Abstract
Listeriosis is a fatal infection for fetuses and newborns with two clinical main morbidities in the neonatal period, meningitis and diffused cutaneous lesions. In this study, we vaccinated pregnant females with two gold glyconanoparticles (GNP) loaded with two peptides, listeriolysin peptide 91–99 (LLO91–99) or glyceraldehyde-3-phosphate dehydrogenase 1–22 peptide (GAPDH1–22). Neonates born to vaccinated mothers were free of bacteria and healthy, while non-vaccinated mice presented clear brain affections and cutaneous diminishment of melanocytes. Therefore, these nanoparticle vaccines are effective measures to offer pregnant mothers at high risk of listeriosis interesting therapies that cross the placenta.
Keywords: glyconanoparticles, listeria peptides, vaccines, melanocytes
1. Introduction
Human listeriosis is a food-borne invasive illness caused by the pathogen Listeria monocytogenes (LM) that causes infections in pregnant women, infants, the elderly, and the immune-compromised. While infections are rare among the healthy population, listeriosis is one of the most lethal bacterial diseases for fetuses and newborns [1,2,3,4,5,6,7]. Nevertheless, pregnant women who get infected experience only mild symptoms, making the diagnosis very difficult, even if fetuses are fatally infected [1]. Therefore, vaccination of pregnant women appears as the most cost-effective measure to deal with this deadly pathogen in pregnancy, as it may be responsible for a high number of non-diagnosed spontaneous abortions.
Our group has been preparing vaccines for this pathogen in different formats. We have successfully developed dendritic cell vaccines loaded with LM peptides and deciphered that two peptides from this pathogen’s virulence factors, listeriolysin O (LLO) and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH), LLO91–99 and GAPDH1–22, conferred significant listeriosis protection in two mice models sensitive and resistant to listeriosis [8,9,10]. We have also prepared a gold glyconanoparticle (GNP) coupled to the LLO91–99 peptide formulated with an adjuvant and achieved good vaccine effectiveness in adult mice [11]. Indeed, the application of nanotechnology in immunology is creating big expectations in the field of vaccination [12] and many examples of nanoparticles (NP) have appeared in the literature [13,14]. Nanoparticle-based vaccine candidates have been developed in order to improve the adjuvant effect of the vaccine formulations [15,16], to deliver immunologically active components to target sites [17] and to trigger immune-modulation of inflammatory responses depending on the mechanisms of NP uptake and interactions with immune cells [18]. Among synthetic NPs, GNPs have recently been reported to be able to potentiate the adaptive immune response towards carbohydrate antigens based on the concept of multivalency and depending on the antigen type and loading [19,20,21]. In fact, nanoparticles are promising vector systems to explore vaccination during pregnancy against infectious agents [22]. The interesting results obtained with GNP-LLO91–99 [11] prompted us to prepare other glyconanoparticles, GNP-GAPDH1–22, and examine the ability of these two vaccine vectors, GNP-LLO91–99 and GNP-GAPDH1–22, to protect fetuses from listeriosis. For this purpose, we vaccinated pregnant mothers with the above-mentioned glyconanoparticles formulated with adjuvants and followed LM infection during pregnancy and the neonatal period as well as the morbidities associated with neonatal listeriosis.
2. Results
2.1. Nanoparticle Vaccine Effectiveness in Pregnancy Amilorates Listeriosis-Associated Morbidities
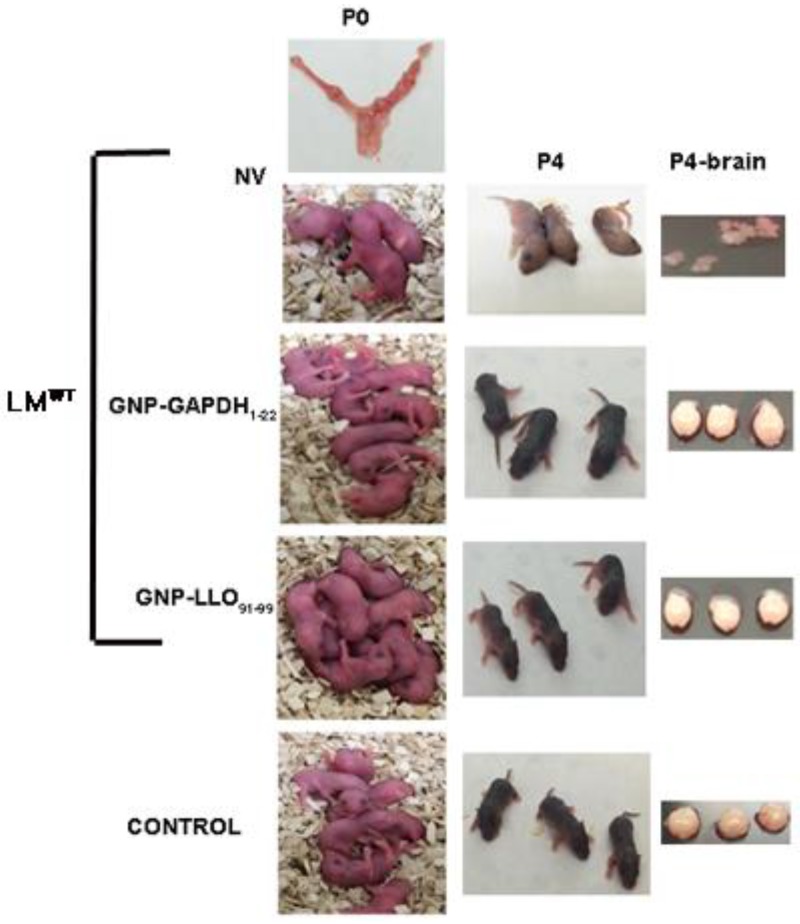
Neonatal listeriosis is characterized by at least three morbidities in newborns: stillbirths, central nervous sytem affection and cutaneous diffused lesions [23,24]. We vaccinated pregnant mothers of C57BL/6 mice (n = 2) at day 9 of gestation (E9) with two prepared nanoparticle vaccines, GNP-LLO91–99 and GNP-GAPDH1–22, formulated with AdvaxTM adjuvant, followed by three days of challenge with LMWT on day 16 of gestation (E16). We prepared three groups of pregnant mothers (n = 2), vaccinated as above, non-vaccinated but challenged with LMWT (NV) and non-vaccinanted and non-infected (control). Control mothers non-vaccinated and non-infected gave birth to nine pups (lower left P0 images in Figure 1), a normal number in C56BL/6 mice that usually deliver six to nine pups. Similarly, GNP-GAPDH1–22- or GNP-LLO91–99-vaccinated and LMWT-challenged mothers delivered nomal numbers of pups, nine and eight, respectively (middle left P0 images in Figure 1). However, NV- and LMWT-challenged mothers only delivered two to three pups (upper P0 images in Figure 1). The reduction of neonates in these groups of NV mothers was due to stillbirths or resorbed fetuses as we detected in the mother’s uterus (upper left P0 image in Figure 1). On day 4 after birth (P4), all pups were explored for clinical data, weight, length, coordination movement tests and general and skin observations with a magnifying lens. Clinical data (Table 1) were normal in the control group and GNP-GAPDH1–22- and GNP-LLO91–99-vaccinated mothers, but they were clearly impaired in the group of NV mothers. The NV group of pups showed weight loss, no ability to move in the metric paper test and a light grey color and wrinkled skin compared to the black and normal skins of vaccinated and control groups (Table 1 and NV images under P4 in Figure 1).
Figure 1.
P0 corresponds to day 1 and P4 to day 4 of pups born to pregnant mothers. Groups of pregnant mothers were the following: non-vaccinated and challenged with Listeria monocytogenes (LMWT) (NV), vaccinated and challenged with LMWT (gold glyconanoparticles listeriolysin peptide 91–99 (GNP-LLO91–99) or gold glyconanoparticles glyceraldehyde-3-phosphate dehydrogenase 1–22 peptide (GNP-GAPDH1–22)) or non-vaccinated and non-infected with LMWT (control). P4-brain corresponds to brains of P4 neonates.
Table 1.
Clinical data of pups (P4) born to vaccinated, non-vaccinated (NV) or control mothers.
| Weight (mg) | Length (cm) | Coordination Movement b (cm) | Cutaneous Test c | |
|---|---|---|---|---|
| NV a | 170 ± 0.5 | 5.0 ± 0.1 | 0.2 ± 0.1 | grey-wrinkled |
| gold glyconanoparticles listeriolysin peptide 91–99 (GNP-LLO91–99) | 280 ± 0.4 | 4.0 ± 0.1 | 9.5 ± 0.8 | black-normal |
| gold glyconanoparticles glyceraldehyde-3-phosphate dehydrogenase 1–22 peptide (GNP-GAPDH1–22) | 295 ± 0.5 | 4.2 ± 0.1 | 10 ± 0.7 | black-normal |
| CONTROL | 300 ± 0.5 | 4.0 ± 0.1 | 10 ± 0.8 | black-normal |
a Four groups of pregnant mothers were prepared: non-vaccinated (NV), GNP-LLO91–99-vaccinated, GNP-GAPDH1–22-vaccinated and controls (non-vaccinated). NV and vaccinated groups were challenged with Listeria monocytogenes (LMWT) but not the control group. All P4 pups were examined for clinical data; b Coordination movement test measures in a 10 cm metric paper the length (cm) achieved by each group of mice in 5 min. Results are the mean ±SD of triplicates; c Cutaneous test with magnifying lens to confirm skin color (black, grey or white) and aspect (normal or wrinkled).
These results suggested that pups born to NV mothers showed central nervous system retardation and enlarged heads and cutaneous immaturation. To confirm these morbidites we sacrificed P4 neonates from the four groups of pregnant mothers. Brains were collected and examined (P4-brain images in Figure 1) and we detected softer cranial covers in pups born to NV mothers (NV images under P4-brain in Figure 1), a significant reduction of blood vessels confirmed by immunohistochemical analysis and a reduced number of melanocytes in the skin with strong apoptotic staining (Table 2).
Table 2.
Number of relative melanocytes and blood vessels.
| Relative Melanocytes a | Apoptotic Melanocytes b (%) | Blood Vessels c | |
|---|---|---|---|
| NV | 0.2 ± 0.1 | 70 ± 0.8 | Few |
| GNP-LLO91–99 | 1 ± 0.1 | 1 ± 0.1 | Normal |
| GNP-GAPDH1–22 | 1 ± 0.1 | 0.9 ± 0.1 | Normal |
| CONTROL | 1 ± 0.1 | 0.9 ± 0.1 | Normal |
a Relative melanocytes, correspond to the number of melanocytes per 1 mm of skin measured by immunohistochemistry; b Apototic melanocytes, correspond to the percentages of melanocytes positive for the TUNNEL staining; c Blood vessels were observed in whole brains with magnifying lens and classified as few or normal.
2.2. Nanoparticles Vaccine Reduced the Number of Viable Bacteria in Microglia and Pro-Inflammatory Cytokine Production
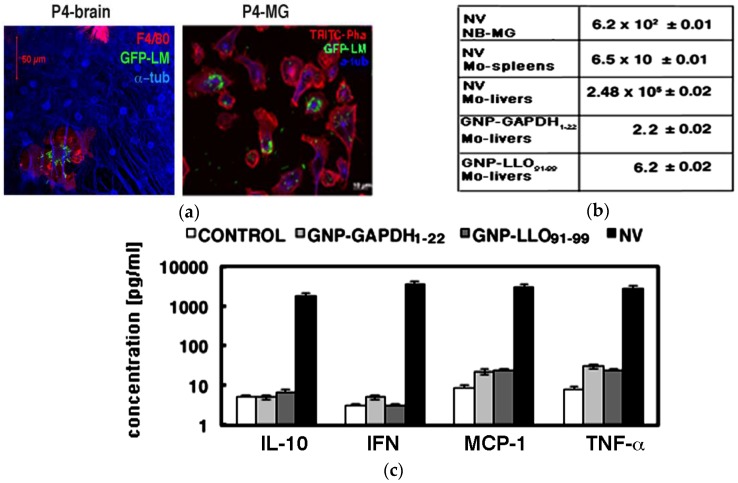
We observed that P4 pups born to NV mothers infected with fluorescent green fluorescence protein Listeria monocytogenes (GFP-LMWT) presented high microglía (MG) infection (green fluorescence in P4-brain, in panel a, Figure 2). MG were characterized by the macrophage F4/80 marker (red fluorescence in P4-brain, panel a, Figure 2). However, we did not observed any bacteria in other brain cells such as neurons as detected with the anti-a-tubuline antibody (blue fluoresnce in P4-brain, panel a, Figure 2), similar to results of in vitro infection of mixed MG cultures [25]. Moreover, purified and isolated MG of P4 pups born to NV mothers presented a severe infection using GFP-LMWT (green fluorescence in P4-MG images, panel a, Figure 2). Numbers of colony-forming-units (CFU) in P4 ewborn pups (NB) born to NV mothers confirmed a high number of viable bacteria, with 6.2 × 102 CFU/mL detected in MG. We detected very few bacteria in MG of P4 pups born to GNP-LLO91–99- and GNP-GADPH1–22-vaccinated mothers, with 6.2 and 2.2 CFU/mL, respectively (panel b, Figure 2). Next, we also explored listeriosis in the mothers and observed no viable CFU in livers or spleens from GNP-LLO91–99- or GNP-GAPDH1–22-vaccinated mothers (data not shown). However, we detected 2.40 × 105 CFU/mL in livers of NV mothers that corresponded with 25-fold growth of bacteria compared to initial inoculation with 104 CFU/mice (panel b, Figure 2) and suggested a normal listeriosis infection in these NV mothers. The low CFU in spleens of NV mothers, 6.5 × 10 CFU/mL, indicated these pregnant mothers cleared the infection, while placental transmission was fatal for their fetuses, and after four to six days all pups died (data not shown).
Figure 2.
(a) P4-brain image corresponds to mixed microglía (MG) cultures of cerebellum brains showing green fluorescence of green fluorescence protein Listeria monocytogenes (GFP-LM), red fluorescence of the macrophage marker F4/80 and blue fluoresecence of neuron tubuline. P4-MG corresponds to purified MG of mixed cultures showing green fluorescence of GFP-LM, red fluorescence of actin cytoskeleton stained with TRITC-phalloidin and blue fluorescence of tubuline; (b) colony-forming-units (CFU)/mL in MG of newborn P4 pups (NB) born to NV mothers, GNP-LLO91–99 or GNP-GAPDH1–22 vaccinated mothers. CFU/mL in livers and spleens of NV mothers are also examined; (c) Cytokines levels (pg/mL) in supernatants of MG of newborn P4 pups born to mothers as in Figure 1.
2.3. Nanoparticle Vaccines Shifted a Th2 Cytokine Pattern to Th1 Production
Cerebral listeriosis in adults is characterized by high levels of Th1 (TNF-a and MCP-1) and Th2 cytokines (IL-6, IL-10) [24,26,27,28]. Vaccination of systemic listeriosis reduced IL-6 levels (Th2 pattern) and presented a significant increase in IL-12 levels (Th1 pattern) [11]. Here, we confirmed that neonatal listeriosis also caused MG to produce high levels of TNF-a (panel c, Figure 2) and IL-6 (Table 3), as reported in most neonatal human meningitis [1,29]. Vaccination of pregant mothers with GNP-LLO91–99 and GNP-GAPDH1–22 vaccines caused a signficant increase of IL-12, and a decrease of IL-6 levels (Table 3).
Table 3.
Cytokine levels of purified microglía (MG) from P4 neonates born to vaccinated or NV mothers.
| IL6 (pg/mL) a | IL-12 (pg/mL) | |
|---|---|---|
| NV | 1650 ± 0.9 | 17 ± 0.8 |
| GNP-LLO91–99 | 37 ± 0.1 | 27 ± 0.1 |
| GNP-GAPDH1–22 | 55 ± 0.1 | 40 ± 0.1 |
| CONTROL | 3 ± 0.1 | 1 ± 0.1 |
a Cytokines are measured by flow cytometry and results expressed as concentration of pg/mL ± SD of triplicates.
3. Discussion
The objective of this study was to identify an optimal vaccine formulation to protect against neonatal listeriosis. Preconceptual vaccination with attenuated L. monocytogenes strains does not protect against fetal wastage or placental-fetal invasion [30], prior to or during pregnancy. Therefore, different vaccination vectors or systems should be used to protect against infections of vertical transmission. In this regard, dendritic vaccines emerged as effective vectors able to confer significant protection against systemic listeriosis in different mice models, either sensitive or more resistant to LM infection [8,9,23]. Further, dendritic vaccines loaded with peptides from virulence factors of LM such as lysteriolysin O (LLO) or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were effective for protection, but they are expensive measures to offer all pregnant women at risk of listeriosis. In this regard, nanoparticles are promising vaccine vectors with interesting properties such as safety, biocompatibility, and versatility to load with different ligands and directionality to dendritic cells with chemical modifications such as carbohydrates [31,32,33].
We previously reported that gold glyconanoparticles loaded with a listeriolysin O peptide, GNP-LLO91–99, when formulated with adjuvants such as AdvaxTM, conferred significant protection against systemic listeriosis in adult animal models [11,23]. Taking advantage of this nanoparticle system, we also designed a similar vector coupled with another peptide of the GAPDH virulence factor, GAPDH1–22, which showed promising properties in dendritic cell vaccines [8,9]. Here, we presented that both GNP-LLO91–99 and GNP-GAPDH1–22, nanovaccines formulated with AdvaxT004D conferred good protection during pregnancy against neonatal listeriosis. GNP-GAPDH1–22 nanovaccines appeared more effective than GNP-LLO91–99 nanovaccines as they showed lower CFU numbers in MG. Moreover, these nanovaccines reduced central nervous system (CNS) and skin-associated morbidities as well as pregnancy complications.
Invasive listeriosis in pregnancy is a fatal infection for the fetuses, causing complications such as spontaneous abortions or stillbirths in 20% of the cases, especially if infection occurs early in the pregnancy; preterm delivery and neonatal infection are also observed [1,34]. 70% of surviving neonates born to mothers infected with LM, presented trans-placental transmission of bacteria. Neonatal listeriosis manifestations imply meningitis, brain abscesses, diffused skin lesions and rash, fever and lethargy [1,2,3,4,5,6,7,23,26]. While not exactly similar, neonatal experimental listeriosis inoculating pregnant mice with pathogenic LM reproduces most of the clinical symptoms [26,27,28,34]. In this regard, our results here detecting resorbed fetuses in mice mothers might reflect human stillbirths; the 2- to 3-fold reduction in neonates at birth might mimic spontaneous abortions, all clinical complications related to pregnancy [1]. We also detected CNS-associated morbidities such as enlarged heads, softer cranial covers and a lack of coordinated movement that resembled CNS impairment [24]. Finally, the lower number of skin melanocytes due to apoptosis we detected in P4 pups born to NV mothers appeared comparable to cutaneous diffused lesions of granulomatosis infantiseptica forms of severe listeriosis in neonates [23]. All these above-mentioned listeriosis-associated morbidities disappeared in pregnant mothers pre-vaccinated with GNP-GAPDH1–22 and GNP-LLO91–99 nanovaccines and in neonates born to these vaccinated mothers. Therefore, they highlighted the ability of these nanovaccines to cross the plancental barrier and protect the fetuses.
Moreover, the ability of these nanovaccines to shift the cytokine pattern of MG [35] towards a Th1-IL-12-dependent pattern is supposed a good benefit since IL-12 production seems associated with vaccine efficiency and long-term protection [8,11,28], but also because acute Th2 cytokines such as IL-6 [29] were significantly reduced after vaccination with GNP-GAPDH1–22 and GNP-LLO91–99 nanovaccines.
We concluded that GNP-LLO91–99 and GNP-GAPDH1–22 nanovaccines are effective measures for vaccination against listeriosis during pregnancy, with GNP-GAPDH1–22 being more potent than GNP-LLO91–99 nanovaccines. The disappearance of listeriosis morbidities is an added value of these nanovaccines that revealed their powerful action.
4. Materials and Methods
Nanoparticles: GNP carrying approximately 90% glucose and 10% LLO91–99 peptide were prepared by reduction in situ of Au(III) salt with sodium borohydride (Figure S1, panel a and b) [11], following previously described procedures [30,31,35,36]. To obtain GNPs carrying GAPDH peptide, an aqueous solution of tetrachloroauric (Strem Chemicals) (0.025 M, 1 eq.) was added to a solution of a mixture of glucose (90%) and GAPDH peptide (10%) thiol ending ligands (0.012 M, 6 eq.) in MeOH/H2O/CH3COOH (3:3:1). An aqueous solution of NaBH4 (1 M, 22 eq.) was then portion-wise added and the mixture was shaken for 2 h at 25 °C. The solvent was evaporated at reduced pressure. The residue was washed with ethanol, re-dissolved in the minimum quantity of milliQ water, loaded into 5–10 cm segments of SnakeSkin® pleated dialysis tubing (Pierce, 3500 MWCO) and purified by dialysis against distilled water (3 L of water, recharging with fresh water every six hours over the course of 72 h). The nanoparticles were obtained as brown powder after lyophilization (Figure S1, panel b shows prepared GNP-LLO91–99 and GNP-GAPDH1–22). The size distribution of the gold nanoparticles was evaluated from several transmission electron microscopy (TEM) micrographs (JEM-2100F, JEOL Ltd., Tokyo, Japan). TEM (average diameter and number of gold atoms): 2.1 ± 0.5 nm (Figure S1, panel c). The presence of glucose and peptide ligands was confirmed by 1H NMR (500 MHz, D2O): 7.3 (br s 4.4 ~5H), 4.4 (br s, ~1H), 4.1–3.1 (br m, ~57H), 2.6 (t, ~3H), 2.5 (t, ~3H), 1.99–1.15 (br m, ~37H), 0.87 (br s ~22H) (Figure S2, panel d). The amount of GAPDH peptide on the GNPs was determined by quantitative NMR (qNMR) in a Bruker AVANCE 500 MHz spectrometer (Bruker Corp., Billerica, MA, USA): 0.234 mg of GNP were dispersed in D2O 99.9% (200 μL). Then 80 μL of this solution were added with 40 μL of a 0.05% 3-(trimethylsilyl)propionic-2,2,3,3-d4 acid sodium salt (TSP-d4) solution in D2O and 60 μL of D2O. Peptide loading was 10.6 μg/0.234 mg GNPs. GNP-LLO91–99 and GNP-GAPDH1–22 stability was performed after following dendritic cells incubation for 8 to 24 h in vitro and FACS analysis of peptides using anti-LLO (Diatheva) and anti-GAPDH1–22-specific antibodies made in rabbit [8]. We observed 90% and 95% double positive CD11c+anti-LLO+ and CD11c+anti-GAPDH1–22+ in DC recovered cells after treatment for 24 h with GNP-LLO91–99 or GNP-GAPDH1–22, respectively.
Bacteria. Daniel A. Portnoy (University of California, Berkeley, CA, USA) provided L. monocytogenes 10403S pathogenic strain (LMWT) and the mutant strains LM∆LLO (DPL-2161) and LM∆ActA (DPL-1942). GFP-LM DH-L1039 (GFP-LM) derived from the 10403S LM strain was a gift from Darren E. Higgins (Harvard Medical School, Boston, MA, USA).
Animals. We used C57BL/6 mice from our animal facilities at the University of Cantabria at 8–12 weeks old. Three female and one male were mated and assessed for the appearance of vaginal plug denoting first embryonic day of pregnancy.
Pre-natal vaccination. Pregnant C57BL/6 female mice were vaccinated or not (NV) at day 9 of gestation (E9) with intravenously (i.v) via the lateral tail vain with GNP-LLO91–99 or GNP-GAPDH1–22 formulated with AdvaxTM (5 µg of nanoparticles and 250 µg of adjuvants/mice). Next, at 16 days of gestation (E16) all mice, vaccinated and NV were inoculated i.v with 100 µL of a LMWT bacterial suspension in saline (1 × 105 CFU/mL) (LMWT infected mothers). We also preserved a group of mothers NV and inoculated with 100 µL of saline (Control). All animals were daily examined. At E20 we detected two to three pups born to LMWT infected mothers and NV, while six to eight pups born to control and vaccinated mice. Four days after birth, P4 postnatal pups born to LMWT infected and vaccinated (GNP-LLO91–99 or GNP-GAPDH1–22) or NV mothers were sacrificed to obtain cerebellum for preparation of mixed microglia and subsequent isolation of primary microglial cultures for CFU quantification and cytokine quantification. LMWT infected and vaccinated, NV and control mothers were bled for cytokine analysis and sacrificed to obtain uterus for observation of resorbed fetuses and spleens and livers for CFU quantification.
Clinical tests. Clinical test were performed on all pups born to mothers vaccinated, NV or control on days P0 and P4. Test include weight, length, coordination-movement assays placing pups in a metric paper and recording the centimeters of movement after 5 min, general and cutaneous screening for lesions and wounds with a magnifying glass.
Microglia isolation. Microglial cultures have been described [37,38] and detailed procedures for obtaining mixed microglial cultures and purified primary microglia were reported [24]. In brief, mixed microglial cell cultures were obtained from cerebellum at P4 until complete neuronal differentiation for seven days as described [37]. Microglial cultures shaked at 200 times/min for 30 min and cells in supernatants re-plated in 24-well plates. MG cells were 90% double positive CD11b+CD45+ and 80% double positive for F4/80+IAb+ as measured by flow cytometry (FACS). Supernatants of MG cells were filtered and stored at −80 °C for cytokine analysis by FACS and next, MG cells were lysed and plated in BHI agar plates for CFU analysis.
Confocal assays. Cells used for confocal microscopy were fixed in 3% paraformaldehyde. Fluorescence labeling and confocal microscopy were performed as previously described [24].
FACS analysis. Cell surface markers of MG were analyzed by FACS using the following antibodies: anti-CD11b-FITC, anti-CD45-PerCP, anti-F4/80-PE and anti-IAb-brilliant blue (Miltenyi Biotech Inc., Auburn, CA, USA). Annexin-V conjugated with APC fluorochrome and 7-ADD (7-amino-actinomycin D) (BD Biosciences, San Jose, CA, USA) and cytokines in MG supernatants were also quantified using the CBA kit (BD Biosciences, San Jose, CA, USA). Samples were analyzed in triplicates and results are expressed as the mean ± SD of two separate experiments.
Immunochemistry. One mice (NB) of each group born of vaccinated, NV or control mothers were immersed in 4% formaldehyde for 24 h. Organs were subsequently embedded in paraffin, processed and sections stained with hematoxylin-eosin and immunohistochemical analysis for apoptotic cells (TUNNEL staining performed according to the manufacturer instructions, Roche), and main antibodies characteristic of different organs (brain, skin, liver, gut, spleen, colon, lungs) as well as with anti-Listeria antiserum (Difco, BD Biosciences, New York, NY, USA) to localize the bacteria. For skin analysis, sections of 1 mm of skin were examined for melanocytes number and apoptosis.
Statistical analysis. For statistical analysis, the Student’s t test was applied. p ≤ 0.05 was considered significant.
Ethics statement. This study was carried out in strict accordance with the recommendations in the guide for the Care and Use of Laboratory Animals of the Spanish Ministry of Science, Research and Innovation. The Committee on the Ethics of Animal Experiments of the University of Cantabria approved this protocol (Permit Number: 2012/06) that follows the Spanish legislation (RD 53/2013). All efforts were made to minimize suffering.
5. Conclusions
GNP-LLO91–99 and GNP-GAPDH1–22 nanovaccines formulated with Advax adyuvant protect against listeriosis when applied during pregnancy. Nanovaccines are effective measures for preganancy vaccination as they are able to cross the placental barrier and diminish listeriosis morbidities.
Acknowledgments
We acknowledge the assistance of Fidel Madrazo-Toca (IDIVAL, Santander, Spain) and Mónica López Fanarraga (Mol Biol Department, Universidad de Cantabria, Santander, Spain) with confocal images and Jesús Navas for critical advices (Mol Biol Department, Universidad de Cantabria, Santander, Spain). AdvaxTM adjuvant was kindly provided by Nikolai Petrovsky (Vaxine Pty Ltd., Flinders Medical Center, Adelaide, Australia). 10403S L. monocytogenes strain was provided by Daniel A. Portnoy (University of California, Berkley, CA, USA) and GFP-Listeria DH-L1039 derived from the 10403S strain was a gift from Darren E. Higgins (Harvard Medical School, Boston, MA, USA). We acknowledge the support of MINECO with grants SAF2009-08695 and SAF2012-34203 (to Carmen Álvarez-Domínguez) and IDIVAL institutional grants INNVAL15/01 (to Carmen Álvarez-Domínguez and Javier Freire) and API2012/03/SAF2009-08695 and AIP2014/14/SAF2012-34203 (to Carmen Álvarez-Domínguez). Ricardo Calderón-Gonzalez and Eva Ferrández-Fernández were supported with the AIP2014/14/SAF2012-34203 grant, Elisabet Frande-Cabanesand with the AIP2012/02/SAF2009-08695 and SAF2009-08695 grants and Héctor Terán-Navarro with the INNVAL15/01 grant.
Abbreviations
The following abbreviations are used in this manuscript:
- GFP
green fluorescence protein
- CFU
colony forming unit
- CNS
central nervous system
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- GNP
glyconanoparticle
- IFN
interferon
- i.v
intravenously
- LLO
listeriolysin O
- LM
Listeria monocytogenes
Supplementary Materials
The following are available online at www.mdpi.com/2079-4991/6/8/151/s1.
Author Contributions
Carmen Álvarez-Domínguez, Sonsoles Yañez-Díaz and Javier Gomez-Román conceived and designed the experiments; Ricardo Calderón-Gonzalez, Héctor Terán-Navarro, Elisabet Frande-Cabanes and Eva Ferrández-Fernández performed the experiments; Javier Freire performed the histochemical analysis; Marco Marradi and Isabel García synthesized all GNP nanovaccines, Soledad Penadés directed GNP naovaccines synthesis and experiments; Carmen Álvarez-Domínguez analyzed the data; Ricardo Calderón-Gonzalez, Héctor Terán-Navarro and Carmen Álvarez-Domínguez wrote the paper.
Conflicts of Interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Mylonakis E., Paliou M., Hohmann E.L., Calderwood S.B., Wing E.J. Listeriosis during pregnancy: A case series and review of 222 cases. Medicine. 2002;81:260–269. doi: 10.1097/00005792-200207000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Allerberger F., Wagner M. Listeriosis: A resurgent foodborne infection. Clin. Microbiol. Infect. 2010;16:16–23. doi: 10.1111/j.1469-0691.2009.03109.x. [DOI] [PubMed] [Google Scholar]
- 3.Magalhaes R., Almeida G., Ferreira V., Santos I., Silva J., Mendes M.M., Pita J., Mariano G., Mancio I., Sousa M.M., et al. Cheese-related listeriosis outbreak, Portugal, March 2009 to February 2012. Euro Surveill. 2015;20:1–6. doi: 10.2807/1560-7917.ES2015.20.17.21104. [DOI] [PubMed] [Google Scholar]
- 4.Pérez-Trallero E., Zigorraga C., Artieda J., Alkorta M., Marimón J.M. Two outbreaks of Listeria monocytogenes infection, Northern Spain. Emerg. Infect. Dis. 2014;20:2155–2157. doi: 10.3201/eid2012.140993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peña-Sagredo J.L., Hernández M.V., Fernandez-Llanio N., Giménez-Ubeda E., Muñoz-Fernandez S., Ortiz A., Gonzalez-Gay M.A., Fariñas M.C., Biobadaser G. Listeria monocytogenes infection in patients with rheumatic diseases on TNF-alpha antagonist therapy: The spanish study group experience. Clin. Exp. Rheumatol. 2008;26:854–859. [PubMed] [Google Scholar]
- 6.Fernandez-Sabe N., Cervera C., Lopez-Medrano F., Llano M., Sáez E., Len O., Fortún J., Blanes M., Laporta R., Torre-Cisneros J. Risk factors, clinical features, and outcomes of listeriosis in solid-organ transplant recipients: a matched case-control study. Clin. Infect. Dis. 2009;49:1153–1159. doi: 10.1086/605637. [DOI] [PubMed] [Google Scholar]
- 7.Ariza-Miguel J., Fernández-Natal M.I., Soriano F., Hernández M., Stessl B., Rodríguez-Lázaro D. Molecular epidemiology of invasive listeriosis due to Listeria monocytogenes in a spanish hospital over a nine-year study period, 2006-2014. Biomed. Res. Int. 2015 doi: 10.1155/2015/191409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calderon-Gonzalez R., Frande-Cabanes E., Bronchalo-Vicente L., Lecea-Cuello M.J., Pareja E., Bosch-Martinez A., Fanarraga M.L., Yañez-Diaz S., Carrasco-Marin E., Alvarez-Dominguez C. Cellular vaccines in listeriosis: Role of the Listeria antigen GAPDH. Front. Cell. Infect. Microbiol. 2014;4 doi: 10.3389/fcimb.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderon-Gonzalez R., Frande-Cabanes E., Tobes R., Pareja E., Alaez-Alvarez L., Alvarez-Dominguez C. A dendritic cell targetted vaccine loaded with a glyceraldehyde-3-phosphate-dehydrogenase peptide proposed for individuals at high risk of listeriosis. J. Vaccines Vaccin. 2015;6 doi: 10.4172/2157-7560.1000266. [DOI] [Google Scholar]
- 10.Calderon-Gonzalez R., Tobes R., Pareja E., Frande-Cabanes E., Alaez-Alvarez L., Petrovsky N., Alvarez-Dominguez C. Identification and characterisation of T-cell epitopes for incorporation into dendritic cell-delivered Listeria vaccines. J. Immunol. Methods. 2015;9:111–119. doi: 10.1016/j.jim.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Del Rio E., Marradi M., Calderon-Gonzalez R., Frande-Cabanes E., Penades S., Petrovsky N., Alvarez-Dominguez C. A gold-glyconanoparticle carrying a listeriolysin O peptide and formulated with Advax™ delta inulin adjuvant induces robust T-cell protection against Listeria infection. Vaccine. 2015;33:1465–1473. doi: 10.1016/j.vaccine.2015.01.062. [DOI] [PubMed] [Google Scholar]
- 12.Smith D.M., Simon J.K., Baker J.R. Applications of nanotechnology for immunology. Nat. Rev. Immunol. 2013;13:592–605. doi: 10.1038/nri3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irvine D.J., Hanso M.C., Rakhra K., Tokatlian T. Synthetic nanoparticles for vaccines and immunotherapy. Chem. Rev. 2015;115:11109–11146. doi: 10.1021/acs.chemrev.5b00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L., Seth A., Wibowo N., Zhao C.X., Mitter N., Yu C.Z., Anton P.J.M. Nanoparticle vaccines. Vaccine. 2014;32:327–337. doi: 10.1016/j.vaccine.2013.11.069. [DOI] [PubMed] [Google Scholar]
- 15.Dykman L.A., Staroverov S.A., Bogatyrev V.A., Shchyogolev S.Y. Adjuvant properties of gold nanoparticles. Nanotechnol. Russia. 2010;5:748–761. doi: 10.1134/S1995078010110029. [DOI] [Google Scholar]
- 16.Prashant C.K., Kumar M., Dinda A.K. Nanoparticle based tailoring of adjuvant function: The role in vaccine development. J. Biomed. Nanotechnol. 2014;10:2317–2331. doi: 10.1166/jbn.2014.1991. [DOI] [PubMed] [Google Scholar]
- 17.Gregory A.E., Titball R., Williamson D. Vaccine delivery using nanoparticles. Front. Cell. Infect. Microbiol. 2013;3 doi: 10.3389/fcimb.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaman M., Good M.F., Toth I. Nanovaccines and their mode of action. Methods. 2013;60:226–231. doi: 10.1016/j.ymeth.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 19.Brinas R.P., Sundgren A., Sahoo P., Morey S., Rittenhouse-Olson K., Wilding G.E., Deng W., Barchi J.J. Design and Synthesis of Multifunctional Gold Nanoparticles Bearing Tumor-Associated Glycopeptide Antigens as Potential Cancer Vaccines. Bioconj. Chem. 2012;23:1513–1523. doi: 10.1021/bc200606s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parry A.L., Clemson N.A., Ellis J., Bernhard S.S.R., Davis B.G., Cameron N.R. ‘Multicopy multivalent’ glycopolymer-stabilized gold nanoparticles as potential synthetic cancer vaccines. J. Am. Chem. Soc. 2013;135:9362–9365. doi: 10.1021/ja4046857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Safari D., Marradi M., Chiodo F., Dekker H.A., Shan Y., Adamo R., Oscarson S., Rijkers G.T., Lahmann M., Kamerling J.P., et al. Gold nanoparticles as carriers for a synthetic Streptococcus pneumoniae type 14 conjugate vaccine. Nanomedicine. 2012;7:651–662. doi: 10.2217/nnm.11.151. [DOI] [PubMed] [Google Scholar]
- 22.Jiménez-Ruiz E., Alvarez-García G., Aguado-Martínez A., Salman H., Irache J.M., Marugán-Hernández V., Ortega-Mora L.M. Low efficacy of NcGRA7, NcSAG4, NcBSR4 and NcSRS9 formulated in poly-ε-caprolactone against neospora caninum infection in mice. Vaccine. 2012;30:4983–4992. doi: 10.1016/j.vaccine.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 23.Godshall C.E., Suh G., Lorber B. Cutaneous listeriosis. J. Clin. Invest. 2013;51:3591–3596. doi: 10.1128/JCM.01974-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drevets D.A., Bronze M.S. Listeria monocytogenes: Epidemiology, human disease, and mechanisms of brain invasion. FEMS Immunol. Med. Microbiol. 2008;53:151–165. doi: 10.1111/j.1574-695X.2008.00404.x. [DOI] [PubMed] [Google Scholar]
- 25.Frande-Cabanes E., Fernandez-Prieto L., Calderon-Gonzalez R., Rodriguez-Del Rio E., Yañez Diaz S., Lopez-Fanarraga M., Alvarez-Dominguez C. Dissociation of innate immune responses in microglia infected with Listeria monocytogenes. Glia. 2014;62:233–246. doi: 10.1002/glia.22602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Disson O., Lecuit M. In vitro and in vivo models to study human listeriosis: Mind the gap. Microbes Infect. 2013;15:971–980. doi: 10.1016/j.micinf.2013.09.012. [DOI] [PubMed] [Google Scholar]
- 27.Schlüter D., Back C., Reiter S., Meyer T., Hof H., Deckert-Schluter M. Immune reactions to Listeria monocytogenes in the brain. Immunobiology. 1999;201:188–195. doi: 10.1016/S0171-2985(99)80058-8. [DOI] [PubMed] [Google Scholar]
- 28.Unanue E.R., Carrero J.A. Studies with Listeria monocytogenes lead the way. Adv. Immunol. 2012;113:1–5. doi: 10.1016/B978-0-12-394590-7.00009-9. [DOI] [PubMed] [Google Scholar]
- 29.Mukai A.O., Krebs V.L.J., Bertoli C.J., Okay T.S. TNF-α and IL-6 in the diagnosis of bacterial and aseptic meningitis in children. Pediatr. Neurol. 2006;34:25–29. doi: 10.1016/j.pediatrneurol.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 30.Clark D.R., Chaturvedi V., Kinder J.M., Jiang T.T., Xin L., Erlet J.M., Way S.S. Perinatal Listeria monocytogenes susceptibility despite preconceptual priming and maintenance of pathogen-specific CD8+ T cells during pregnancy. Cell. Mol. Immunol. 2014;11:595–605. doi: 10.1038/cmi.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Ávila O., Hijazi K., Marradi M., Clavel C., Campion C., Kelly C., Spenades S. Gold manno-glyconanoparticles: Multivalent systems to block HIV-1 gp120 binding to the lectin DC-SIGN. Chem. Eur. J. 2009;15:9874–9888. doi: 10.1002/chem.200900923. [DOI] [PubMed] [Google Scholar]
- 32.Marradi M., Chiodo F., García I., Penadés S. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem. Soc. Rev. 2013;42:4728–4745. doi: 10.1039/c2cs35420a. [DOI] [PubMed] [Google Scholar]
- 33.Calderon-Gonzalez R., Garcia I., Marradi M., Petrovsky N., Alvarez-Dominguez C. Novel nanoparticles vaccines. Hum. Vaccin. Immunother. 2015;11:2501–2503. doi: 10.1080/21645515.2015.1063756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardy J., Kirkendoll B., Zhao H., Pisani L., Luong R., Switzer A., McConnell M.V., Contag C.H. Infection of pregnant mice with Listeria monocytogenes induces fetal bradicardia. Pediatr. Res. 2012;71:539–545. doi: 10.1038/pr.2012.2. [DOI] [PubMed] [Google Scholar]
- 35.Franco R., Fernandez-Suarez D. Alternatively activated microglia and macrophages in the central nervous system. Prog. Neurobiol. 2015;131:65–86. doi: 10.1016/j.pneurobio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 36.De la Fuente J.M., Penadés S. Glyconanoparticles: Types, synthesis and applications in glycoscience, biomedicine and material science. Biochim. Biophys. Acta. 2006;1760:636–651. doi: 10.1016/j.bbagen.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Fanarraga M., Carranza G., Bellido J., Kortazar D., Villegas J.C., Zabala J.C. Tubulin cofactor B plays a role in the neuronal growth cone. J. Neurochem. 2007;100:1680–1687. doi: 10.1111/j.1471-4159.2006.04328.x. [DOI] [PubMed] [Google Scholar]
- 38.Ribes S., Ebert S., Regen T., Agarwal A.M., Tauber S.C., Czesnik D., Spreer A., Bunkowski S., Eiffert S., Hanischm U.W., et al. Toll-like receptor stimulation enhances phagocytosis and intracellular killing of nonencapsulated Streptococcus pneumonia by murine microglia. Infect. Immun. 2010;78:865–871. doi: 10.1128/IAI.01110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.