi: 10.1016/j.schres.2010.05.012. The first use of D-serine in doses more than 30 mg/kg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Norberg MM. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottlieb JD, Cather C, Shanahan M, et al. D-cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;35:1901–1907. doi: 10.1016/j.schres.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪.Kinon BJ, Zhang L, Millen BA, et al. A multicenter, inpatient, phase 2, double blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol. 2011;31:349–355. doi: 10.1097/JCP.0b013e318218dcd5. A failed clinical trial of LY2140023 monohydrate in schizophrenia. [DOI] [PubMed] [Google Scholar]
- 34.Stefani MR, Moghaddam B. Activation of type 5 metabotropic glutamate receptors attenuates deficits in cognitive flexibility induced by NMDA receptor blockade. Eur J Pharmacol. 2010;639:26–32. doi: 10.1016/j.ejphar.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmentier-Batteur S, Obrien JA, Doran S, et al. Differential effects of the mGluR5 positive allosteric modulator CDPPB in the cortex and striatum following repeated administration. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.11.013. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36▪.Miyake N, Skinbjerg M, Easwaramoorthy B, et al. Imaging changes in glutamate transmission in vivo with the metabotropic glutamate receptor 5 tracer [11C] ABP688 and N-acetylcysteine challenge. Biol Psychiatry. 2011;69:822–824. doi: 10.1016/j.biopsych.2010.12.023. A connection of N-acetylcysteine and MGluR receptor. [DOI] [PubMed] [Google Scholar]
- 37.Hackler EA, Byun NE, Jones CK, et al. Selective potentiation of the metabotropic glutamate receptor subtype 2 blocks phencyclidine-induced hyper-locomotion and brain activation. Neuroscience. 2010;168:209–218. doi: 10.1016/j.neuroscience.2010.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiusaroli R, Garofalo P, Espinoza S, et al. Antipsychotic-like effects of the N-methyl-D-aspartate receptor modulator neboglamine: an immunohistochemical and behavioural study in the rat. Pharmacol Res. 2010;61:430–436. doi: 10.1016/j.phrs.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Smith SM, Uslaner JM, Hutson PH. The therapeutic potential of D-amino acid oxidase (DAAO) inhibitors. Open Med Chem J. 2010;4:3–9. doi: 10.2174/1874104501004020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferraris DV, Tsukamoto T. Recent advances in the discovery of D-amino acid oxidase inhibitors and their therapeutic utility in schizophrenia. Curr Pharm Des. 2011;17:103–111. doi: 10.2174/138161211795049633. [DOI] [PubMed] [Google Scholar]
- 41.Strick CA, Li C, Scott L, et al. Modulation of NMDA receptor function by inhibition of D-amino acid oxidase in rodent brain. Neuropharmacology. 2011;61:1001–1015. doi: 10.1016/j.neuropharm.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 42.DeVito LM, Balu DT, Kanter BR, et al. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 2011;10:210–222. doi: 10.1111/j.1601-183X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43▪.Bado P, Madeira C, Vargas-Lopes C, et al. Effects of low-dose D-serine on recognition and working memory in mice. Psychopharmacology (Berl) 2011;218:461–470. doi: 10.1007/s00213-011-2330-4. This is an evaluation of the cognitive enhancing properties of D-serine. [DOI] [PubMed] [Google Scholar]
- 44.Shimazaki T, Kaku A, Chaki S. D-Serine and a glycine transporter-1 inhibitor enhance social memory in rats. Psychopharmacology (Berl) 2010;209:263–270. doi: 10.1007/s00213-010-1794-y. [DOI] [PubMed] [Google Scholar]