Abstract
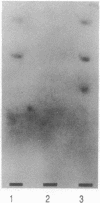
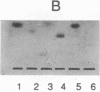
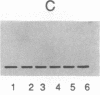
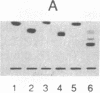
This report demonstrates that galactosyl ceramide (GalCer) or a molecule derived from it may serve as an alternative receptor for human immunodeficiency virus in the nervous system. Recombinant gp120, an envelope glycoprotein of human immunodeficiency virus type 1, specifically binds to GalCer and its derivatives. This specificity was studied by inhibiting binding of radioiodinated gp120 to GalCer with antibodies to GalCer, antibodies to gp120, and an excess of unlabeled gp120. Binding activity was also removed by absorbing gp120 with liposomes containing GalCer. In addition, studies using natural and semisynthetic lipids indicate that the linkage between galactose and ceramide is essential for binding. The significance of an alternative receptor for human immunodeficiency virus in the nervous system is discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger J. R., Sheremata W. A., Resnick L., Atherton S., Fletcher M. A., Norenberg M. Multiple sclerosis-like illness occurring with human immunodeficiency virus infection. Neurology. 1989 Mar;39(3):324–329. doi: 10.1212/wnl.39.3.324. [DOI] [PubMed] [Google Scholar]
- Bhat S., Silberberg D. H. Oligodendrocyte cell adhesion molecules are related to neural cell adhesion molecule (N-CAM). J Neurosci. 1986 Nov;6(11):3348–3354. doi: 10.1523/JNEUROSCI.06-11-03348.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi F., Fuerstenberg S., Gidlund M., Asjö B., Fenyö E. M. Infection of brain-derived cells with the human immunodeficiency virus. J Virol. 1987 Apr;61(4):1244–1247. doi: 10.1128/jvi.61.4.1244-1247.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Collman R., Godfrey B., Cutilli J., Rhodes A., Hassan N. F., Sweet R., Douglas S. D., Friedman H., Nathanson N., Gonzalez-Scarano F. Macrophage-tropic strains of human immunodeficiency virus type 1 utilize the CD4 receptor. J Virol. 1990 Sep;64(9):4468–4476. doi: 10.1128/jvi.64.9.4468-4476.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Sakai K., Bresser J., Stevenson M., Evinger-Hodges M. J., Volsky D. J. Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV. J Virol. 1987 Dec;61(12):3774–3782. doi: 10.1128/jvi.61.12.3774-3782.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman S. H., Fry J. M., Silberberg D. H., Grose C., Manning M. C. Cerebroside antibody titers in antisera capable of myelination inhibition and demyelination. Brain Res. 1978 May 26;147(2):410–415. doi: 10.1016/0006-8993(78)90854-5. [DOI] [PubMed] [Google Scholar]
- Dyer C. A., Benjamins J. A. Antibody to galactocerebroside alters organization of oligodendroglial membrane sheets in culture. J Neurosci. 1988 Nov;8(11):4307–4318. doi: 10.1523/JNEUROSCI.08-11-04307.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth G. S., Walsh F. S., Nirenberg M. Monoclonal antibody to a plasma membrane antigen of neurons. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorkey F., Melnick J. L., Gyorkey P. Human immunodeficiency virus in brain biopsies of patients with AIDS and progressive encephalopathy. J Infect Dis. 1987 May;155(5):870–876. doi: 10.1093/infdis/155.5.870. [DOI] [PubMed] [Google Scholar]
- Harouse J. M., Kunsch C., Hartle H. T., Laughlin M. A., Hoxie J. A., Wigdahl B., Gonzalez-Scarano F. CD4-independent infection of human neural cells by human immunodeficiency virus type 1. J Virol. 1989 Jun;63(6):2527–2533. doi: 10.1128/jvi.63.6.2527-2533.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm L., Elwing H., Fredman P., Strannegård O. Sendai virus receptor: proposed recognition structure based on binding to plastic-adsorbed gangliosides. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1947–1950. doi: 10.1073/pnas.77.4.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B. A., Rao P. E., Kong L. I., Hahn B. H., Shaw G. M., Hood L. E., Kent S. B. Location and chemical synthesis of a binding site for HIV-1 on the CD4 protein. Science. 1988 Jun 3;240(4857):1335–1339. doi: 10.1126/science.2453925. [DOI] [PubMed] [Google Scholar]
- Jones H. R., Jr, Ho D. D., Forgacs P., Adelman L. S., Silverman M. L., Baker R. A., Locuratolo P. Acute fulminating fatal leukoencephalopathy as the only manifestation of human immunodeficiency virus infection. Ann Neurol. 1988 May;23(5):519–522. doi: 10.1002/ana.410230515. [DOI] [PubMed] [Google Scholar]
- Karlsson K. A. Animal glycosphingolipids as membrane attachment sites for bacteria. Annu Rev Biochem. 1989;58:309–350. doi: 10.1146/annurev.bi.58.070189.001521. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kleihues P., Lang W., Burger P. C., Budka H., Vogt M., Maurer R., Lüthy R., Siegenthaler W. Progressive diffuse leukoencephalopathy in patients with acquired immune deficiency syndrome (AIDS). Acta Neuropathol. 1985;68(4):333–339. doi: 10.1007/BF00690837. [DOI] [PubMed] [Google Scholar]
- Larkin M., Childs R. A., Matthews T. J., Thiel S., Mizuochi T., Lawson A. M., Savill J. S., Haslett C., Diaz R., Feizi T. Oligosaccharide-mediated interactions of the envelope glycoprotein gp120 of HIV-1 that are independent of CD4 recognition. AIDS. 1989 Dec;3(12):793–798. doi: 10.1097/00002030-198912000-00003. [DOI] [PubMed] [Google Scholar]
- Ledeen R. W., Yu R. K. Gangliosides: structure, isolation, and analysis. Methods Enzymol. 1982;83:139–191. doi: 10.1016/0076-6879(82)83012-7. [DOI] [PubMed] [Google Scholar]
- Li X. L., Moudgil T., Vinters H. V., Ho D. D. CD4-independent, productive infection of a neuronal cell line by human immunodeficiency virus type 1. J Virol. 1990 Mar;64(3):1383–1387. doi: 10.1128/jvi.64.3.1383-1387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J. D., Reyes G. R., McGrath M. S., Stein B. S., Engleman E. G. AIDS retrovirus induced cytopathology: giant cell formation and involvement of CD4 antigen. Science. 1986 May 30;232(4754):1123–1127. doi: 10.1126/science.3010463. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Kennedy M. S., Sligh J. M., Cort S. P., Mawle A., Nicholson J. K. Binding of HTLV-III/LAV to T4+ T cells by a complex of the 110K viral protein and the T4 molecule. Science. 1986 Jan 24;231(4736):382–385. doi: 10.1126/science.3001934. [DOI] [PubMed] [Google Scholar]
- McKeating J. A., Griffiths P. D., Weiss R. A. HIV susceptibility conferred to human fibroblasts by cytomegalovirus-induced Fc receptor. Nature. 1990 Feb 15;343(6259):659–661. doi: 10.1038/343659a0. [DOI] [PubMed] [Google Scholar]
- Price R. W., Brew B., Sidtis J., Rosenblum M., Scheck A. C., Cleary P. The brain in AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science. 1988 Feb 5;239(4840):586–592. doi: 10.1126/science.3277272. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Mirsky R., Fields K. L., Lisak R. P., Dorfman S. H., Silberberg D. H., Gregson N. A., Leibowitz S., Kennedy M. C. Galactocerebroside is a specific cell-surface antigenic marker for oligodendrocytes in culture. Nature. 1978 Aug 24;274(5673):813–816. [PubMed] [Google Scholar]
- Sattentau Q. J., Dalgleish A. G., Weiss R. A., Beverley P. C. Epitopes of the CD4 antigen and HIV infection. Science. 1986 Nov 28;234(4780):1120–1123. doi: 10.1126/science.2430333. [DOI] [PubMed] [Google Scholar]
- Sharpe A. H., Hunter J. J., Chassler P., Jaenisch R. Role of abortive retroviral infection of neurons in spongiform CNS degeneration. Nature. 1990 Jul 12;346(6280):181–183. doi: 10.1038/346181a0. [DOI] [PubMed] [Google Scholar]
- Stoler M. H., Eskin T. A., Benn S., Angerer R. C., Angerer L. M. Human T-cell lymphotropic virus type III infection of the central nervous system. A preliminary in situ analysis. JAMA. 1986 Nov 7;256(17):2360–2364. [PubMed] [Google Scholar]
- Tateno M., Gonzalez-Scarano F., Levy J. A. Human immunodeficiency virus can infect CD4-negative human fibroblastoid cells. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4287–4290. doi: 10.1073/pnas.86.11.4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner A., Winskowsky G., Cichutek K., Norley S. G., Kurth R. Productive infection of both CD4+ and CD4- human cell lines with HIV-1, HIV-2 and SIVagm. AIDS. 1990 Jun;4(6):537–544. doi: 10.1097/00002030-199006000-00007. [DOI] [PubMed] [Google Scholar]
- de la Monte S. M., Ho D. D., Schooley R. T., Hirsch M. S., Richardson E. P., Jr Subacute encephalomyelitis of AIDS and its relation to HTLV-III infection. Neurology. 1987 Apr;37(4):562–569. doi: 10.1212/wnl.37.4.562. [DOI] [PubMed] [Google Scholar]