Abstract
Background
Esophagogastric junction (EGJ) distensibility and distension-mediated peristalsis can be assessed with the functional lumen imaging probe (FLIP) during a sedated upper endoscopy. We aimed to describe esophageal motility assessment using FLIP topography in patients presenting with dysphagia.
Methods
145 patients (ages 18 – 85, 54% female) with dysphagia that completed upper endoscopy with a 16-cm FLIP assembly and high-resolution manometry (HRM) were included. HRM was analyzed according to the Chicago Classification of esophageal motility disorders; major esophageal motility disorders were considered ‘abnormal’. FLIP studies were analyzed using a customized program to calculate the EGJ-distensibility index (DI) and generate FLIP topography plots to identify esophageal contractility patterns. FLIP topography was considered ‘abnormal’ if EGJ-DI was < 2.8 mm2/mmHg or contractility pattern demonstrated absent contractility or repetitive, retrograde contractions.
Results
HRM was abnormal in 111 (77%) patients: 70 achalasia (19 type I, 39 type II, 12 type III), 38 EGJ outflow obstruction, and three jackhammer esophagus. FLIP topography was abnormal in 106 (95%) of these patients, including all 70 achalasia patients. HRM was ‘normal’ in 34 (23%) patients: five ineffective esophageal motility and 29 normal motility. 17 (50%) had abnormal FLIP topography including 13 (37%) with abnormal EGJ-DI.
Conclusions
FLIP topography provides a well-tolerated method for esophageal motility assessment (especially to identify achalasia) at the time of upper endoscopy. FLIP topography findings that are discordant with HRM may indicate otherwise undetected abnormalities of esophageal function, thus FLIP provides an alternative and complementary method to HRM for evaluation of non-obstructive dysphagia.
Introduction
An evaluation of esophageal motility is indicated for the evaluation of non-obstructive dysphagia. Esophageal manometry is the primary methodology employed and typically evaluates primary peristalsis and esophagogastric junction (EGJ) pressures following standardized swallows. However, the trans-nasal catheter placement required for esophageal manometry is associated with patient discomfort. Additionally, the standard manometric evaluation may not identify an etiology for patients’ symptoms.(1) Thus, an alternate method to objectively evaluate measure esophageal motility would be valuable.
The functional lumen imaging probe (FLIP) employs high-resolution impedance planimetry to measure the relationship of luminal dimensions and distensive pressure (i.e. distensibility) during controlled, volumetric distension. The distensibility of the esophagogastric junction (EGJ) is assessed by a metric termed the EGJ distensibility index (DI), which was demonstrated to be abnormally low in treatment-naïve achalasia.(2-4) Distension-mediated contractility was reported in initial studies utilizing esophageal impedance planimetry and later observed as a complicating factor during FLIP-distensibility assessment of the esophageal body in eosinophilic esophagitis.(5-8) However, by employing customized software to generate FLIP topography plots, organized patterns of distension-associated contractions were identified.(9-11) In asymptomatic controls, the predominant contractile pattern involved repetitive, antegrade contractions (RACs), which we proposed was a secondary-peristaltic response to the sustained volumetric distension that occurs during the FLIP study. Esophageal contractions were also frequently observed with FLIP topography among achalasia patients and the patterns of contractility differed by subtype.(11) The majority of non-spastic (type I and II) achalasia patients demonstrated absent or non-patterned contractility. Patients with spastic (type III) achalasia typically demonstrated repetitive, retrograde contractions (RRCs), a pattern not observed in asymptomatic controls, suggesting that RRCs are a pathologic finding.
Although awake trans-nasal placement of the FLIP can be employed, the FLIP study can be performed in less than five minutes during a sedated upper endoscopy. Thus, FLIP represents a well-tolerated method that can be employed at the time of an upper endoscopy to evaluate non-obstructive dysphagia. Analogous to the hierarchical analysis of high-resolution manometry (HRM) studies that first evaluate deglutitive EGJ pressures and then the associated esophageal contractility pattern,(12, 13) we hypothesized that a similar scheme could be applied to FLIP topography using EGJ-DI and distension-induced contractility. Thus, we aimed to evaluate the diagnostic utility of FLIP topography to assess esophageal motility in patients with non-obstructive dysphagia.
Methods
Subjects
Patients presenting to the Esophageal Center of Northwestern for evaluation of dysphagia between November, 2012 and April, 2016 that completed HRM and FLIP during upper endoscopy were prospectively included. Upper endoscopy was completed using sedation with midazolam (2 - 15 mg) and fentanyl (0 - 300 mcg); propofol (in addition to midazolam and fentanyl) was used with anesthesiologist assistance at the discretion of the performing endoscopist in some cases. Patients with previous upper gastrointestinal surgery, significant medical co-morbidities, eosinophilic esophagitis, severe reflux esophagitis (LA-classification C or D), or large hiatal hernia were excluded. Patients were often identified by referral for manometry, thus FLIP was commonly included with the endoscopic evaluation if an esophageal motility disorder was suspected. Enrollment of achalasia patients was prioritized, but limited to 70 patients: 49 of the achalasia patients were previously described.(11) We intentionally included an excess of achalasia patients to evaluate the diagnostic effectiveness of FLIP topography for this important esophageal motility disorder. Additional clinical evaluation (e.g. barium esophagram) were obtained and management decisions made at the discretion of the primary treating gastroenterologist. The study protocol was approved by the Northwestern University Institutional Review Board.
High resolution manometry
Manometry studies were completed using a 4.2-mm outer diameter solid-state assembly with 36 circumferential pressure sensors at 1-cm intervals (Medtronic Inc, Shoreview, MN). After a minimum 6-hour fast, the HRIM assembly was placed trans-nasally and positioned to record from the hypopharynx to the stomach with approximately three intragastric pressure sensors. The HRM protocol included a 5-minute baseline recording, ten 5-ml swallows in a supine position using 50% saline for test swallows at 20-30 second intervals.
Functional lumen imaging probe
The FLIP assembly consisted of a 240-cm long, 3-mm outer diameter catheter with an 18-CM, infinitely compliant balloon (up to a distension volume of 60 mL) mounted near the distal end of the catheter (EndoFLIP®; Crospon, Inc, Galway, Ireland). The balloon tapered at both ends to assume a 16-cm long cylindrical shape in the center that housed 17 impedance planimetry ring electrodes spaced at 1-cm intervals and a solid-state pressure transducer positioned at the distal end to provide simultaneous measurement of 16 channels of cross-sectional area (CSA) converted to diameter based on the assumption of circular geometry and intra-balloon pressure. The impedance planimetry segment had a range of measureable diameters of 5.2 - 22 mm within the infinitely compliant limits of the balloon. Diameter and pressure values could be measured when the balloon was distended beyond a 22-mm diameter, but mechanical properties of the balloon would be engaged. Measurements from the impedance planimetry electrode pairs and the pressure transducer were sampled at 10 Hz with the data acquisition system and transmitted to the recording unit.
Subjects underwent sedated upper endoscopy in the left lateral decubitus position. The FLIP probe was placed trans-orally and positioned with the distal 2-3 impedance sensors beyond the EGJ as confirmed by demonstration of a waist in the impedance planimetry segment at a balloon distension volume of 20-30 ml. The endoscope was withdrawn before initiation of the FLIP study protocol. The FLIP assembly position was adjusted by the endoscopist during the study to maintain placement relative to the EGJ as visualized on real-time output. Simultaneous CSAs and intra-balloon pressures were measured during 5 - 10 ml stepwise distensions beginning with 5 ml and increasing to target volume of 60 or 70 ml; each incremental distension volume was maintained for 10 – 30 seconds. The distension protocol was modified during the course of the evaluation period, initially with a limit of 60ml and later to a limit of 70 ml, as well as initially with 5-ml stepwise distensions and later with 10-ml stepwise distensions. The recording unit was set to stop infusing and display an alarm message if the intra-balloon pressure exceeded 60 mmHg, which sometimes limited the extent of balloon distension.
Data analysis
Manometry studies were analyzed using ManoView version 3.0 analysis software to measure basal EGJ pressure (EGJP) at end-expiration, the 4-s integrated relaxation pressure (IRP), distal contractile integral (DCI), and distal latency. Esophageal motility diagnoses was generated from the ten supine swallows according to the Chicago Classification v3.0, using a median IRP of 15 mmHg as the upper-limit of normal.(13) Achalasia, EGJ outflow obstruction (EGJOO), absent contractility, distal esophageal spasm, or jackhammer esophagus were considered major motility disorders (i.e. those not observed in asymptomatic controls).(13)
FLIP data including distension volume, intra-balloon pressure, and 16 channels of luminal diameter for each subject were exported to MATLAB (The Math Works, Natick, MA, USA) for analysis using a customized MATLAB program.(14) This program applied a filter to minimize vascular and respiratory artifact and then generated tracings of each channel’s luminal diameter. Interpolation between channels was applied to generate color-coded topography plots by time with corresponding plots of volume distension and intra-balloon pressure. The program identified the EGJ-midline by searching for the minimal diameter of the distal impedance planimetry channels. The EGJ-distensibility index (EGJ-DI) was calculated by measuring the narrowest EGJ CSA and intra-balloon pressure at each data sample obtained during the time course at the 60-ml distension volume, which corresponds with the 40-ml distension volumes previously reported using a shorter (8-cm) FLIP assembly.(3) The median values for narrowest EGJ CSA and intra-balloon pressure were then divided to calculate the EGJ-DI (CSA/pressure; mm2/mmHg). If a 60-ml distension volume was not achieved, values obtained at the maximum fill volume were utilized to calculate the EGJ-DI. An EGJ-DI < 2.8 mm2/mmHg was considered abnormal based on the lower range of values from previously evaluated normal controls.(11) This value was also reported as a reliable threshold using shorter (6.4 and 8-cm) FLIP assemblies.(2, 3)
Esophageal body contractions were identified by a transient decrease of ≥ 5 mm in the measured luminal diameter detected in ≥2 adjacent axial impedance planimetry channels using the FLIP topography plots and 16 channel diameter tracing output.(10, 11) Contractions were described in terms of propagation direction (antegrade or retrograde) based on the tangent line placed on the onset of contraction. Contractions were considered repetitive (RACs or RRCs based on propagation direction) when ≥3 occurred consecutively. The presence of RACs and RRCs was not mutually exclusive, thus both could be present in a single patient over the course of the FLIP study.
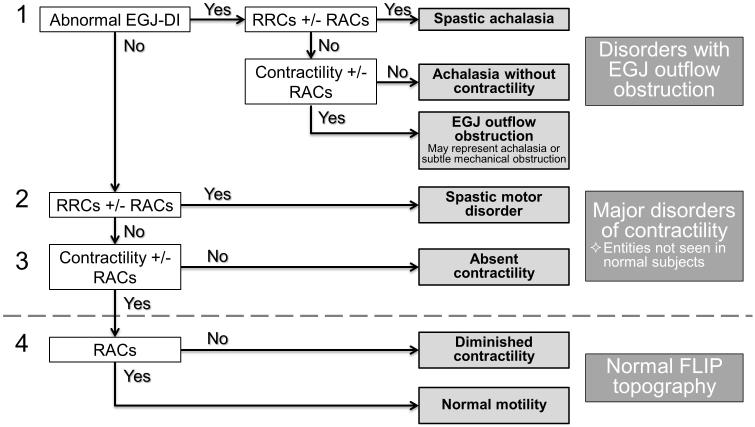
A FLIP topography esophageal motility diagnosis was generated for each patient using the classification scheme detailed in Figure 1. Motility diagnoses were classified using a hierarchical scheme designating 1) Presence or absence of EGJ outflow obstruction as defined by an abnormal EGJ-DI and 2) Contractility pattern (Figure 2): a) Absent contractility, b) Contractility without RACs nor RRCs, c) RACs without RRCs, and d) RRCs, with or without RACs. Findings not observed in asymptomatic controls (abnormal EGJ-DI, absent contractility, or RRCs) constituted a major motor disorder and were considered ‘abnormal’ FLIP topography.(10, 11)
Figure 1. FLIP topography motility classification.
FLIP topography motility diagnoses were generated using a hierarchical analysis scheme based on 1) EGJ-distensibility index (DI) and 2) Contractile pattern. Motility diagnoses above the dashed line represent patterns not observed in asymptomatic controls and therefore considered major motor disorders, i.e. ‘abnormal’ FLIP topography. RACs – repetitive, antegrade contractions. RRCs – repetitive, retrograde contractions.
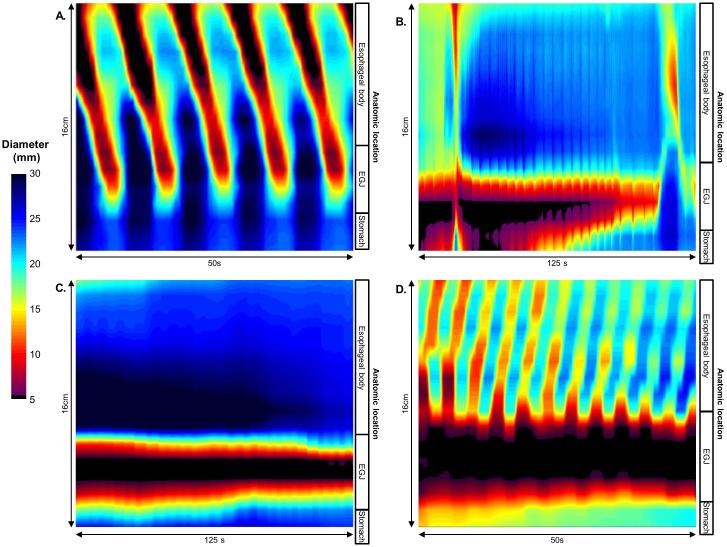
Figure 2. FLIP topography contractile patterns.
A) Repetitive, antegrade contractions (RACs) from a patient with normal motility on high-resolution manometry (HRM). RACs were the most commonly observed contractile pattern in asymptomatic controls.(10, 11) B) Contractility without RAC or repetitive, retrograde contractions in a patients with a HRM demonstrating EGJ outflow obstruction with a peristaltic pattern of ineffective esophageal motility. C) Absent contractility in a patient with type I achalasia on HRM. D). Repetitive, retrograde contractions (RRCs) in a patient with type III achalasia on HRM.
Statistical analysis
Values are expressed as median (interquartile range, IQR), unless otherwise specified. Groups were compared using Χ2 or Kruskal-Wallis for categorical and continuous variables, respectively. Analyses assumed a 5% level of statistical significance; a post-hoc Bonferroni correction was applied for multiple comparisons.
Results
Subjects
One-hundred forty-five patients (mean age 52 years, range 18 – 85; 54% female) were included in the analysis. A major motility disorder on HRM was observed in 111 (77%) patients; HRM motility diagnoses included 70 (48%) patients with achalasia [19 (13%) type I, 39 (27%) type II, and 12 (8%) type III], 38 (26%) with EGJOO, and three (2%) with jackhammer esophagus. The remaining 34 (23%) patients without major motility disorders included 5 (4%) patients with ineffective esophageal motility (IEM) and 29 (19%) patients with normal motility. Two patients were diagnosed with achalasia with an IRP less than 15 mmHg via Chicago Classification caveats: one had absent contractility with an IRP of 12 mmHg (type I achalasia) the other had pan-esophageal pressurization with an IRP of 12 mmHg (type II achalasia). Patient characteristics by esophageal motility diagnosis are displayed in Table 1. Though the majority of patients underwent endoscopy with conscious sedation, 10 (7%) patients had an endoscopic-sedation regimen including propofol. There were no differences in sedation dosages or propofol usage between patients with normal and abnormal EGJ-DI or among patients based on FLIP topography motility classification (Table 2). Seven patients, five with achalasia (three type III) and two with normal motility on HRM, were on chronic opiate therapy at the time of evaluation.
Table 1.
Subject characteristics by high-resolution manometry motility diagnosis. Values are median (IQR) unless otherwise specified.
| Type I achalasia |
Type II achalasia |
Type III achalasia |
EGJOO | Jackhammer | IEM | Normal motility |
|
|---|---|---|---|---|---|---|---|
| n | 19 | 39 | 12 | 38 | 3 | 5 | 29 |
| Age (years) | 57 (40 - 67) | 47 (28 - 62) | 65 (58 - 67) | 60 (49 - 67) | 52, 60, 79 | 50 (45 - 63) | 48 (31 - 59) |
| Gender (F/M) | 8/11 | 17/22 | 4/8 | 23/15 | 2/1 | 3/2 | 21/8 |
| Median IRP (mmHg) | 38 (24 - 56) | 33 (25 - 42) | 32 (22 - 42) | 22(19-26) | 12, 12, 13 | 12(6-13) | 9(6-12) |
|
Basal EGJ pressure
(mmHg) |
30(19-45) | 26(23-41) | 38 (29 - 46) | 32 (24 - 44) | 32, 49, 55 | 8(8-21) | 16(11 -23) |
Table 2.
Sedation dosages and utilization among FLIP topography findings. EGJ-DI – esophagogastric junction distensibility index. EGJOO – EGJ outflow obstruction.
| FLIP classification | n | Midazolam, mg; median (IQR) |
Fentanyl, mcg; median (IQR) |
Propofol, n (%) |
|---|---|---|---|---|
| Abnormal EGJ-DI | 117 | 7 (5 – 9) | 150 (125 – 200) | 8 (7) |
| Normal EGJ-DI | 28 | 8 (5 – 9) | 150 (125 – 200) | 2 (7) |
|
Abnormal FLIP
topography |
123 | 7 (5 – 9) | 150 (125 – 200) | 8 (7) |
|
Normal FLIP
topography |
22 | 8 (5 – 9) | 150 (119 – 200) | 2 (9) |
|
Achalasia without
contractility |
29 | 7 (6 – 10) | 163 (125 – 200) | 3 (10) |
| Spastic achalasia | 44 | 5 (5 – 9) | 125 (125 – 200) | 4 (9) |
| EGJOO | 44 | 7 (5 – 8) | 150 (125 – 175) | 1 (2) |
|
Spastic motor
disorder |
5 | 9 (7 – 10) | 200 (175 – 200) | 0 |
|
Absent
contractility |
1 | 5 | 125 | 0 |
|
Diminished
contractility |
1 | 10 | 200 | 0 |
| Normal motility | 21 | 8 (5 – 9) | 150 (113 – 188) | 2 (10) |
During the study period, two patients were unable to tolerate HRM but had FLIP performed that both demonstrated abnormal EGJ-DI (0.7 and 1.0 mm2/mmHg) and absent contractility. They were both subsequently diagnosed as achalasia and treated with 30-mm balloon dilation. Without corresponding HRM, they were not included in subsequent analysis. Additionally, there were 13 patients excluded for poor FLIP catheter placement (i.e. distal migration of the catheter that prevented contractility evaluation) and six excluded for technical-limitations.
Relationship of HRM and FLIP topography
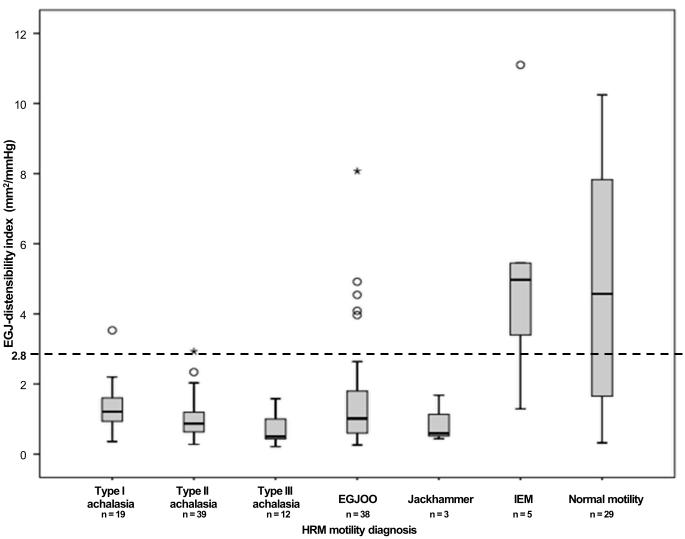
Of the 105 patients with an EGJ outflow obstruction (IRP > 15 mmHg) on HRM, 98 (85%) also had an EGJ outflow obstruction (EGJ-DI < 2.8 mm2/mmHg) on FLIP. EGJ-DI differed among HRM motility diagnoses (p < 0.001, Figure 3). A post-hoc Bonferroni adjustment for multiple comparisons assumed statistical significance at a p-value of 0.003; due to small sample size, jackhammer patients were not subjected to pair-wise group comparison. Achalasia (all three sub-types) and EGJOO had lower EGJ-DI compared with normal motility (p–values ≤ 0.001). Type II achalasia and type III achalasia had lower EGJ-DI compared with IEM (p–values ≤ 0.001); there was a numeric trend toward lower EGJ-DI in type I achalasia and EGJOO than IEM (p-values = 0.005). Trends toward differences in EGJ-DIs were also detected between type I and type II achalasia (p = 0.034), type I and type III achalasia (p = 0.011), type II and type III achalasia (p = 0.087), and type III achalasia and EGJOO (p = 0.038).
Figure 3. EGJ – distensibility index by manometric esophageal motility diagnosis.
The dashed line indicates the lower range of normal based on previous study of asymptomatic controls.(10, 11) EGJOO – esophagogastric junction outflow obstruction. IEM – ineffective esophageal motility. HRM – high resolution manometry.
Among all patients, FLIP topography contractility patterns consisted of 30 (19%) patients with no contractility, 25 (16%) patients with contractility, but without RACs or RRCs, 77 (53%) patients with RACs, and 50 (34%) patients with RRCs; 27 (17%) had both RACs and RRCs. FLIP topography contractility patterns differed among HRM esophageal motility diagnoses (p < 0.001). Frequencies of FLIP topography classifications by HRM diagnosis are displayed in Table 3. The majority (68%) of type I achalasia patients had abnormal EGJ-DI with absent contractility (achalasia without contractility) and the majority (83%) of type III achalasia patients had abnormal EGJ-DI with RRCs (spastic achalasia). There was a more substantial amount of heterogeneity of FLIP topography classifications among patients with type II achalasia, EGJOO, IEM, and normal motility.
Table 3.
FLIP topography classification by manometric motility diagnosis. Values represent number of patients and percentage within each high-resolution manometry (HRM) motility diagnosis. Previously evaluated asymptomatic controls are included as a reference.(10, 11). EGJOO – esophagogastric junction outflow obstruction. IEM – ineffective esophageal motility.
| FLIP topography motility classification n (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| HRM motility diagnosis |
n | Achalasia without contractility |
Spastic achalasia |
EGJOO (achalasia or subtle mechanical obstruction) |
Spastic motor disorder |
Absent contractility |
Diminished contractility |
Normal motility |
| Type I achalasia | 19 | 13 (68) | 2 (11) | 3 (16) | 0 | 1 (5) | 0 | 0 |
| Type II achalasia | 39 | 14 (36) | 13 (33) | 12 (31) | 1 (3) | 0 | 0 | 0 |
| Type III achalasia | 12 | 0 | 10 (83) | 2 (17) | 0 | 0 | 0 | 0 |
| EGJOO | 38 | 2 (5) | 13 (34) | 18 (47) | 0 | 0 | 0 | 5 (13) |
| Jackhammer | 3 | 0 | 3 (100) | 0 | 0 | 0 | 0 | 0 |
| IEM | 5 | 0 | 0 | 1 (20) | 1 (20) | 0 | 1 (20) | 2 (40) |
| Normal | 29 | 0 | 4 (14) | 8 (28) | 3 (10) | 0 | 0 | 14 (48) |
| Controls(10, 11) | 10 | 0 | 0 | 0 | 0 | 0 | 2 (20) | 8 (80) |
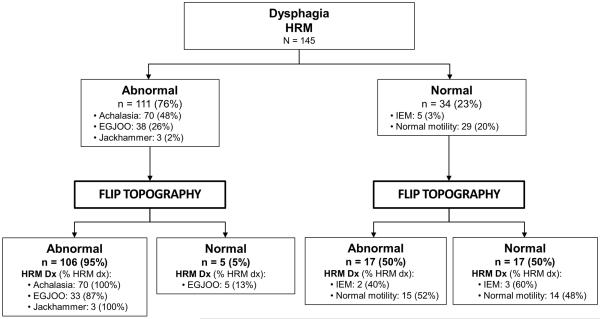
Among the 111 patients with a major motility disorder on HRM, 106 (95%) had a FLIP topography pattern consistent with a major motility disorder, i.e. abnormal FLIP topography (Figure 4). Of the 34 patients without a major motility disorder on HRM, 17 (50%) had abnormal FLIP topography.
Figure 4. Flow diagram of high-resolution manometry (HRM) and FLIP topography motility diagnoses.
“Abnormal” indicated detection of a major esophageal motility disorder. DX – diagnosis. EGJOO – EGJ outflow obstruction. IEM – ineffective esophageal motility.
All 70 patients with achalasia by HRM had abnormal FLIP topographies. Sixty-eight (97%) had an abnormal EGJ-DI. Of the remaining two achalasia patients with normal EGJ-DI, one (type I HRM) had absent contractility; the other (type II HRM) had RRCs (spastic motor disorder). Both of these patients had a median intra-balloon pressure during the 60-ml fill volume of 11 mmHg, while the median CSAs were only 33 and 38 mm2 (i.e. diameters of 6.5 and 7 mm). Additionally, the two-included achalasia patients with IRP < 15 mmHg had EGJ-DIs of 1.6 (Figure 5A) and 1.7 mm2/mmHg.
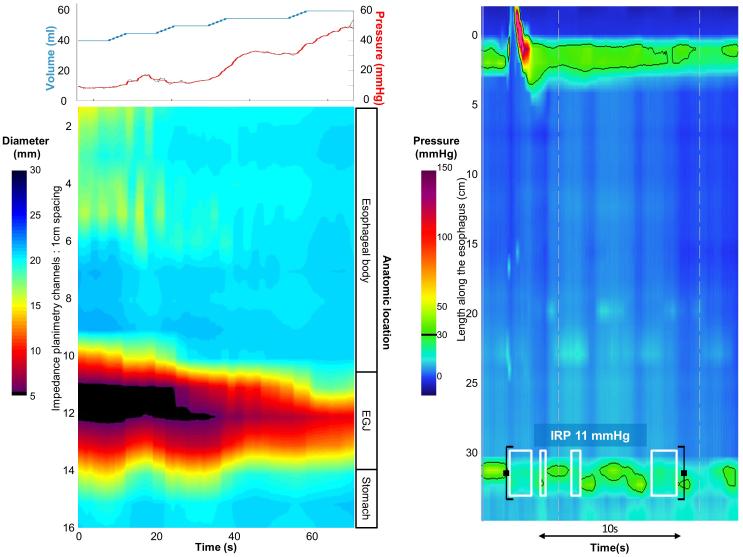
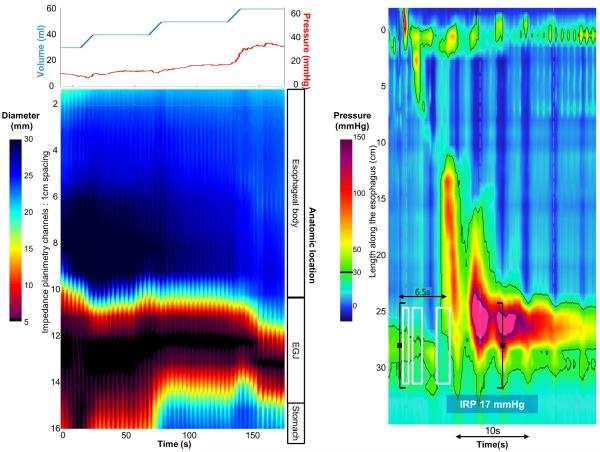
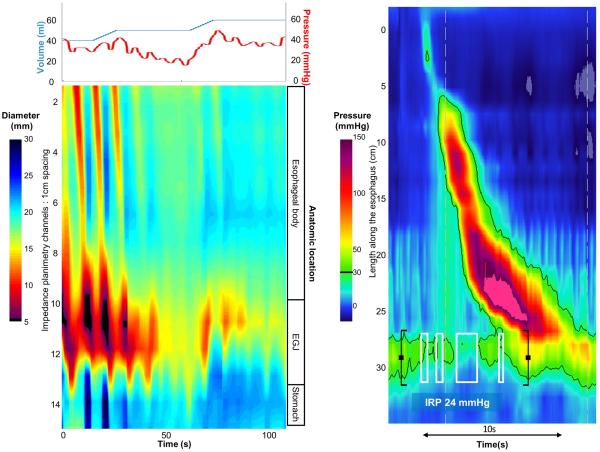
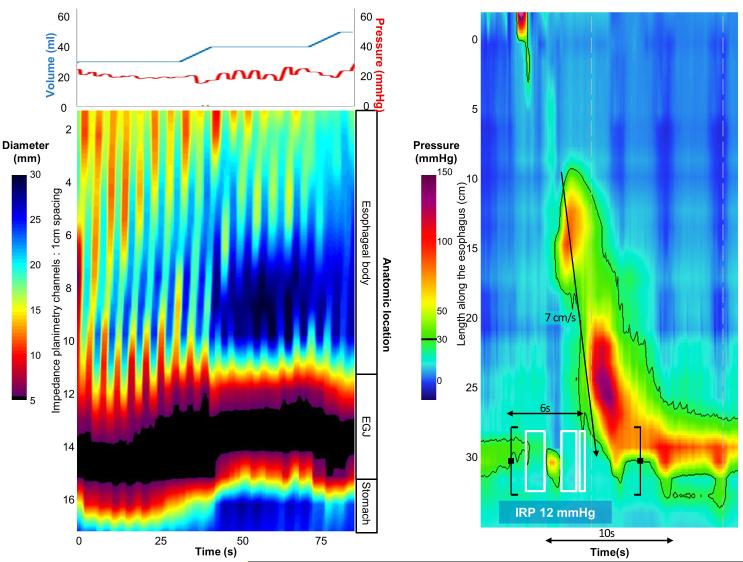
Figure 5. Case examples of FLIP topography.
Left panels represent portions of the FLIP study: distension volume (top, blue line), and intra-balloon pressure (top, red line), and FLIP topography (bottom). A swallow from the corresponding HRM is included in the right panels. A) A patient with type I achalasia, but borderline IRP (median 12 mmHg). FLIP topography demonstrated an abnormal EGJ-DI of 1.6 mm2/mmHg and absent contractility. B) A patient with EGJOO, and suspected evolving achalasia, on HRM. FLIP topography demonstrated abnormal EGJ-DI and absent contractility, supporting the diagnosis of achalasia. The patients was treated with a botulinum-toxin injection to the lower esophageal sphincter that resulted in improvement in dysphagia. C) A patient with EGJOO on HRM. FLIP topography was essentially normal with a normal EGJ-DI (4.9 mm2/mmHg) and RACs. An esophagram showed normal clearance of barium. Dysphagia had resolved without intervention at 6-month follow-up. D) A patient with normal motility on HRM (median IRP 13 mmHg), but with an abnormal EGJ-DI (0.46 mm2/mmHg) and RRCs on FLIP topography. Esophagram demonstrated persistent esophageal barium column height of 4.4-cm after 5 minutes and impaction of a 12.5 mm barium tablet at the EGJ. The patient underwent endoscopic ultrasound with diffusely thickened distal esophageal muscle layers supporting diagnosis of a primary esophageal motor disorder. A per-oral endoscopic myotomy was recommended
An abnormal FLIP topography was observed in 33 of 38 (87%) patients with EGJOO on HRM. An achalasia without contractility pattern was observed in two (5%) EGJOO patients; in both, the global clinical impression was of evolving achalasia: one was treated with botulinum toxin injection (Figure 5B), and the other is being evaluated for lower esophageal sphincter myotomy. Five (13% of 38) patients with EGJOO on HRM (median IRPs ranging from 19 – 26 mmHg) had normal EGJ-DI; all five also had RACs and thus normal FLIP topography. Median intra-balloon pressures at the 60-ml fill volume were > 30 mmHg and median CSA was > 180 mm2 (diameter 15 mm) for all five. Three of these patients had barium esophagrams judged to be normal either on the basis of normal 12.5 mm tablet passage (n = 2) or rapid clearance of 200 ml of a thin liquid barium challenge (n = 1; Figure 5C). For these five patients, one was treated with hyoscyamine, one was referred for cognitive behavioral therapy, and the remaining three patients were managed with observation.
An abnormal FLIP topography was observed in all three patients with jackhammer on HRM. All three had an abnormal EGJ-DI and RRCs.
Abnormal FLIP topography was observed in 50% (17/34) of patients with normal motility or IEM on HRM. An EGJ outflow obstruction was the most common criterion for FLIP topography abnormality observed in 13/17 (76%). Four patients had abnormal EGJ-DI and RRCs (i.e. a FLIP-topography spastic-achalasia pattern); among these, two had a single hypercontractile (DCI > 8,000 mmHg•cm•s) swallow, one had a barium esophagram and endoscopic ultrasound consistent with achalasia (Figure 5D), and one an esophagram with tertiary contractions, but normal passage of a 12.5 mm tablet. Among the remaining nine patients with an abnormal EGJ-DI and normal motility or IEM on HRM, four completed an esophagram: two were normal, one had transient delay of a 12.5 mm barium tablet at the EGJ, and one patient was unable to swallow a 12.5 mm barium tablet. Among the remaining five patients, one had dysphagia improvement following empiric bougie dilation, two were treated with hyoscyamine, and observation was recommended in two (including a patient on chronic opiate therapy).
Finally, four patients (11% of 35) with normal motility or IEM on HRM had RRCs and a normal EGJ-DI on FLIP topography. Among these patients, one had a history of food impaction resulting in endoscopic disimpaction, but esophageal mucosal biopsies, HRM, and esophagram that were normal. The remaining three patients with RRCs had reflux or esophageal hypersensitivity suspected as the etiology for their symptoms.
Discussion
The main finding of our study was that esophageal motility evaluation with FLIP topography generated during a sedated upper endoscopy accurately detected major esophageal motility disorders and achalasia: 95% of patients with a major motility disorder on HRM had an abnormal FLIP topography including 100% of patients with achalasia on HRM. Additionally, FLIP topography appeared to enhance the esophageal functional evaluation of non-obstructive dysphagia by detecting an abnormal response to esophageal distension in 50% of patients with IEM or a normal HRM study. Furthermore, FLIP topography may help arbitrate whether a true EGJOO exists, as opposed to a recording artifact. Thus, FLIP topography may evolve to a disruptive technology for the evaluation of non-obstructive dysphagia that can be performed in conjunction with a sedated upper endoscopy leading to greater patient acceptance.
While the high proportion of achalasia patients in our cohort may not be representative of typical clinical practice, we thought it was important to adequately assess the diagnosis of this important esophageal motility disorder. In doing so, we demonstrated that all 70 achalasia patients had abnormal FLIP topography. Similar to the significance placed on the IRP for the HRM evaluation by the Chicago Classification, the EGJ-DI is paramount in the FLIP topography evaluation. A caveat of the Chicago Classification dictates that HRM with absent contractility and borderline IRP may be considered as type I achalasia, but it can be difficult to clinically differentiate between achalasia and absent contractility, such as in systemic sclerosis.(13) FLIP may help as an arbiter in these borderline cases (as in Figure 5A). Among our 70 achalasia patients (by HRM diagnosis), only two (3%) had a normal EGJ-DI. As the EGJ-DI is a calculated value with the denominator consisting of the intra-balloon pressure, the EGJ-DI may be prone to error from pressure readings at extremes of the range. Thus, a caveat that low intra-balloon pressures (e.g. < 15 mmHg) associated with narrow EGJ-diameters (e.g. < 12.5 mm) may generate a normal EGJ-DI, but still reflect an EGJ outflow obstruction should be implemented.
Though we described the correspondence of the FLIP topography motility classification with HRM-defined esophageal motility disorders, we recognize that HRM is not a perfect diagnostic tool in the evaluation of non-obstructive dysphagia. HRM may be non-diagnostic and remains susceptible to pressure artifacts that can complicate motility diagnoses, particularly with EGJOO.(1) Thus, the objective is not necessarily to reproduce HRM findings, but rather to detect functional abnormalities with clinical significance. While our assessment of clinical outcomes is limited without a standardized treatment or follow-up protocol, we describe numerous clinical examples (Figure 5) from even a relatively small patient cohort in which FLIP topography enhanced the clinical evaluation beyond what was provided by HRM. Hence, we propose that distinct motility patterns of achalasia without contractility and normal motility identified on FLIP topography may be sufficient to characterize esophageal motility during endoscopy. Further, FLIP topography may supplement the manometric evaluation in non-obstructive dysphagia patients with other less well-defined esophageal motility diagnoses, such as EGJOO.
An HRM diagnosis of EGJOO reflects a heterogeneous patient population, and thus often presents a challenging clinical scenario.(13, 15) The elevated IRP may not always reflect a primary esophageal motor disorder, but instead can be related to other causes (including hiatal hernia and recording artifact).(13) While EGJOO may represent evolving achalasia, it can also represent a benign clinical condition in which symptoms spontaneously remit.(15-17) We demonstrated that FLIP topography helps improve characterization of HRM-defined EGJOO patients. EGJOO patients with confirmed abnormal EGJ-response to distension with FLIP topography can be treated with EGJ-directed interventions. The EGJOO patients with normal EGJ-DI in our study did not have evidence of EGJ obstruction on subsequent imaging (as in Figure 5C), and thus may represent patients more likely to carry a benign course and thus warrant expectant management.
The majority (13/23, 57%) of our FLIP-HRM discordance to detect major motility disorders resulted from abnormal EGJ-DI in patients with a normal IRP on HRM (normal motility and IEM). In most of these patients, subtle abnormalities were also appreciated in other tests (HRM or esophagram) that supported an abnormality in esophageal function in these symptomatic patients. However, future study to refine and validate the measurement of EGJ-distensibility in patients without achalasia is needed.
Given the reported association of esophageal motility disorders (particularly type III achalasia) with chronic opiate use, the reliability of motility evaluation during sedated endoscopy may also be questioned.(18-20) While rapid-acting opiates and benzodiazepines may cause transient changes in esophageal motility parameters (with some inconsistencies, previous studies reported opiates generally increased EGJ pressures and benzodiazepines generally reduced or did not affect EGJ pressures), short-term opiate use is unlikely to alter the neural networks that are hypothesized to account for the association of esophageal motility disorders with chronic opiate use.(19, 21-25) Additionally, our analysis paradigm is based on control subjects also evaluated during endoscopy with conscious sedation and we did not observe differences in dosage or utilization of sedating agents across FLIP topography findings (Table 2). Regardless of the use during sedation, FLIP topography motility classifications ultimately appeared to accurately characterize patients. Esophageal motility evaluation during sedated endoscopy was also reported using other methods including visual inspection of lower esophageal sphincter characteristics and esophageal contractility and through-the-scope manometry catheters.(26, 27) However, the subjective nature of endoscopic-visual inspection and the reliance on cooperation of a sedated patient to swallow on command are limitations. Thus the clinical application of these techniques was not broadly adopted. Endoscopic motility evaluation using the FLIP, on the other hand, evaluates distension-induced esophageal contractility and provides objective measurement of esophageal function. Thus, reliance on patient cooperation is minimized and an additional component of esophageal function that is not well-evaluated with standard methods is assessed: the esophageal response to distension.
A limitation of our study is the paucity of major disorders of peristalsis other than achalasia (e.g. jackhammer and distal esophageal spasm). To some degree, this reflects the overall rarity of these disorders. This may particularly affect the reliance on RRCs as an abnormal finding in the proposed FLIP topography paradigm. Based on the frequent observation of RRCs in type III achalasia patients and the absence of RRCs in the cohort of ten asymptomatic controls, RRCs were hypothesized as a potential manifestation of dysfunctional esophageal inhibitory innervation (i.e. spastic contractions).(11) However, it remains possible that RRCs may represent a manifestation of the secondary-peristaltic response to esophageal outflow obstruction; alternatively, RRCs in isolation (i.e. without co-existing esophageal outflow obstruction) may not reflect a pathologic finding. In the present study, all three jackhammer patients (and two additional patients classified as ‘normal motility’, but with hypercontractility not meeting criteria for jackhammer on HRM), had an abnormal EGJ-DI despite normal IRP, which may support the hypothesis that hypercontractile esophagus is related to an EGJ outflow obstruction.(28) Alternatively, as all five of these patients demonstrated RRCs, the potential remains that hypercontractility (and retrograde contractions as a response to distension) are related to an imbalance of inhibitory and excitatory neural regulation.(29, 30) Future studies evaluating a greater number of asymptomatic controls and patients with HRM-defined spastic motor disorders, as well as patients with mechanical esophageal obstruction, will clarify the clinical significance of RRCs and aid refinement of the FLIP topography motility classification.
Generalizability of our findings to broad clinical practice is also presently limited by the need for the FLIP system and more so, the requisite customized MATLAB program to generate the topographic plots. Thus, greater distribution and experience with FLIP and development of commercially-available analytic software will aid future evaluation of the broader application of FLIP topography.
In conclusion, although further study and refinement of diagnostic parameters are needed, esophageal motility evaluation during endoscopy with FLIP topography is an appealing concept for patient-centered care. Incorporation of FLIP topography into clinical practice could reduce the need for esophageal manometry (a test associated with patient discomfort) and also be more convenient for patients (i.e. negate the need for a return visit to complete manometry). Additionally, although HRM is often considered the gold standard for esophageal motility diagnoses, HRM may be non-diagnostic in non-obstructive dysphagia and sometimes, esophageal motility diagnoses can still be missed (such as in Figure 5D). Thus, supplementary diagnostic information may be obtained from cases with discordant FLIP and HRM motility diagnoses. Ultimately, FLIP topography has the potential to become a valuable tool in the diagnostic evaluation of non-obstructive dysphagia.
Study highlights.
What is current knowledge?
Esophageal manometry is indicated for evaluation of non-obstructive dysphagia, though manometry catheter placement is associated with patient discomfort.
The functional lumen imaging probe (FLIP) study can be performed during sedated endoscopy to measure the esophageal response to distension.
What is new here?
Esophageal motility can be classified using the FLIP by assessing 1) esophagogastric junction distensibility and 2) distension-induced contractility by employing a novel, analytic paradigm: FLIP topography.
FLIP topography identified abnormalities in esophageal motility in the vast majority (95%) of patients with abnormal motility on high-resolution manometry, including 100% of achalasia patients.
FLIP topography may enhance the diagnostic evaluation of non-obstructive dysphagia by clarifying equivocal manometric diagnoses and identifying abnormalities not appreciated on manometry.
Acknowledgments
Grant support: This work was supported by T32 DK101363 (JEP) and R01 DK079902 (JEP) from the Public Health service.
Footnotes
Disclosures:
John E. Pandolfino: Given Imaging (Consultant, Grant, Speaking), Sandhill Scientific (Consulting, Speaking), Takeda (Speaking), Astra Zeneca (Speaking)
Ikuo Hirano: Receptos, Inc (Consulting); Regeneron Pharmaceutical, Inc (Consulting), Shire (Consulting)
Nirmala Gonsalves: Nutricia (speaking)
Dustin A. Carlson, Zhiyue Lin, Peter J. Kahrilas, Zoe Listernick, Katherine Ritter, Michael Tye, Fraukje A. Ponds, Ian Wong: None
Author contributions: DAC contributed to study concept and design, data analysis, data interpretation, drafting of the manuscript, and approval of the final version. PJK contributed to data acquisition, revising the manuscript critically, and approval of the final version. ZL contributed to data analysis and approval of the final version. IH, NG, ZL, KR, MT contributed to recruitment of patients, data acquisition, and approval of the final version. FAP and IW contributed to data analysis and approval of the final version. JEP contributed to study concept and design, revising the manuscript critically, and approval of the final version.
References
- 1.Xiao Y, Kahrilas PJ, Nicodeme F, et al. Lack of correlation between HRM metrics and symptoms during the manometric protocol. Am J Gastroenterol. 2014;109(4):521–6. doi: 10.1038/ajg.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology. 2012;143(2):328–35. doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 3.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013;25(6):496–501. doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smeets FG, Masclee AA, Keszthelyi D, et al. Esophagogastric junction distensibility in the management of achalasia patients: relation to treatment outcome. Neurogastroenterol Motil. 2015;27(10):1495–503. doi: 10.1111/nmo.12651. [DOI] [PubMed] [Google Scholar]
- 5.Orvar KB, Gregersen H, Christensen J. Biomechanical characteristics of the human esophagus. Dig Dis Sci. 1993;38(2):197–205. doi: 10.1007/BF01307535. [DOI] [PubMed] [Google Scholar]
- 6.Mujica VR, Mudipalli RS, Rao SS. Pathophysiology of chest pain in patients with nutcracker esophagus. Am J Gastroenterol. 2001;96(5):1371–7. doi: 10.1111/j.1572-0241.2001.03791.x. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140(1):82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicodeme F, Hirano I, Chen J, et al. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2013;11(9):1101–7. doi: 10.1016/j.cgh.2013.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Kahrilas PJ, Xiao Y, et al. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therap Adv Gastroenterol. 2013;6(2):97–107. doi: 10.1177/1756283X12470017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson DA, Lin Z, Rogers MC, et al. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterol Motil. 2015;27(7):981–9. doi: 10.1111/nmo.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015;149(7):1742–51. doi: 10.1053/j.gastro.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Ghosh SK, Rice J, et al. Classifying esophageal motility by pressure topography characteristics: a study of 400 patients and 75 controls. Am J Gastroenterol. 2008;103(1):27–37. doi: 10.1111/j.1572-0241.2007.01532.x. [DOI] [PubMed] [Google Scholar]
- 13.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2015;27(2):160–74. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Z, Nicodeme F, Boris L, et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP) Neurogastroenterol Motil. 2013;25(11):e765–71. doi: 10.1111/nmo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scherer JR, Kwiatek MA, Soper NJ, et al. Functional esophagogastric junction obstruction with intact peristalsis: a heterogeneous syndrome sometimes akin to achalasia. J Gastrointest Surg. 2009;13(12):2219–25. doi: 10.1007/s11605-009-0975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Hoeij FB, Smout AJ, Bredenoord AJ. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol Motil. 2015;27(9):1310–6. doi: 10.1111/nmo.12625. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Fernandez MT, Santander C, Marinero A, et al. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol Motil. 2016;28(1):116–26. doi: 10.1111/nmo.12708. [DOI] [PubMed] [Google Scholar]
- 18.Kraichely RE, Arora AS, Murray JA. Opiate-induced oesophageal dysmotility. Aliment Pharmacol Ther. 2010;31(5):601–6. doi: 10.1111/j.1365-2036.2009.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ratuapli SK, Crowell MD, DiBaise JK, et al. Opioid-Induced Esophageal Dysfunction (OIED) in Patients on Chronic Opioids. Am J Gastroenterol. 2015;110(7):979–84. doi: 10.1038/ajg.2015.154. [DOI] [PubMed] [Google Scholar]
- 20.Ravi K, Murray JA, Geno DM, et al. Achalasia and chronic opiate use: innocent bystanders or associated conditions? Dis Esophagus. 2016;29(1):15–21. doi: 10.1111/dote.12291. [DOI] [PubMed] [Google Scholar]
- 21.Mittal RK, Frank EB, Lange RC, et al. Effects of morphine and naloxone on esophageal motility and gastric emptying in man. Dig Dis Sci. 1986;31(9):936–42. doi: 10.1007/BF01303214. [DOI] [PubMed] [Google Scholar]
- 22.Penagini R, Picone A, Bianchi PA. Effect of morphine and naloxone on motor response of the human esophagus to swallowing and distension. Am J Physiol. 1996;271(4):G675–80. doi: 10.1152/ajpgi.1996.271.4.G675. Pt 1. [DOI] [PubMed] [Google Scholar]
- 23.Hall AW, Moossa AR, Clark J, et al. The effects of premedication drugs on the lower oesophageal high pressure zone and reflux status of rhesus monkeys and man. Gut. 1975;16(5):347–52. doi: 10.1136/gut.16.5.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rushnak MJ, Leevy CM. Effect of diazepam on the lower esophageal sphincter. A double-blind controlled study. Am J Gastroenterol. 1980;73(2):127–30. [PubMed] [Google Scholar]
- 25.Marsh JK, Hoffman SM, Dmuchowski CF. Effect of intravenous midazolam on esophageal motility testing in normal human volunteers. Am J Gastroenterol. 1993;88(6):860–3. [PubMed] [Google Scholar]
- 26.Cameron AJ, Malcolm A, Prather CM, et al. Videoendoscopic diagnosis of esophageal motility disorders. Gastrointest Endosc. 1999;49(1):62–9. doi: 10.1016/s0016-5107(99)70447-5. [DOI] [PubMed] [Google Scholar]
- 27.Kwo PY, Cameron AJ, Phillips SF. Endoscopic esophageal manometry. Am J Gastroenterol. 1995;90(11):1985–8. [PubMed] [Google Scholar]
- 28.Burton PR, Brown W, Laurie C, et al. The effect of laparoscopic adjustable gastric bands on esophageal motility and the gastroesophageal junction: analysis using high-resolution video manometry. Obes Surg. 2009;19(7):905–14. doi: 10.1007/s11695-009-9845-3. [DOI] [PubMed] [Google Scholar]
- 29.Jung HY, Puckett JL, Bhalla V, et al. Asynchrony between the circular and the longitudinal muscle contraction in patients with nutcracker esophagus. Gastroenterology. 2005;128(5):1179–86. doi: 10.1053/j.gastro.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Kim HS, Park H, Lim JH, et al. Morphometric evaluation of oesophageal wall in patients with nutcracker oesophagus and ineffective oesophageal motility. Neurogastroenterol Motil. 2008;20(8):869–76. doi: 10.1111/j.1365-2982.2008.01128.x. [DOI] [PubMed] [Google Scholar]