Abstract
Neuropathic pain results from diseases or trauma affecting the nervous system. This pain can be devastating and is poorly controlled. The pathophysiology is complex, and it is essential to understand the underlying mechanisms in order to identify the relevant targets for therapeutic intervention. In this article, we focus on the recent research investigating neuro-immune communication and epigenetic processes, which gain particular attention in the context of neuropathic pain. Specifically, we analyze the role of glial cells, including microglia, astrocytes, and oligodendrocytes, in the modulation of the central nervous system inflammation triggered by neuropathy. Considering epigenetics, we address DNA methylation, histone modifications, and the non-coding RNAs in the regulation of ion channels, G-protein-coupled receptors, and transmitters following neuronal damage. The goal was not only to highlight the emerging concepts but also to discuss controversies, methodological complications, and intriguing opinions.
Keywords: neuropathic pain, glial cells, microglia, astrocytes, oligodendrocytes
Introduction
Neuropathic pain is a severe chronic disease that can develop following lesions to the central nervous system (CNS) (spinal cord injury, stroke, and multiple sclerosis) or to peripheral nerves. The peripheral nerve damage can be caused by diseases (diabetes and herpes zoster) or trauma (compression, stretch, and amputation) 1. Patients experience spontaneous burning or shooting pain, which can be amplified by mechanical and thermal stimuli, both noxious (pressure, heat, and cold) and normally innocuous (touch and warm or cool temperatures) 2. Given that current therapies are unsatisfactory 3, the investigation of mechanisms by which nerve damage triggers pain remains the focus of research, the goal of which is to find new targets for better treatment. The neuropathic pain pathophysiology is complex and includes peripheral and central neuronal alterations 4– 6 as well as neuro-immune interactions 7– 11. In this article, we focus on the findings of the last 3 to 4 years in the very dynamic and expanding areas of epigenetics and glia, immune cells of the CNS, including sex differences in neuropathic pain.
Glia in the modulation of neuropathic pain
It has been established that neuropathic pain is not exclusively a sensory phenomenon but also involves immune reactions. Glia represent immune cells of the CNS; microglia and astrocytes are the most investigated 9– 11, and oligodendrocytes have emerged as new players relevant to neuropathic pain.
Microglia
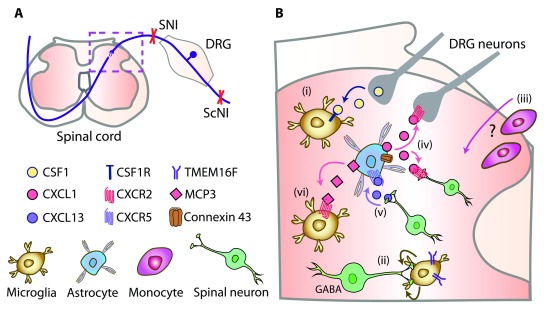
Following peripheral nerve injury, microglia proliferate, become hypertrophic and activated, and secrete molecules which sensitize sensory neurons in the spinal cord. The best-characterized mechanism involves the activation of microglial purinergic receptors P2X4 by adenosine triphosphate (ATP) secreted from damaged neurons or astrocytes or both. This stimulates microglia to release brain-derived neurotrophic factor (BDNF), which activates its neuronal receptors. Ultimately, inhibitory signaling mediated by γ-aminobutyric acid A (GABA A) receptors is diminished and this culminates in enhanced pain 11– 14. The microglia–neuronal interactions are bidirectional, and there is some dispute of the initial signal that activates microglia. In addition to the ATP/P2X4 pathway, the dorsal root ganglion (DRG) neuron central endings (which terminate in the spinal cord dorsal horn) ( Figure 1A) secrete chemokines such as CX3CL1, CCL2, and CCL21, which activate their respective receptors on microglia 13. The previous concepts have recently been challenged by studies suggesting that it is a cytokine colony-stimulating factor 1 (CSF1) which is upregulated in injured DRG neurons, transported, and secreted in the spinal cord, where it activates CSF1 receptors in microglia, leading to their proliferation and neuropathic pain induction 15, 16 ( Figure 1Bi). Selective neuronal CSF1 deletion reduced microglia proliferation and prevented neuropathy-triggered hypersensitivity in mice. Downstream of CSF1 receptor, the microglial membrane adaptor protein DAP12 was required for hypersensitivity but surprisingly not for microglia proliferation. Hence, CSF1-mediated hypersensitivity engaged DAP12-dependent upregulation of pain-related genes (including the gene encoding P2X4 receptor) and DAP12-independent microglia proliferation 15.
Figure 1. Novel neuro-glial mechanisms of neuropathic pain.
( A) Schematic innervation of the dorsal horn spinal cord (dashed box) by DRG neurons. Neuropathy was induced by SNI or ScNI in mice or rats. ( B) Neuro-glial mechanisms in the dorsal horn spinal cord (dashed box in A): (i) CSF1 released from injured DRG neurons activates CSF1 receptor (CSF1R) in microglia 15, 16. (ii) TMEM16F in microglia mediates their phagocytosis of GABAergic-interneuron terminals 17. (iii) Whether monocytes infiltrate spinal cord and their relative role versus resident microglia are still unclear 22, 26, 28, 29, 32. (iv) Upregulated connexin 43 in astrocytes triggers the secretion of CXCL1 to activate CXCR2 on DRG neuron central terminals and spinal interneurons 33. (v) Spinal neuron-derived CXCL13 activates CXCR5 in astrocytes 34. (vi) MCP-3 secreted from astrocytes activates CXCR2 in microglia 74. CSF1, colony-stimulating factor 1; CXCR, chemokine CXC receptor; DRG, dorsal root ganglion; GABA, γ-aminobutyric acid; MCP-3, monocyte chemotactic protein-3; ScNI, sciatic nerve injury; SNI, spinal nerve injury.
As phagocytic cells, microglia remove apoptotic tissue following injury. New work proposes that a Ca 2+-dependent channel, TMEM16F, in microglia mediates their phagocytic activity, which leads to the shortening of GABAergic interneuron terminals, subsequent loss of GABA-mediated inhibitory transmission, and neuropathic pain 17 ( Figure 1Bii). The concept is in line with recently postulated loss of synaptic contacts between spinal parvalbumin-expressing inhibitory interneurons and excitatory interneurons leading to their disinhibition 18, and this represents an alternative mechanism to the controversial apoptosis of inhibitory interneurons 19, 20. Selective depletion of microglial TMEM16F diminished microglia-mediated phagocytosis, preserved neuronal GABA immunoreactivity, and abolished hypersensitivity following sciatic nerve injury (ScNI) in mice. Thus, microglia phagocytic activity may contribute to neuropathic pain 17.
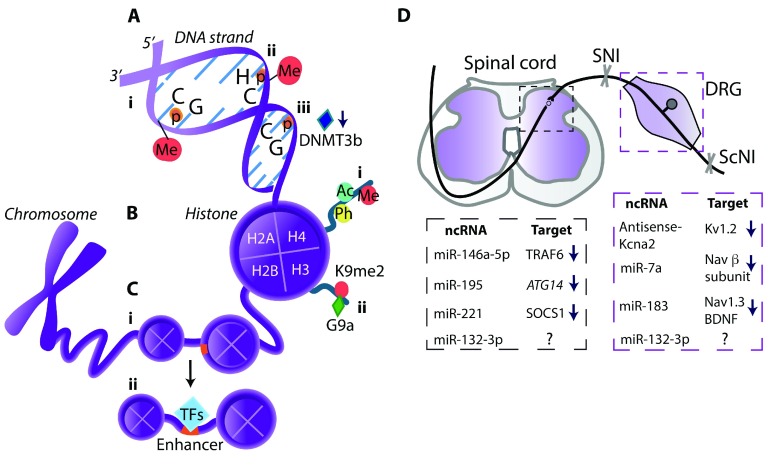
Mechanisms underlying neuropathic pain chronification gain increasing attention. This was recently addressed by analyzing latent enhancers. Enhancers are DNA regulatory regions that bind transcription factors to influence gene expression 21. Following ScNI in mice, the increased binding at enhancers close to genes encoding complement system member C4b and chemokines CCL5 and CCL12 was identified in spinal microglia ( Figure 2C). Since these effects lasted for a relatively long time (4 weeks) after the injury, when most transcriptional changes returned to normal, the enhancer profile alterations may account for pain persistency 22. Intriguingly, the study made several observations contradicting previous findings, including the absence of the gene encoding BDNF considered a crucial microglia–neuronal signaling molecule 13, 14, 23 (see also above). Furthermore, in contrast to previous data 24– 27, there was no spinal cord infiltration by blood-derived monocytes/macrophages and T lymphocytes, and thus resident microglia were regarded as the major spinal immune cells (99%) orchestrating neuropathic pain. Suggested reasons for discrepancies include the use of unspecific cell markers in previous studies versus microglia-specific purinergic receptor P2Y12 antibody 22.
Figure 2. Epigenetic modifications in neuropathic pain.
( A) DNA methylation (Me) occurs mostly at the cytosine of CpG sites 55, 61 (i) but can also happen at the cytosine of CpH sites (H = A, T, or C) 67 (ii). (iii) DNA methyltransferase 3b (DNMT3b) downregulation leads to the demethylation at the CpG sites in the purinergic P2X3 receptor gene promoter 70. ( B) Histone modifications: (i) DNA is wrapped around histone octamers that consist of histones H2A, H2B, H3, and H4. Histone tails, particularly on H3 and H4, can be acetylated (Ac), phosphorylated (Ph), or Me; (ii) Dimethyltransferase G9a mediates dimethylation (me2) of lysine 9 (K9me2) on histone H3 of the promoters of genes encoding K + channels 72 or MOR 73. ( C) Enhancers (orange) are non-coding DNA regions that can be activated by local nucleosome remodeling and binding of transcription factors (TFs), thereby influencing gene expression 21: (i) An inactive enhancer is packed in compact chromatin and cannot be bound by TFs. (ii) Following nerve injury, increased TF binding at spinal microglia enhancers was identified for genes encoding complement system member C4b and chemokines CCL5 and CCL12 [22]. ( D) ncRNAs and their targets in the spinal cord and DRG (listed in the respective dashed boxes) involved in neuropathic pain following SNI or ScNI in mice or rats. ncRNAs include analgesic miRNAs (miR-7a, miR-183, miR-146a-5p) 80– 82 and pain-promoting long non-coding RNA (Kcna2 antisense RNA) 79 or miRNAs (miR-195, miR-221, miR-132-3p) 83– 85. ↓, downregulation; ↑, upregulation; ?, unidentified target; ATG14, gene encoding protein that facilitates autophagy; BDNF, brain-derived neurotrophic factor; CpG, cytosine–phosphate–guanine dinucleotide; DRG, dorsal root ganglion; Kv, voltage-gated potassium channel; miRNA, microRNA; MOR, µ-opioid receptor; Nav, voltage-gated sodium channel; ncRNA, non-coding RNA; ScNI, sciatic nerve injury; SNI, spinal nerve injury; SOCS1, suppressor of cytokine signaling 1, a protein suppressing cytokine signaling; TRAF6, tumor necrosis factor (TNF) receptor associated factor 6, an adapter protein mediating TNF-α signaling.
The exclusive role of resident spinal microglia was explored by Tashima et al., who proposed that irradiation and bone marrow transplantation, often employed in earlier studies, possibly impair the blood–spinal cord barrier, which represents an experimental artifact underlying monocyte/macrophage extravasation in the spinal cord 28. This was supported by the absence of spinal cord infiltration by circulating monocytes by using less toxic irradiation protocols, parabiotic mice, and mice with genetically labeled microglia. Also, Gu et al. postulated preferential involvement of resident microglia by employing P2Y12 immunostaining and double transgenic mice in which microglia and monocytes were differently labeled 29. Importantly, inhibition of microgliosis by an anti-mitotic drug diminished hypersensitivity following spinal nerve injury (SNI). However, the emphasis on microglia proliferation contradicts findings on the dissociation between microgliosis and pain 30, 31. Indeed, the upregulation of purinergic receptors and BDNF, and p38 mitogen-activated protein kinase phosphorylation in spinal microglia, but not their morphological alterations, are considered critical for neuropathic pain 27. Nevertheless, despite proposing the sole role of resident microglia 29, the concurrent study of this group claims the synergistic action of microglia and monocytes 32. Depletion of chemokine CX3C receptor (R) 1-expressing cells (including spinal microglia and peripheral monocytes/macrophages) attenuated SNI-induced hypersensitivity in mice. Postulated selective microglia depletion (using diphtheria toxin receptor transgenic mice) produced only transient analgesia. However, this transgenic approach caused compensatory elevations in astrocyte numbers, which could account for the re-emergence of hypersensitivity. Additionally, clodronate liposome-induced depletion of blood monocytes did not attenuate hypersensitivity, suggesting that they were not involved; monocytes were not detected in the spinal cord either. Furthermore, liposome treatment caused a compensatory increase in a circulating mononuclear cell subset 32. Together, these studies yielded conflicting findings, and the relative contribution of resident microglia versus astrocytes and infiltrating monocytes ( Figure 1Biii) and the relevance of microglia proliferation to neuropathic pain remain inconclusive.
Astrocytes
Following peripheral nerve damage, spinal astrocytes proliferate and produce pro-inflammatory cytokines (for example, interleukin-1β [IL-1β]), matrix metalloproteinases, and chemokines CCL2, CCL7, and CXCL1 27, 30. An additional mechanism involves connexin 43, a protein which forms gap junctions and exerts hemichannel activity. Thus, (presumably) microglia-derived tumor necrosis factor-α (TNF-α) selectively upregulated connexin 43 in astrocytes and triggered the secretion of CXCL1 to activate CXCR2 on neurons ( Figure 1Biv), which resulted in hypersensitivity following ScNI in mice. Inhibition of this pathway, including astrocyte depletion, attenuated the hypersensitivity. Since these effects occurred at later neuropathy stages, targeting astrocytic connexin 43 may be therapeutically more promising than interfering with early, microglia-mediated responses 33. In addition to astroglial–neuronal interactions 33, novel neuronal–astroglial communication has been proposed; it involves neuron-derived chemokine CXCL13, which activates CXCR5 in astrocytes 34 ( Figure 1Bv). Blocking the CXCL13/CXCR5 pathway suppressed SNI-induced hypersensitivity in mice. Nevertheless, the neuronal–astrocytic interaction involving CXCL13/CXCR5 will need confirmation since CXCL13 was also found in microglia and macrophages. Furthermore, CXCR5 was also expressed in spinal neurons 34 and thus direct neuronal CXCR5 activation, without astrocytic contribution, cannot be excluded.
Astrocytes are not electrically excitable, but they induce metabotropic glutamate receptor (mGluR)-mediated Ca 2+ oscillations and synaptogenic thrombospondin 1 (TSP-1) release, which are involved in neuronal circuit formation during development but typically are downregulated in adulthood. New work elegantly shows that the ScNI resulted in the re-emergence of mGluR5 signaling in cortical astrocytes in the adult mouse brain. Nerve injury enhanced levels of neuron-derived glutamate, which activated mGluR5 in astrocytes in the cortex. This elicited Ca 2+ transients and the release of astrocytic TSP-1, which in turn activated neuronal α2δ1 receptors to induce new synapse formation. Blocking astrocytic Ca 2+ elevation or synaptic formation (using α2δ1 blocker gabapentin) suppressed neuropathy-triggered hypersensitivity 35. These findings are interesting since gabapentin is used clinically. However, as some patients benefit from the treatment, the analgesic effects are moderate and side effects (dizziness, somnolence) may lead to therapy termination 36– 38. Hence, indications of novel therapeutic targets resulting from this research still need to be defined.
Oligodendrocytes
Classically, oligodendrocytes produce myelin for axonal insulation in the CNS, and their role in pain modulation is just emerging. Genetic oligodendrocyte ablation in naïve mice (employing a diphtheria toxin receptor system) induced mechanical and cold but not heat hypersensitivity. This was associated with axonal degeneration and swelling (but not neuronal loss) in the spinal cord and was independent of demyelination, lymphocyte infiltration, and gliosis. In summary, oligodendrocyte loss induced symptoms of central neuropathic pain, supporting their requirement for normal somatosensation 39.
On the other hand, oligodendrocytes may contribute to the generation of neuropathic pain by secretion of IL-33, which can activate its receptors in microglia and astrocytes (and possibly neurons), leading to the release of other pro-inflammatory cytokines (TNF-α and IL-1β). This has been suggested in a mouse ScNI model and is based mostly on the IL-33 immunohistochemical detection in oligodendrocytes; however, the direct contribution of oligodendrocytes to hypersensitivity was not demonstrated 40.
Glia and sex differences
Pain-related sex differences are receiving increased attention 41– 43. An intriguing recent suggestion is that microglia play a role in males only but that in females T lymphocytes infiltrating the spinal cord mediate neuropathic pain 44. This was supported by the reversal of ScNI-induced hypersensitivity by glia inhibitors (minocycline, fluorocitrate, and propentofylline) in male, but not female, mice. Hence, the classic pathway involving P2X4–p38–BDNF and disinhibition of spinal GABA A-mediated transmission contributed to neuropathic pain in males only. In females, T lymphocytes expressing peroxisome proliferator-activated receptor-γ (PPAR-γ) drove the hypersensitivity. Importantly, microglial hypertrophy and proliferation did not differ between sexes 31, 44. The male-specific p38 activation and signaling in spinal microglia, independent of proliferation, were also described in mice and rats following nerve damage in another study 45. These sex-related differences are suggested to be spinal cord specific. Attenuation of neuropathy-induced hypersensitivity in female mice following systemic minocycline 46 or in both sexes after systemic p38 inhibitor 45 was attributed to actions on peripheral immune cells 45, 47.
Nevertheless, other authors found neuropathy-induced upregulation of microglia and astrocytes expressing phosphorylated p38 also in female mice 48, indicating that spinal p38 activation is not always male specific 45. Additionally, spinally administered PPAR-γ agonist attenuated hypersensitivity in male rats 49, contrasting the predominant role of PPAR-γ in female mice 44. Furthermore, other studies found no differences between female and male mice in microglia-mediated hypersensitivity 17, 32. Future research will need to clarify whether the sex-related role of glia is restricted to selected mechanisms or models of neuropathic pain and how these findings are relevant to clinical pain. Clearly, there is increasing pressure to consider sex in preclinical research. Whereas many scientists are concerned, this will double the number of animals used, an intriguing opinion claims that this is unjustified since “there is no need to use enough animals to ensure that there is sufficient statistical power to detect all quantitative sex differences” 50. Although statistical significance should not be overestimated, the quality of statistical analysis is a strong focus of current recommendations 51– 54 and in many cases is strictly required. For example, according to German regulations, the sample size estimation and statistical power analysis are prerequisites for study approval by ethics committees.
Epigenetic modifications and neuropathic pain
Epigenetic modifications, which include DNA methylation, histone modifications, and expression of non-coding RNAs (ncRNAs), lead to changes in gene expression but do not alter the actual DNA sequence. They are induced by stress, tissue damage, and disease and can affect both physiological and pathological processes 55– 57. During pain progression, the activity of genes encoding ion channels, receptors, neurotransmitters, and modulators is enhanced or suppressed abnormally 58. Here, we address recent findings on epigenetic modifications along the neuro-immune pain pathways.
DNA methylation
DNA is mostly methylated at the cytosine of cytosine–phosphate–guanine (CpG) dinucleotide ( Figure 2Ai). CpG sites are distributed throughout the DNA sequence, including promoters, gene bodies, and transposable elements 55, 59. DNA methylation is tissue/cell type specific and forms differentially methylated regions (DMRs), which reflect tissue-specific function 60. Epigenome analysis of monozygotic twins who have almost identical genetic material but different methylation profiles might help in revealing DMRs associated with low or high pain sensitivity. Genome-wide DNA methylation analysis in blood leukocytes revealed that individuals with lower heat pain thresholds had increased DNA methylation in the promoter of the gene encoding transient receptor potential ankyrin 1 (TRPA1). In skin biopsies, the TRPA1 gene was upregulated in individuals with higher pain thresholds 61. Similarly, burning pain sensation was correlated with TRPA1 gene hypermethylation, and the hypermethylation was associated with TRPA1 mRNA downregulation in blood cells of a mixed group of patients with back pain and post-herpetic neuralgia 62. These findings are intriguing since, in mammals, nociceptor TRPA1 senses noxious stimuli and may be activated by cold but not heat 63, 64. Interestingly, another study found that high DMR numbers (72%) overlapped between blood T lymphocytes and prefrontal cortex following ScNI in rats 65. This suggests the feasibility of DNA methylation changes in T lymphocytes as non-invasive biomarkers of neuropathic pain. Nevertheless, only 43% of the promoters were altered in the same direction in both tissues 65 and thus careful tissue/cell type-specific analysis of methylation patterns is needed to adequately correlate them with pain intensity. Additionally, precise DNA sequence assessment may be important; for example, methylated CpGs are also located in intergenic regions, where they have no effect on gene activity. Moreover, although CpG methylation in the promoters typically represses transcription, methylation in the gene bodies can increase mRNA expression 66. Though not yet addressed in pain conditions, methylation also occurs at the CpH sites (H = A, T, or C) ( Figure 2 Aii) and was shown to be the dominant form of DNA methylation in human and mouse frontal cortex neurons 67. Notably, recent technical advances enable the generation of high-resolution epigenetic libraries and precise epigenetic analysis 68, 69.
Pain can also be associated with DNA demethylation. Demethylation in the purinergic P2X3 receptor gene promoter ( Figure 2Aiii) and its binding of transcription factor p65 resulted in P2X3 receptor protein upregulation in the DRG and diabetic neuropathic pain in rats. As these data are mechanistically interesting, their therapeutic relevance seems less clear since interfering with this pathway by blocking p65 appeared to be less effective than the P2X3 receptor antagonist treatment in producing analgesia 70.
Histone modifications
Genomic DNA is wrapped around histone proteins, which can be post-translationally modified by methylation, acetylation, or phosphorylation 56 ( Figure 2Bi). Generally, histone acetylation and phosphorylation increase transcription whereas histone methylation can either silence or increase gene expression, depending on the methylated lysine 71. Laumet et al. 72 examined histone methylation at genes encoding voltage-gated (Kv1.4, Kv4.2, and Kv7.2) and Ca 2+-activated K + channels; opening of these channels results in decreased neuronal excitability. Following SNI in rats, while DNA methylation was unaltered, the dimethyltransferase G9a-mediated lysine 9 dimethylation on histone H3 (H3K9me2) of the promoters of genes encoding K + channels in the DRG was increased ( Figure 2Bii), resulting in the channels’ downregulation and correlating with hypersensitivity. Consistently, the G9a inhibition normalized the expression of K + channels and attenuated hypersensitivity 72. The G9a was also shown to methylate the promoter of the gene encoding µ-opioid receptor (MOR), which is activated by opioids such as morphine. SNI-induced downregulation of MOR gene and protein was associated with highly enriched levels of G9a product H3K9me2 in the MOR gene promoter. The G9a blockers restored MOR expression and enhanced morphine-induced analgesia. Additionally, in mice with G9a conditional knockout in the DRG neurons, neuropathy did not reduce MOR levels and morphine-induced analgesia 73. In summary, the G9a can silence several K + channels and MOR on the transcriptional level. Blocking G9a activity may improve the efficacy of opioid analgesics and prevent the chronification of neuropathic pain.
Histone modifications can also influence astrocyte–microglia interactions. ScNI in mice elevated the expression of pro-inflammatory cytokine monocyte chemotactic protein-3 (MCP-3) (also known as chemokine CCL7) exclusively in spinal astrocytes ( Figure 1Bvi). This was associated with the decreased histone methylation on MCP-3 promoter. MCP-3 application activated microglia, which was reversed by MCP-3 antibody, resulting in diminished neuropathy-induced hypersensitivity. Thus, MCP-3 promoter histone hypomethylation-mediated microglia activation by MCP-3 secreted from astrocytes in the spinal cord may represent a new target for neuropathic pain treatment 74.
Non-coding RNAs
ncRNAs are RNAs that do not encode proteins. They are classified according to their size and function, and the major members include microRNAs (miRNAs) and long ncRNAs (lncRNAs). miRNAs contain 20 to 24 nucleotides and post-transcriptionally suppress protein expression by mRNA degradation or inhibition of translation. lncRNAs are longer than 200 nucleotides and can interfere with transcription and post-transcriptional processes by interacting with DNA, RNA, or protein 56, 75. There is abundant evidence on pain modulation by miRNAs, but the role for lncRNAs is much less known 76– 78. Below, we discuss recent research exploring the targets and functional relevance of ncRNAs in neuropathy.
A single study examining lncRNAs in neuropathic pain found that increased expression of a lncRNA named Kcna2 antisense RNA (as it is complementary to Kcna2 mRNA which encodes the Kv1.2 channel) led to Kcna2 mRNA downregulation in DRG neurons following SNI in rats ( Figure 2D). Blocking this lncRNA restored Kcna2 expression and attenuated SNI-triggered hypersensitivity 79.
There are more data on the role of both analgesic and pain-inducing miRNAs ( Figure 2D). miR-7a suppressed DRG neuron excitability by targeting the β2 subunit of voltage-gated Na + (Nav) channels after SNI in rats. SNI-induced miR-7a downregulation was associated with the β2 subunit protein upregulation in injured DRG. Locally injected virus expressing miR-7a diminished β2 subunit expression and alleviated hypersensitivity 80. miR-183 exerted analgesia by targeting Nav1.3 and BDNF in DRG. SNI-induced miR-183 downregulation was restored by spinal viral miR-183 delivery, resulting in decreased expression of Nav1.3 and BDNF mRNAs in DRG and diminished hypersensitivity in rats 81. Also, spinally applied miR-146a-5p mimic (a synthetic double-stranded RNA that mimics endogenous miR-146a-5p) reduced SNI-induced hypersensitivity in mice, probably by suppressing TNF receptor-associated factor 6, an adapter protein mediating signaling of TNF-α 82. Together, these studies indicate the restoration of analgesic miR-7a, miR-183, and miR-146a-5p as potential neuropathic pain treatments.
Alternatively, beneficial effects may be achieved by inhibition of pain-inducing miRNAs ( Figure 2D). miR-195 was upregulated in the spinal cord microglia, which was associated with decreased microglial autophagy (self-degradation) and enhanced SNI-induced hypersensitivity in rats. Consistently, spinal miR-195 inhibition enhanced microglial autophagy and decreased hypersensitivity. The miR-195-mediated actions were suggested to result from targeting gene ATG14 which regulates autophagy, since miR-195 overexpression downregulated ATG14 and inhibited autophagy. Thus, promoting microglia autophagy via miR-195 inhibition may attenuate neuropathic pain 83. Analgesia was also produced by inhibition of miR-221, which resulted from the upregulation of suppressor of cytokine signaling 1, a protein suppressing cytokine signaling. miR-221 was upregulated in the spinal cord microglia following ScNI. Spinal application of miR-221 inhibitor downregulated pro-inflammatory cytokines and diminished neuropathy-induced hypersensitivity 84. Pain-promoting actions of miR-132-3p were addressed in both neuropathic pain patients and rats. miR-132-3p was upregulated in blood leukocytes and peripheral nerve biopsies of neuropathic pain patients. A similar effect was found in the spinal cord and DRG (but not nerve) in rats with ScNI, and spinal miR-132-3p antagonism reversed hypersensitivity. However, the miR-132-3p target responsible for its pain-promoting effects remains elusive since, in contrast to the expected upregulation, it downregulated the expression of the glutamate receptor 85.
Open questions, opportunities, and challenges
Recent research resulted in interesting novel findings that considerably advance our understanding of neuropathic pain pathogenesis and offer numerous new intervention targets, including cytokines (CSF1) 15, 16; TMEM16F-mediated microglial phagocytosis 17; microglial enhancers 22; astrocytic connexin 43, CXCL13/CXCR5, or cortical synaptic plasticity 33– 35; oligodendrocyte-based signaling 40; G9a-mediated histone methylation 72, 73; and many ncRNAs 79– 85. The key questions are which of these effects will be replicated and tested in patients and will any of them prove clinically effective. Elaborated transgenic approaches help to address specific cell type or signaling pathways but may result in compensatory changes. This is particularly true when manipulating the immune system 32, 86, whose role is to rapidly react to alterations in the body. Not surprisingly, several controversies emerged 22, 29, 32, and they certainly will stimulate further research. Additional remaining questions include the relative role of different glia types and of resident versus circulating leukocytes infiltrating the CNS, exploration and promotion of protective effects of glia, understanding human glia pathology, and the precise identification of epigenetic mechanisms. Given that chronic pain is mediated by a plethora of functionally redounding mediators and signaling pathways, the ability of a single epigenetic tool to modify multiple gene transcripts offers a possibility to target multiple pain mediators simultaneously and this has higher potential for successful pain treatment compared with targeting single molecules. On the other hand, this multimodal feature of epigenetic mechanisms carries risks of compensatory changes and side effects. An additional challenge is to achieve sufficient and tissue-relevant delivery of epigenetic modulators without inducing off-target actions.
Previous clinical trials, including those which tested glial modulators, have failed to produce sufficient pain relief in patients with neuropathic conditions 13, 30, 87. It is essential to acknowledge that chronic pain is a biopsychosocial experience and that not only biologic but also psychological and social factors result in pain persistence and illness behavior. A search for better drug targets is needed, but pharmacological treatment alone will not be sufficient; interdisciplinary management combining pharmacologic and non-pharmacologic approaches, including physical, cognitive behavioral, meditative, and occupational therapies, needs to be considered for chronic pain treatment 88– 93.
Abbreviations
ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CNS, central nervous system; CpG, cytosine–phosphate–guanine dinucleotide; CSF1, colony-stimulating factor 1; CXCR, chemokine CXC receptor; DMR, differentially methylated region; DRG, dorsal root ganglion; GABA, γ-aminobutyric acid; GABA A receptor, γ-aminobutyric acid A receptor; IL, interleukin; Kv, voltage-gated potassium channel; lncRNA, long non-coding RNA; MCP-3, monocyte chemotactic protein-3; mGluR, metabotropic glutamate receptor; miRNA, microRNA; MOR, µ-opioid receptor; Nav, voltage-gated sodium channel; ncRNA, non-coding RNA; PPAR-γ, peroxisome proliferator-activated receptor-γ; ScNI, sciatic nerve injury; SNI, spinal nerve injury; TNF-α, tumor necrosis factor-α; TRPA1, transient receptor potential ankyrin 1; TSP-1, thrombospondin 1.
Acknowledgments
We thank Philip Stötzner for critical reading of the manuscript.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Makoto Tsuda, Graduate School of Pharmaceutical Sciences, Kyushu University, Fukuoka, Japan
Venetia Zachariou, Department of Neuroscience, Friedman Brain Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Baron R: Mechanisms of disease: neuropathic pain--a clinical perspective. Nat Clin Pract Neurol. 2006;2(2):95–106. 10.1038/ncpneuro0113 [DOI] [PubMed] [Google Scholar]
- 2. Jensen TS, Finnerup NB: Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 2014;13(9):924–35. 10.1016/S1474-4422(14)70102-4 [DOI] [PubMed] [Google Scholar]
- 3. Finnerup NB, Sindrup SH, Jensen TS: Recent advances in pharmacological treatment of neuropathic pain. F1000 Med Rep. 2010;2:52. 10.3410/M2-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen SP, Mao J: Neuropathic pain: mechanisms and their clinical implications. BMJ. 2014;348:f7656. 10.1136/bmj.f7656 [DOI] [PubMed] [Google Scholar]
- 5. Tibbs GR, Posson DJ, Goldstein PA: Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol Sci. 2016;37(7):522–42. 10.1016/j.tips.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 6. Busserolles J, Tsantoulas C, Eschalier A, et al. : Potassium channels in neuropathic pain: advances, challenges, and emerging ideas. Pain. 2016;157(Suppl 1):S7–14. 10.1097/j.pain.0000000000000368 [DOI] [PubMed] [Google Scholar]
- 7. Austin PJ, Moalem-Taylor G: The neuro-immune balance in neuropathic pain: involvement of inflammatory immune cells, immune-like glial cells and cytokines. J Neuroimmunol. 2010;229(1–2):26–50. 10.1016/j.jneuroim.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 8. Machelska H: Dual peripheral actions of immune cells in neuropathic pain. Arch Immunol Ther Exp (Warsz). 2011;59(1):11–24. 10.1007/s00005-010-0106-x [DOI] [PubMed] [Google Scholar]
- 9. Mika J, Zychowska M, Popiolek-Barczyk K, et al. : Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716(1–3):106–19. 10.1016/j.ejphar.2013.01.072 [DOI] [PubMed] [Google Scholar]
- 10. McMahon SB, La Russa F, Bennett DL: Crosstalk between the nociceptive and immune systems in host defence and disease. Nat Rev Neurosci. 2015;16(7):389–402. 10.1038/nrn3946 [DOI] [PubMed] [Google Scholar]
- 11. Tsuda M, Inoue K: Neuron-microglia interaction by purinergic signaling in neuropathic pain following neurodegeneration. Neuropharmacology. 2016;104:76–81. 10.1016/j.neuropharm.2015.08.042 [DOI] [PubMed] [Google Scholar]
- 12. Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. : P2X 4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–83. 10.1038/nature01786 [DOI] [PubMed] [Google Scholar]
- 13. Grace PM, Hutchinson MR, Maier SF, et al. : Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14(4):217–31. 10.1038/nri3621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malcangio M: Microglia and chronic pain. Pain. 2016;157(5):1002–3. 10.1097/j.pain.0000000000000353 [DOI] [PubMed] [Google Scholar]
- 15. Guan Z, Kuhn JA, Wang X, et al. : Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19(1):94–101. 10.1038/nn.4189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Okubo M, Yamanaka H, Kobayashi K, et al. : Macrophage-Colony Stimulating Factor Derived from Injured Primary Afferent Induces Proliferation of Spinal Microglia and Neuropathic Pain in Rats. PLoS One. 2016;11(4):e0153375. 10.1371/journal.pone.0153375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Batti L, Sundukova M, Murana E, et al. : TMEM16F Regulates Spinal Microglial Function in Neuropathic Pain States. Cell Rep. 2016;15(12):2608–15. 10.1016/j.celrep.2016.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petitjean H, Pawlowski SA, Fraine SL, et al. : Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015;13(6):1246–57. 10.1016/j.celrep.2015.09.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Polgár E, Gray S, Riddell JS, et al. : Lack of evidence for significant neuronal loss in laminae I-III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111(1–2):144–50. 10.1016/j.pain.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 20. Scholz J, Broom DC, Youn DH, et al. : Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25(32):7317–23. 10.1523/JNEUROSCI.1526-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heinz S, Romanoski CE, Benner C, et al. : The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol. 2015;16(3):144–54. 10.1038/nrm3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Denk F, Crow M, Didangelos A, et al. : Persistent Alterations in Microglial Enhancers in a Model of Chronic Pain. Cell Rep. 2016;15(8):1771–81. 10.1016/j.celrep.2016.04.063 [DOI] [PubMed] [Google Scholar]
- 23. Coull JA, Beggs S, Boudreau D, et al. : BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438(7070):1017–21. 10.1038/nature04223 [DOI] [PubMed] [Google Scholar]
- 24. Cao L, DeLeo JA: CNS-infiltrating CD4 + T lymphocytes contribute to murine spinal nerve transection-induced neuropathic pain. Eur J Immunol. 2008;38(2):448–58. 10.1002/eji.200737485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Costigan M, Moss A, Latremoliere A, et al. : T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity. J Neurosci. 2009;29(46):14415–22. 10.1523/JNEUROSCI.4569-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Echeverry S, Shi XQ, Rivest S, et al. : Peripheral nerve injury alters blood-spinal cord barrier functional and molecular integrity through a selective inflammatory pathway. J Neurosci. 2011;31(30):10819–28. 10.1523/JNEUROSCI.1642-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ji RR, Xu ZZ, Gao YJ: Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13(7):533–48. 10.1038/nrd4334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tashima R, Mikuriya S, Tomiyama D, et al. : Bone marrow-derived cells in the population of spinal microglia after peripheral nerve injury. Sci Rep. 2016;6: 23701. 10.1038/srep23701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gu N, Peng J, Murugan M, et al. : Spinal Microgliosis Due to Resident Microglial Proliferation Is Required for Pain Hypersensitivity after Peripheral Nerve Injury. Cell Rep. 2016;16(3):605–14. 10.1016/j.celrep.2016.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alfonso Romero-Sandoval E, Sweitzer S: Nonneuronal central mechanisms of pain: glia and immune response. Prog Mol Biol Transl Sci. 2015;131:325–58. 10.1016/bs.pmbts.2014.11.007 [DOI] [PubMed] [Google Scholar]
- 31. Mapplebeck JC, Beggs S, Salter MW: Sex differences in pain: a tale of two immune cells. Pain. 2016;157(Suppl 1):S2–6. 10.1097/j.pain.0000000000000389 [DOI] [PubMed] [Google Scholar]
- 32. Peng J, Gu N, Zhou L, et al. : Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 2016;7: 12029. 10.1038/ncomms12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen G, Park CK, Xie RG, et al. : Connexin-43 induces chemokine release from spinal cord astrocytes to maintain late-phase neuropathic pain in mice. Brain. 2014;137(Pt 8):2193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang BC, Cao DL, Zhang X, et al. : CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5. J Clin Invest. 2016;126(2):745–61. 10.1172/JCI81950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim SK, Hayashi H, Ishikawa T, et al. : Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 2016;126(5):1983–97. 10.1172/JCI82859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cruccu G: Treatment of painful neuropathy. Curr Opin Neurol. 2007;20(5):531–5. 10.1097/WCO.0b013e328285dfd6 [DOI] [PubMed] [Google Scholar]
- 37. Moore RA, Wiffen PJ, Derry S, et al. : Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database Syst Rev. 2014; (4):CD007938. 10.1002/14651858.CD007938.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Finnerup NB, Attal N, Haroutounian S, et al. : Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–73. 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gritsch S, Lu J, Thilemann S, et al. : Oligodendrocyte ablation triggers central pain independently of innate or adaptive immune responses in mice. Nat Commun. 2014;5: 5472. 10.1038/ncomms6472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zarpelon AC, Rodrigues FC, Lopes AH, et al. : Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016;30(1):54–65. 10.1096/fj.14-267146 [DOI] [PubMed] [Google Scholar]
- 41. Greenspan JD, Craft RM, LeResche L, et al. : Studying sex and gender differences in pain and analgesia: a consensus report. Pain. 2007;132(Suppl 1):S26–45. 10.1016/j.pain.2007.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mogil JS: Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–66. 10.1038/nrn3360 [DOI] [PubMed] [Google Scholar]
- 43. Bartley EJ, Fillingim RB: Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 2013;111(1):52–8. 10.1093/bja/aet127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sorge RE, Mapplebeck JC, Rosen S, et al. : Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–3. 10.1038/nn.4053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taves S, Berta T, Liu DL, et al. : Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. 10.1016/j.bbi.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bastos LF, Godin AM, Zhang Y, et al. : A minocycline derivative reduces nerve injury-induced allodynia, LPS-induced prostaglandin E2 microglial production and signaling via toll-like receptors 2 and 4. Neurosci Lett. 2013;543:157–62. 10.1016/j.neulet.2013.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sorge RE, Totsch SK: Sex Differences in Pain. J Neurosci Res. 2016. 10.1002/jnr.23841 [DOI] [PubMed] [Google Scholar]
- 48. Vacca V, Marinelli S, Pieroni L, et al. : Higher pain perception and lack of recovery from neuropathic pain in females: a behavioural, immunohistochemical, and proteomic investigation on sex-related differences in mice. Pain. 2014;155(2):388–402. 10.1016/j.pain.2013.10.027 [DOI] [PubMed] [Google Scholar]
- 49. Griggs RB, Donahue RR, Morgenweck J, et al. : Pioglitazone rapidly reduces neuropathic pain through astrocyte and nongenomic PPARγ mechanisms. Pain. 2015;156(3):469–82. 10.1097/01.j.pain.0000460333.79127.be [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mogil JS: Perspective: Equality need not be painful. Nature. 2016;535(7611):S7. 10.1038/535S7a [DOI] [PubMed] [Google Scholar]
- 51. Kilkenny C, Browne WJ, Cuthill IC, et al. : Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8(6):e1000412. 10.1371/journal.pbio.1000412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Landis SC, Amara SG, Asadullah K, et al. : A call for transparent reporting to optimize the predictive value of preclinical research. Nature. 2012;490(7419):187–91. 10.1038/nature11556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sutherland WJ, Spiegelhalter D, Burgman MA: Policy: Twenty tips for interpreting scientific claims. Nature. 2013;503(7476):335–7. 10.1038/503335a [DOI] [PubMed] [Google Scholar]
- 54. Colquhoun D: An investigation of the false discovery rate and the misinterpretation of p-values. R Soc Open Sci. 2014;1(3):140216. 10.1098/rsos.140216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smith ZD, Meissner A: DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14(3):204–20. 10.1038/nrg3354 [DOI] [PubMed] [Google Scholar]
- 56. Descalzi G, Ikegami D, Ushijima T, et al. : Epigenetic mechanisms of chronic pain. Trends Neurosci. 2015;38(4):237–46. 10.1016/j.tins.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bai G, Ren K, Dubner R: Epigenetic regulation of persistent pain. Transl Res. 2015;165(1):177–99. 10.1016/j.trsl.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. LaCroix-Fralish ML, Austin JS, Zheng FY, et al. : Patterns of pain: meta-analysis of microarray studies of pain. Pain. 2011;152(8):1888–98. 10.1016/j.pain.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 59. Lander ES, Linton LM, Birren B, et al. : Initial sequencing and analysis of the human genome. Nature. 2001;409(6822):860–921. 10.1038/35057062 [DOI] [PubMed] [Google Scholar]
- 60. Ziller MJ, Gu H, Muller F, et al. : Charting a dynamic DNA methylation landscape of the human genome. Nature. 2013;500(7463):477–81. 10.1038/nature12433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bell JT, Loomis AK, Butcher LM, et al. : Differential methylation of the TRPA1 promoter in pain sensitivity. Nat Commun. 2014;5: 2978. 10.1038/ncomms3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sukenaga N, Ikeda-Miyagawa Y, Tanada D, et al. : Correlation Between DNA Methylation of TRPA1 and Chronic Pain States in Human Whole Blood Cells. Pain Med. 2016;17(10):1906–1910, pii: pnv088. 10.1093/pm/pnv088 [DOI] [PubMed] [Google Scholar]
- 63. Kwan KY, Allchorne AJ, Vollrath MA, et al. : TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–89. 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 64. Caspani O, Heppenstall PA: TRPA1 and cold transduction: an unresolved issue? J Gen Physiol. 2009;133(3):245–9. 10.1085/jgp.200810136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Massart R, Dymov S, Millecamps M, et al. : Overlapping signatures of chronic pain in the DNA methylation landscape of prefrontal cortex and peripheral T cells. Sci Rep. 2016;6: 19615. 10.1038/srep19615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laird PW: Principles and challenges of genomewide DNA methylation analysis. Nat Rev Genet. 2010;11(3):191–203. 10.1038/nrg2732 [DOI] [PubMed] [Google Scholar]
- 67. Lister R, Mukamel EA, Nery JR, et al. : Global epigenomic reconfiguration during mammalian brain development. Science. 2013;341(6146): 1237905. 10.1126/science.1237905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lara-Astiaso D, Weiner A, Lorenzo-Vivas E, et al. : Immunogenetics. Chromatin state dynamics during blood formation. Science. 2014;345(6199):943–9. 10.1126/science.1256271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Brind'Amour J, Liu S, Hudson M, et al. : An ultra-low-input native ChIP-seq protocol for genome-wide profiling of rare cell populations. Nat Commun. 2015;6: 6033. 10.1038/ncomms7033 [DOI] [PubMed] [Google Scholar]
- 70. Zhang HH, Hu J, Zhou YL, et al. : Promoted Interaction of Nuclear Factor-κB With Demethylated Purinergic P2X3 Receptor Gene Contributes to Neuropathic Pain in Rats With Diabetes. Diabetes. 2015;64(12):4272–84. 10.2337/db15-0138 [DOI] [PubMed] [Google Scholar]
- 71. Géranton SM, Tochiki KK: Regulation of gene expression and pain states by epigenetic mechanisms. Prog Mol Biol Transl Sci. 2015;131:147–83. 10.1016/bs.pmbts.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 72. Laumet G, Garriga J, Chen SR, et al. : G9a is essential for epigenetic silencing of K + channel genes in acute-to-chronic pain transition. Nat Neurosci. 2015;18(12):1746–55. 10.1038/nn.4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhang Y, Chen SR, Laumet G, et al. : Nerve Injury Diminishes Opioid Analgesia through Lysine Methyltransferase-mediated Transcriptional Repression of μ-Opioid Receptors in Primary Sensory Neurons. J Biol Chem. 2016;291(16):8475–85. 10.1074/jbc.M115.711812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Imai S, Ikegami D, Yamashita A, et al. : Epigenetic transcriptional activation of monocyte chemotactic protein 3 contributes to long-lasting neuropathic pain. Brain. 2013;136(Pt 3):828–43. [DOI] [PubMed] [Google Scholar]
- 75. Chen LL: Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci. 2016;41(9):761–72. 10.1016/j.tibs.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 76. Kress M, Huttenhofer A, Landry M, et al. : microRNAs in nociceptive circuits as predictors of future clinical applications. Front Mol Neurosci. 2013;6:33. 10.3389/fnmol.2013.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bali KK, Kuner R: Noncoding RNAs: key molecules in understanding and treating pain. Trends Mol Med. 2014;20(8):437–48. 10.1016/j.molmed.2014.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sakai A, Suzuki H: Emerging roles of microRNAs in chronic pain. Neurochem Int. 2014;77:58–67. 10.1016/j.neuint.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 79. Zhao X, Tang Z, Zhang H, et al. : A long noncoding RNA contributes to neuropathic pain by silencing Kcna2 in primary afferent neurons. Nat Neurosci. 2013;16(8):1024–31. 10.1038/nn.3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sakai A, Saitow F, Miyake N, et al. : miR-7a alleviates the maintenance of neuropathic pain through regulation of neuronal excitability. Brain. 2013;136(Pt 9):2738–50. [DOI] [PubMed] [Google Scholar]
- 81. Lin CR, Chen KH, Yang CH, et al. : Intrathecal miR-183 delivery suppresses mechanical allodynia in mononeuropathic rats. Eur J Neurosci. 2014;39(10):1682–9. 10.1111/ejn.12522 [DOI] [PubMed] [Google Scholar]
- 82. Lu Y, Cao DL, Jiang BC, et al. : MicroRNA-146a-5p attenuates neuropathic pain via suppressing TRAF6 signaling in the spinal cord. Brain Behav Immun. 2015;49:119–29. 10.1016/j.bbi.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 83. Shi G, Shi J, Liu K, et al. : Increased miR-195 aggravates neuropathic pain by inhibiting autophagy following peripheral nerve injury. Glia. 2013;61(4):504–12. 10.1002/glia.22451 [DOI] [PubMed] [Google Scholar]
- 84. Xia L, Zhang Y, Dong T: Inhibition of MicroRNA-221 Alleviates Neuropathic Pain Through Targeting Suppressor of Cytokine Signaling 1. J Mol Neurosci. 2016;59(3):411–20. 10.1007/s12031-016-0748-1 [DOI] [PubMed] [Google Scholar]
- 85. Leinders M, Uceyler N, Pritchard RA, et al. : Increased miR-132-3p expression is associated with chronic neuropathic pain. Exp Neurol. 2016;283(Pt A):276–86. 10.1016/j.expneurol.2016.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ghasemlou N, Chiu IM, Julien JP, et al. : CD11b +Ly6G - myeloid cells mediate mechanical inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 2015;112(49):E6808–17. 10.1073/pnas.1501372112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kissin I: Scientometrics of drug discovery efforts: pain-related molecular targets. Drug Des Devel Ther. 2015;9:3393–404. 10.2147/DDDT.S85633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stein C, Kopf A: Anesthesia and treatment of chronic pain. In: Miller’s Anesthesia 7th edition. Edited by Miller RD. Philadelphia: Churchill Livingstone;2010;1797–818. Reference Source [Google Scholar]
- 89. Makris UE, Abrams RC, Gurland B, et al. : Management of persistent pain in the older patient: a clinical review. JAMA. 2014;312(8):825–36. 10.1001/jama.2014.9405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cherkin DC, Sherman KJ, Balderson BH, et al. : Effect of Mindfulness-Based Stress Reduction vs Cognitive Behavioral Therapy or Usual Care on Back Pain and Functional Limitations in Adults With Chronic Low Back Pain: A Randomized Clinical Trial. JAMA. 2016;315(12):1240–9. 10.1001/jama.2016.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jacob JA: As Opioid Prescribing Guidelines Tighten, Mindfulness Meditation Holds Promise for Pain Relief. JAMA. 2016;315(22):2385–7. 10.1001/jama.2016.4875 [DOI] [PubMed] [Google Scholar]
- 92. Macfarlane GJ, Kronisch C, Dean LE, et al. : EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2016; pii: annrheumdis-2016-209724. 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 93. Hilton L, Hempel S, Ewing BA, et al. : Mindfulness Meditation for Chronic Pain: Systematic Review and Meta-analysis. Ann Behav Med. 2016. 10.1007/s12160-016-9844-2 [DOI] [PMC free article] [PubMed] [Google Scholar]