Abstract
Objective:
Renal dysfunction has been linked with increased risk for cognitive impairment and dementia, but studies are conflicting. For that reason, the aim of the present systematic review and meta-analysis is to summarize the best available evidence on the prospective association between potential markers of renal dysfunction and development of cognitive impairment or dementia.
Methods:
Medline, Embase, and Cochrane Database of Systematic Reviews were searched for potential publications until August 1, 2016. Studies were eligible if they fulfilled the following criteria: population-based study, prospective design, ≥100 participants, aged ≥45 years, ≥1 year follow-up, and cognition/dementia outcomes. Where appropriate, random effects meta-analyses were conducted yielding pooled odds ratios (OR) and 95% confidence intervals (CI).
Results:
Twenty-two out of 8,494 abstracts fulfilled the eligibility criteria. Sufficient evidence was found for albuminuria, mixed results for estimated glomerular filtration rate (eGFR), insufficient support for cystatin C, and tentative evidence for serum creatinine and creatinine clearance. Meta-analyses of 5 studies representing 27,805 persons showed a 35% increased risk of cognitive impairment or dementia in those with albuminuria (OR 1.35, 95% CI 1.06–1.73, p = 0.015), whereas eGFR <60 mL/min/1.73 m2 showed no significant association (OR 1.28, 95% CI 0.99–1.65, p = 0.063). No meta-analyses could be done for serum creatinine, creatinine clearance, or cystatin C.
Conclusions:
The overall evidence for an association between renal dysfunction and cognitive impairment or dementia is modest. Evidence suggests that albuminuria is associated with higher odds of developing cognitive impairment or dementia.
Renal dysfunction has been considered a candidate risk factor for cognitive impairment and dementia.1–3 The kidneys and the brain, both being end organs, are susceptible to vascular damage due to broadly similar anatomic and hemodynamic features.4 Chronic kidney disease (CKD) and dementia share a similar risk factor profile including hypertension, diabetes mellitus, or hyperlipidemia, and in both conditions a high prevalence of small vessel disease, silent brain infarcts, white matter pathology, and microbleeds was reported.2,3,5–8 Hence, several pathways may underlie the association between CKD and cognitive impairment, including shared vascular factors or a direct neurotoxic effect of uremia.2,9
Worldwide, the number of people with dementia has increased.10 Identification of determinants of dementia is important given the absence of effective treatments. There is sufficient evidence to support the associations between modifiable risk factors and cognitive decline or dementia later in life.11,12 The global prevalence of CKD in the general population is estimated to be 8%–16%, with the highest prevalence in older people.13 Both CKD and dementia are important public health problems with associated poor health outcomes and rising health care costs for our society.14,15
A previous meta-analysis found a 39% increased odds for cognitive impairment in patients with CKD.16 Yet this study included only 6 prospective studies and only reported on estimated glomerular filtration rate (eGFR) as a marker of renal dysfunction, potentially excluding relevant studies using other markers like albuminuria or cystatin C. Therefore, we took a broader approach and systematically reviewed the best available evidence on the prospective association between potential markers of renal dysfunction and development of cognitive impairment or dementia.
METHODS
Data sources and searches.
The literature search was conducted in Medline, Embase, and Cochrane Database of Systematic Reviews. A deliberate choice was made for a broad search with minimal restrictions in order to harvest all potentially interesting publications. Some markers of renal function were specified within the search term due to their wide use in clinical practice.17 The search strategy included (1) terms related to predictors (e.g., renal, kidney, albuminuria, creatinine, cystatin), (2) terms for the outcomes (e.g., dementia, Alzheimer disease [AD], cognitive impairment), and (3) specific limitations (i.e., humans, language restrictions). See appendix e-1 at Neurology.org for the complete search strategy.
Study selection.
All publications until August 1, 2016, were included that fulfilled the following inclusion criteria: population-based study, prospective design, ≥100 participants, age ≥45 years, ≥1 year follow-up, and cognition/dementia outcomes. Secondary literature (review articles, conference abstracts) and reference lists of publications were also scrutinized.
Data extraction and quality assessment.
The selection process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (appendix e-2). First, 2 independent raters (S.K., K.D.) screened titles and abstracts for broad suitability and eligibility criteria. Second, 2 independent investigators (I.C., K.D.) reviewed the full-text versions of potentially relevant citations and extracted information such as sample size, setting, age range, follow-up period, outcome (dementia, cognitive impairment, or cognitive decline), predictors (marker of renal dysfunction), and effect estimate, according to a standardized data collection form. Discrepancies were resolved through consensus and discussion with the third reviewer (S.K.). Corresponding authors were contacted if full-text articles were not available or additional information was required (with 2 reminders in case of nonresponse). Quality aspects were assessed with the Newcastle-Ottawa Scale (NOS).18
Markers of renal dysfunction.
eGFR is expressed as milliliter blood filtered per minute by the functioning nephrons in the kidney, with <60 mL/min/1.73 m2 as moderately impaired and <45 mL/min/1.73 m2 as moderately to severely impaired kidney function. An eGFR of 60–90 mL/min/1.73 m2 represents mildly reduced kidney function (stage 2 CKD). In this study, we examine more advanced stages of CKD, namely eGFR levels <45, 45–59, and <60 mL/min/1.73 m2. eGFR <60 mL/min/1.73 m2 is the most common indicator of CKD.19 eGFR can be derived according to different formulas, usually from serum creatinine (SCr).20 Albuminuria or proteinuria refers to an abnormal amount of proteins (e.g., albumin) present in the urine.21 Normally, proteins are retained during the filtration process in the kidneys. Microalbuminuria is defined as excretion of 30–300 mg/24 h of albumin, whereas macroalbuminuria is defined as an excretion of more than 300 mg/24 h of albumin.22 The value of acceptable albumin excretion lies between 2 and 30 mg/24 hours.23 In addition, albuminuria can be measured by using an albumin-to-creatinine ratio on a random (spot) urine sample. An albumin-to-creatinine ratio of 30 mg/g is considered clinically significant.24 Cystatin C, a cysteine proteinase inhibitor, is a very small protein produced by all nucleated cells. It is freely filtered by the glomerulus and then metabolized in the tubules. Normal kidney functioning is characterized by a steady cystatin C blood level, whereas high levels of cystatin C indicate kidney dysfunction.25 Creatinine is a waste product of creatine phosphate in muscles, and is relatively stable over time. An elevated level of SCr (for men >1.2 mg/dL; for women 1.0 mg/dL) may indicate that the kidneys are not working properly.7 However, SCr does not correlate linearly with eGFR and is a relatively poor measure of renal function.19 Creatinine clearance (CCl) is the measurement of the amount of creatinine excreted in the urine per unit of time (usually based on a 24-hour urine collection).17
Data synthesis and analysis.
Random-effects meta-analysis was used to generate pooled odds ratios (OR) and their 95% confidence intervals (CI). Tests were 2-sided at an α level of 0.05. Estimates from crude as well as most fully adjusted models available were used. Heterogeneity among studies was assessed using the I2 statistic. Possible publication bias was assessed by funnel plots and the Egger test. Separate meta-analyses were only conducted for different levels of eGFR and albuminuria. All analyses were done with Stata 13.1 (StataCorp, College Station, TX).
RESULTS
We identified 8,494 abstracts, of which 86 (1%) were included for full-text scrutiny. Of these, 64 were excluded due to article type (e.g., review, editorial), study design (e.g., cross-sectional study), or they were conference abstracts or duplicate records. We contacted 17 authors to obtain full-text articles that were not available to us. Of these, 11 authors responded. The 6 unavailable full-text articles were patient-based studies, conference abstracts, or review articles. In addition, we contacted authors of 20 studies for additional information that was not included in full text, and 10 authors responded. This resulted in 22 prospective population-based studies (figure 1). One additional study was found from cross-references, but this study was excluded after full-text screening. Quality assessment of 22 prospective studies was sufficient (mean NOS score 8.00, SD 0.62, range 7–9).
Figure 1. Flow diagram.
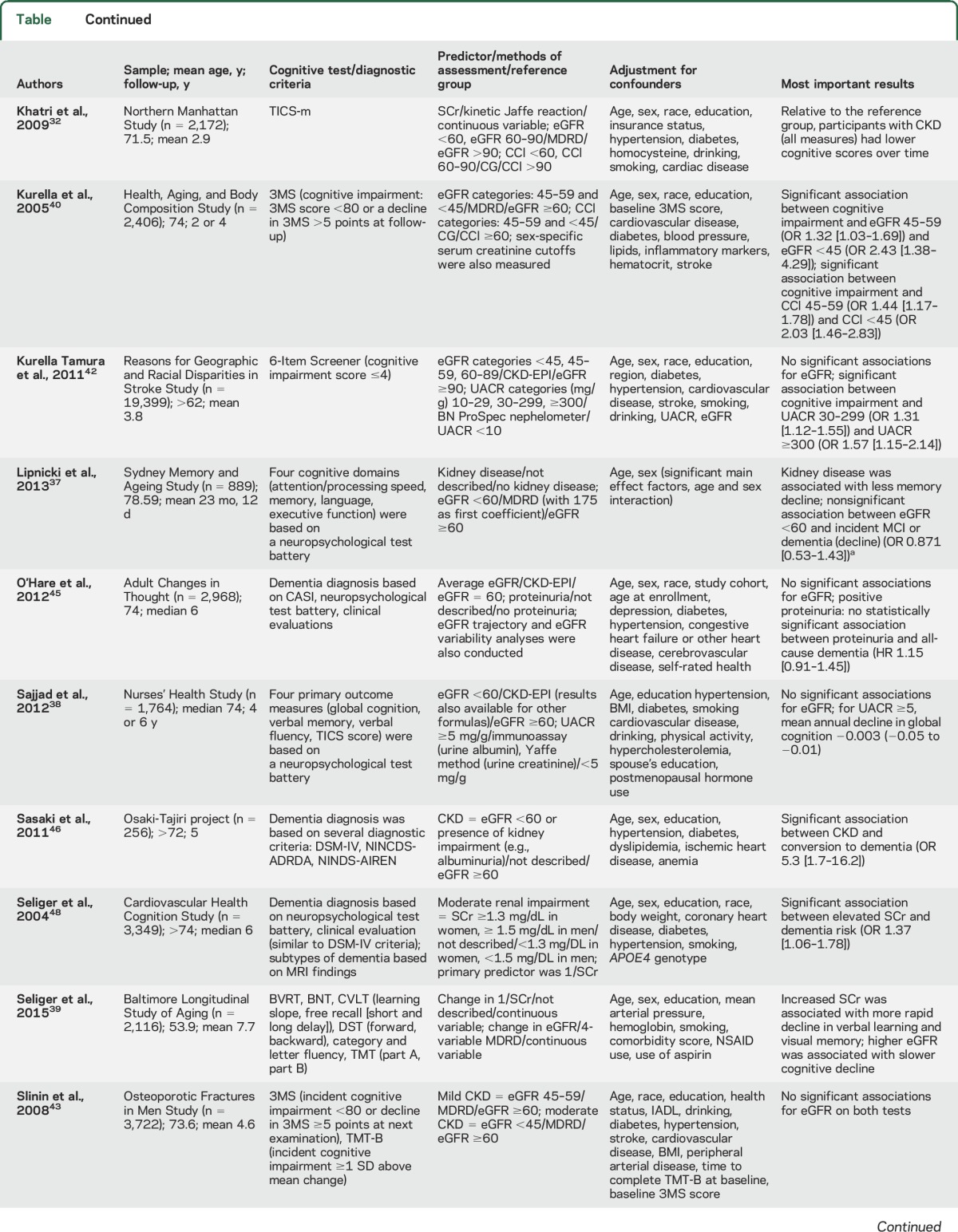
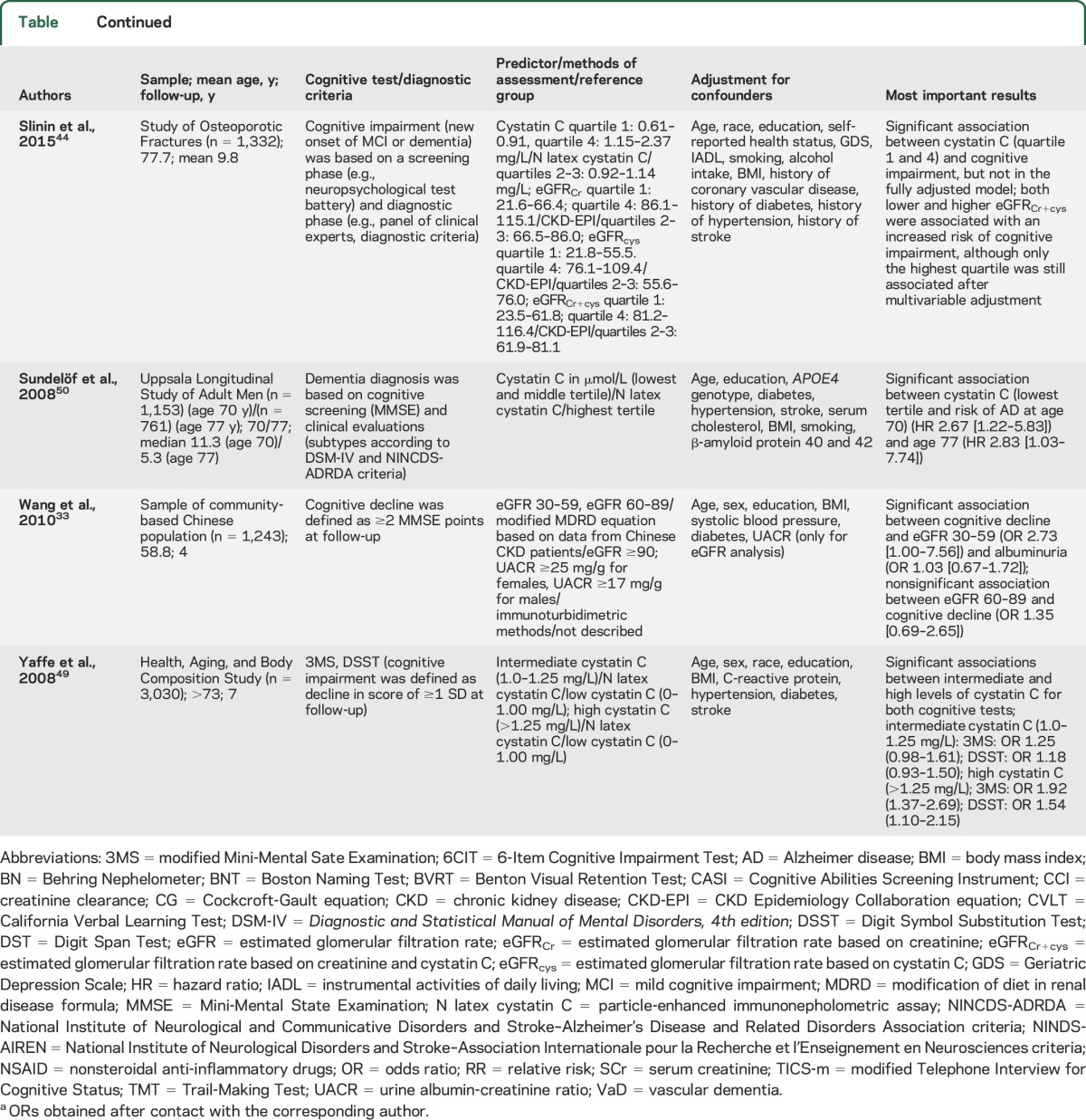
Renal dysfunction in the population-based studies was based on eGFR, albuminuria, cystatin C, SCr, and CCl. Cognitive functioning was most often defined as a decline in cognition between 2 time points (e.g., change in Mini-Mental State Examination [MMSE] scores). Other outcome measures such as cognitive impairment or dementia were diagnosed based on findings from neuropsychological examinations, clinical evaluations, diagnostic criteria (e.g., DSM-IV26), and review of medical records. If studies investigated dementia subtypes, this was generally based on different criteria for AD dementia (National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association criteria27) and vascular dementia (National Institute of Neurological Disorders and Stroke–Association Internationale pour la Recherche en l’Enseignement en Neurosciences criteria28). All 22 studies and their results are summarized in the table and appendix e-3.
Table.
Characteristics of population-based studies assessing the relation between (markers of) renal dysfunction and cognitive impairment or dementia

eGFR.
Seventeen studies investigated the association between (change in) eGFR and cognitive decline or risk of cognitive impairment/dementia. Ten studies focused on cognitive decline, of which 5 found more decline in cognitive capacity with lower eGFR as time progresses,29–33 and 5 studies found no effect.34–38 Two of these studies and 1 additional study looked at the association between longitudinal changes in eGFR and cognitive decline.34,35,39 One study found that faster eGFR decline was associated with cognitive decline and incident dementia with a vascular component,35 1 study reported an association between declining eGFR and a decline in several cognitive domains,34 and 1 study found that higher eGFR was associated with slower cognitive decline.39 Five studies focused on cognitive impairment, of which 1 study found an increased risk,40 and 4 studies found no association.41–44 Three studies examined the relation between eGFR and dementia risk, 2 studies found no association between eGFR and dementia risk,35,45 while 1 study found an increased risk of all-cause dementia.46 Rather than eGFR at baseline, age-related change in eGFR and type of dementia might play a role.
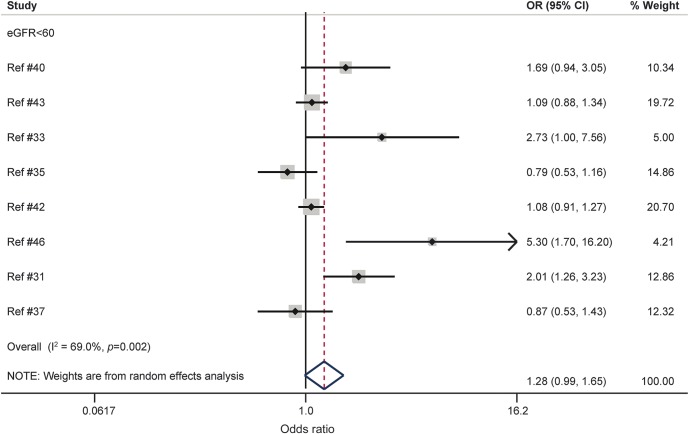
In the meta-analysis, a total of 8 studies, representing 36,636 persons, could be included.31,33,35,37,40,42,43,46 For this, 4 studies reporting both eGFR <45 mL/min/1.73 m2 and eGFR 45–59 mL/min/1.73 m2 levels were pooled individually (within 1 study) in order to be included in the eGFR <60 mL/min/1.73 m2 meta-analysis.35,40,42,43 eGFR <60 mL/min/1.73 m2 was not associated with risk of cognitive impairment or dementia (p = 0.063; figure 2). There were signs of heterogeneity based on the I2 statistic (I2 = 69.0%, p = 0.002). The funnel plot of the adjusted estimates was broadly symmetrical (see figure 1 of appendix e-4) and the Egger test (p = 0.105) was not significant, suggesting no signs of publication bias. Exclusion of 2 relatively small influential studies (logarithm of standard error >0.4) reduced heterogeneity modestly (OR 1.13, 95% CI 0.91–1.40, p = 0.258; I2 = 58.8%, p = 0.033). Separate meta-analyses were conducted for eGFR <45 mL/min/1.73 m2 and eGFR 45–59 mL/min/1.73 m2. No significant associations were observed in these analyses (appendix e-5). It was not possible to perform a meta-analysis for eGFR 60–89 mL/min/1.73 m2 since there were only 2 studies.33,42 Overall, heterogeneity in outcomes across studies was reduced in all analyses for adjusted estimates in comparison with unadjusted estimates (e.g., eGFR <60 mL/min/1.73 m2: I2 = 69.0% vs I2 = 91.6%, respectively). See figures 1–3 of appendix e-6 for the results of the unadjusted estimates.
Figure 2. Forest plot of population-based prospective studies assessing the relation between estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 and cognitive impairment or dementia (fully adjusted estimates).
CI = confidence interval; OR = odds ratio.
Albuminuria.
Seven studies reported on albuminuria, of which 4 found a faster rate of cognitive decline,33,36,38,47 1 found an increased risk of cognitive impairment,42 and 3 showed mixed results for incident dementia.35,45,47
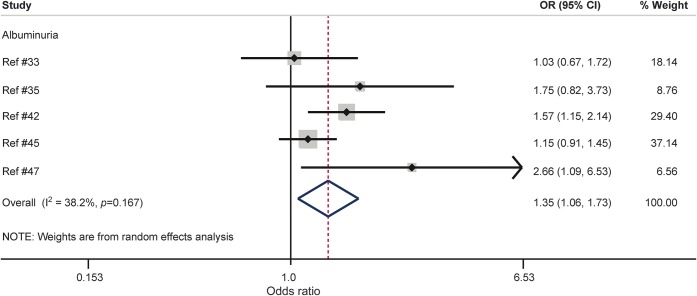
In the meta-analysis, 5 studies, representing 27,805 persons, could be included.33,35,42,45,47 Based on the fully adjusted estimates, albuminuria was associated with a 35% increased risk of cognitive impairment or dementia (p = 0.015; figure 3). There was mild to moderate heterogeneity (I2 = 38.2). There were no signs of publication bias based on visual inspection of the funnel plot (see figure 2 of appendix e-5) and the Egger test (p = 0.274). Four of these studies reported unadjusted estimates (see figure 4 of appendix e-6). Heterogeneity in outcomes across studies was reduced for adjusted estimates in comparison with unadjusted estimates (I2 = 38.2% vs I2 = 74.8%, respectively).
Figure 3. Forest plot of population-based prospective studies assessing the relation between albuminuria and cognitive impairment or dementia (fully adjusted estimates).
CI = confidence interval; OR = odds ratio.
Creatinine.
Five studies reported on SCr, of which 2 found an increased risk of cognitive impairment,40,41 1 found an increased risk of dementia,48 1 found a faster rate of cognitive decline,32 and 1 found that an increase in SCr was associated with more rapid cognitive decline.39 No meta-analysis could be conducted for this association due to different methods of exposure and outcome formulations across studies.
Creatinine clearance.
Three studies investigated the association between CCl and cognitive decline or cognitive impairment. The Northern Manhattan Study in 2,172 community-dwelling participants found that persons with a baseline CCl <60 mL/min showed significantly more decline compared with individuals with a CCl >90 mL/min over the 2.9 years of follow-up.32 The Health ABC Study in 2,406 elderly individuals found a significant association between CCl (45–59 and <45 mL/min/1.73 m2) and cognitive impairment.40 The INVADE study demonstrated a significant association between CCl <45 mL/min/1.73 m2 and cognitive impairment after 2 years of follow-up.41 No meta-analysis could be conducted because of differences in exposure and outcome definitions between studies.
Cystatin C.
Three studies focused on cystatin C and cognitive impairment or dementia. The Health ABC Study found an increased risk of cognitive impairment among 3,030 older adults if they had high levels of cystatin C.49 In contrast, in men aged 70 and 77 years from the Uppsala Longitudinal Study of Adult Men, high levels of serum cystatin C were associated with a decreased risk of AD.50 The Study of Osteoporotic Fractures in 1,332 elderly women found a U-shape association between cystatin C and cognitive impairment, but after adjustment for covariates, these associations were no longer significant.44 No valid meta-analysis could be conducted due to different methods of exposure (e.g., cutoffs, tertiles, quartiles) and outcome formulations across studies.
DISCUSSION
This systematic review and meta-analysis suggested that individuals with albuminuria have, on average, a 35% increased risk of cognitive impairment or dementia. Separate meta-analyses for different levels of eGFR yielded nonsignificant results, possibly due to substantial heterogeneity among studies. The literature on eGFR is mixed in general, with studies showing a positive or no association between low levels of eGFR with cognitive decline or dementia risk. For SCr, tentative evidence suggests an association between elevated SCr levels with faster cognitive decline and higher risk for dementia, but too few high-quality studies exist for meta-analysis. In contrast, the review on cystatin C yielded only 3 studies with different methodology and contradictory results.
The substantial heterogeneity observed in the meta-analyses of eGFR might be due to several methodologic issues, such as (1) the difference in formulas used to measure kidney function (e.g., Modification of Diet in Renal Disease formula, Chronic Kidney Disease Epidemiology Collaboration equation); (2) the variation in study population (e.g., sex-specific, age range, concurrent medication); (3) the inclusion of possible confounders (e.g., including a few [sociocultural demographics] or the choice of covariates [exercise, depression, genotype]); and (4) the assessment of cognitive functioning (e.g., generic screening [MMSE], multidomain test battery, dementia diagnosis).2,9
The exact mechanisms relating renal dysfunction to dementia are not fully understood, but may include shared risk factors, some of which are better documented than others.9 Traditional risk factors include cardiovascular disease (e.g., myocardial infarction, atrial fibrillation), stroke, type 2 diabetes mellitus, isolated systolic hypertension, age, smoking, and hypercholesterolemia.9,51 Other factors include anemia, albumin, and hyperhomocysteinemia, whereas inflammation, oxidative stress, cerebral small vessel disease, silent brain infarcts, microbleeds, and white matter lesions are possible underlying mechanisms leading to cognitive impairment or dementia.2,9 It seems not surprising that the prevalence of the abovementioned risk factors is higher in patients with CKD than in the general population.2,3,6–8 On the other hand, CKD also appears to be a risk factor for cardiovascular or cerebrovascular diseases. For instance, persons with renal insufficiency have an increased risk of stroke or carotid atherosclerosis.52,53 In addition, a recent MRI study found that renal dysfunction was associated with poor cognitive performance and volume deficits in the brain's white matter.7 A recent longitudinal study in 600 patients with vascular risk factors found that CKD was related to all-cause dementia independent from vascular risk factors and baseline cerebral small vessel disease.54 These findings suggest that vascular damage is not the only possible explanation of the association.
We were not able to pool enough studies that reported on cystatin C. A growing body of evidence demonstrated the involvement of cystatin C in neuroprotective processes in the brain, including its colocalization with β-amyloid (Aβ) in parenchymal and vascular amyloid deposits, and the inhibition of Aβ aggregation and deposition by binding to the amyloid precursor protein or the Aβ40 and Aβ42 peptides.55 In addition, the Icelandic form of the hereditary cystatin C amyloid angiopathy is caused by a mutation in the cystatin C gene and is characterized by low levels of serum and CSF cystatin C, intracerebral hemorrhages, stroke, dementia, and death before the age of 40 years.56 The study by Yaffe et al.49 found that individuals with high levels of serum cystatin C had a 92% increased risk of developing cognitive impairment over 7 years of follow-up. This is in line with results from the Cardiovascular Health Study Cognition Study, which found that high serum levels of cystatin C were associated with poorer cognitive performance 6 years later, greater prevalence of brain infarcts, more white matter lesions, and lower gray matter volume. However, this study only measured cognition at a single time point.57
In contrast, the study by Sundelöf et al.50 indicated that lower serum levels of cystatin C were associated with increased odds of AD. A recent study demonstrated the presence of low CSF levels of cystatin C in patients with AD in comparison with healthy controls.58 Although serum levels of cystatin C are presumably more related to renal dysfunction and CSF levels of cystatin C more to AD pathology, it is difficult to establish a one-to-one relationship due to intercorrelations.
On the other hand, it is also possible that a direct neuronal toxicity of the uremic state is involved.9 Concentrations of uremic toxins in brain areas related to cognition (e.g., thalamus, cerebral cortex) are about 10 times higher in patients with CKD than in healthy people.59 The aforementioned association with cystatin C might also support the idea of a direct neural toxic effect.60 Yet it is important to note that the pathways linking CKD and dementia are not mutually exclusive but might work additively or even synergistically. Clearly, more studies into plausible underlying pathways are needed.
The present results for various eGFR levels differ substantially from the findings of a previous meta-analysis by Etgen et al.16 This inconsistency in findings can be explained by the fact that we only included the most rigorously adjusted model for each study in our analysis of fully adjusted estimates, while some of the risk estimates reported by Etgen et al. were based on crude data,35,41 including the large study by Helmer et al.,35 which found almost a significant protective effect of eGFR.
Our study has a number of strengths. By using large population-based studies with prospective designs including long follow-up periods and adjustment for a large number of known confounders, our study adds to the growing evidence that renal dysfunction is an independent risk factor for cognitive impairment or dementia. A number of limitations in this study must also be mentioned. First, most of the included studies used different methods for estimating renal function (e.g., formulas, equations, or sex-specific cutoffs), probably increasing heterogeneity among studies. In addition, most studies assessed kidney function only once, usually at study entry. Yet renal function is complex and dynamic (e.g., day-to-day variability) and cannot be fully captured at one point in time, potentially leading to exposure misclassification. Since such misclassification is likely nondifferential (i.e., independent from dementia outcome), this might have diluted stronger associations in the population. Future studies should use multiple baselines. In addition, it would be informative to study longitudinal changes in renal functioning in relation to changes in cognitive performance to learn how trajectories correlate over time. We could identify only 3 prospective studies that reported on longitudinal measures of renal function, but due to the differences in applied methodology (e.g., annual eGFR decline, change in logged eGFR), we were unable to pool these results.34,35,39 Second, studies adjusted for different sets of possible confounders (all adjusted minimally for age, sex, and education). For instance, some studies reporting on non-eGFR markers adjusted for eGFR levels while others did not. However, adjusting for eGFR levels had virtually no effect on risk estimates within individual studies. Third, we were unable to include 6 studies (30% of the total included studies) that incorporated markers of renal function as continuous variables in the meta-analyses because of the differences in scaling exposure (e.g., per 0.1 mg/dL increase, decrease of 1 SD), and outcomes (e.g., β coefficients, change per unit increase), next to diversity in cognitive domains tested (e.g., MMSE, Trail-Making Test B). Fourth, various measures were used across studies to operationalize the dichotomous cognitive impairment or dementia outcomes (e.g., MMSE cutoffs, diagnostic criteria). Fifth, although we used fully adjusted models, it is possible that the association is influenced by residual confounding. Sixth, we did not contact authors to conduct additional analyses (e.g., to provide results for specific cutoffs of markers of renal dysfunction not provided in the original article). While this could have increased the number of studies for pooling of data, using only reported estimates increases transparency. Finally, both renal function and cognition may decline with age, making it difficult to separate the effects of aging from a direct effect of renal function on cognition.
Albuminuria was associated with a modestly increased risk of cognitive impairment or dementia. Results for different levels of eGFR were nonsignificant, probably due to heterogeneity across studies. More research is needed to examine whether the association with albuminuria is causal or due to shared mechanisms, and to establish the underlying pathophysiology.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- CCl
creatinine clearance
- CI
confidence interval
- CKD
chronic kidney disease
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- eGFR
estimated glomerular filtration rate
- MMSE
Mini-Mental State Examination
- NOS
Newcastle-Ottawa Scale
- OR
odds ratio
- SCr
serum creatinine
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Kay Deckers: study concept and design, acquisition of data, analysis and interpretation of data, drafting the manuscript. Ileana Camerino: acquisition of data, critical revision of manuscript for intellectual content. Martin P.J. van Boxtel: study concept and design, critical revision of manuscript for intellectual content. Frans R.J. Verhey: study concept and design, critical revision of manuscript for intellectual content. Kate Irving: critical revision of manuscript for intellectual content. Carol Brayne: critical revision of manuscript for intellectual content. Miia Kivipelto: critical revision of manuscript for intellectual content. John M. Starr: critical revision of manuscript for intellectual content. Kristine Yaffe: critical revision of manuscript for intellectual content. Peter W. de Leeuw: critical revision of manuscript for intellectual content. Sebastian Köhler: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content, study supervision.
STUDY FUNDING
Supported by the In-MINDD (Innovative Midlife Intervention for Dementia Deterrence) project. In-MINDD is funded by the European Union's Framework Programme Seven (FP7) under contract number 304979. M.K. was funded by the Center for Innovative Medicine (CIMED) at Karolinska Institutet.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Deckers K, van Boxtel MP, Schiepers OJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry 2015;30:234–246. [DOI] [PubMed] [Google Scholar]
- 2.Madero M, Gul A, Sarnak MJ. Cognitive function in chronic kidney disease. Semin Dial 2008;21:29–37. [DOI] [PubMed] [Google Scholar]
- 3.Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis 2008;15:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mogi M, Horiuchi M. Clinical interaction between brain and kidney in small vessel disease. Cardiol Res Pract 2011;2011:306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011;10:819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikram MA, Vernooij MW, Hofman A, Niessen WJ, van der Lugt A, Breteler MM. Kidney function is related to cerebral small vessel disease. Stroke 2008;39:55–61. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopalan P, Refsum H, Hua X, et al. Mapping creatinine- and cystatin C-related white matter brain deficits in the elderly. Neurobiol Aging 2013;34:1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seliger SL, Sarnak MJ. Subclinical vascular disease of the brain in dialysis patients. Am J Kidney Dis 2007;50:8–10. [DOI] [PubMed] [Google Scholar]
- 9.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol 2013;24:353–363. [DOI] [PubMed] [Google Scholar]
- 10.Ferri CP, Prince M, Brayne C, et al. Global prevalence of dementia: a Delphi consensus study. The Lancet 2005;366:2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 12.Plassman BL, Williams JW, Jr., Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 2010;153:182–193. [DOI] [PubMed] [Google Scholar]
- 13.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–272. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Dementia: A Public Health Priority. Geneva: WHO; 2012. [Google Scholar]
- 15.Tonelli M, Riella M. Chronic kidney disease and the ageing population. Lancet 2014;383:1278–1279. [DOI] [PubMed] [Google Scholar]
- 16.Etgen T, Chonchol M, Forstl H, Sander D. Chronic kidney disease and cognitive impairment: a systematic review and meta-analysis. Am J Nephrol 2012;35:474–482. [DOI] [PubMed] [Google Scholar]
- 17.Traynor J, Mactier R, Geddes CC, Fox JG. How to measure renal function in clinical practice. BMJ 2006;333:733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wells G, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2000. [Google Scholar]
- 19.O'Riordan P, Stevens PE, Lamb EJ. Estimated glomerular filtration rate. BMJ 2014;348:g264. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Browne OT, Bhandari S. Interpreting and investigating proteinuria. BMJ 2012;344. [DOI] [PubMed] [Google Scholar]
- 22.Barzilay JI, Gao P, O'Donnell M, et al. Albuminuria and decline in cognitive function: the ONTARGET/TRANSCEND studies. Arch Intern Med 2011;171:142–150. [DOI] [PubMed] [Google Scholar]
- 23.Chavan VU, Sayyed AK, Durgawale PP, Sontakke AV, Nilakhe SD. Practical aspects of calculation, expression and interpretation of urine albumin measurement. NJIRM 2011;2:29–34. [Google Scholar]
- 24.Keane WF, Eknoyan G. Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): a position paper of the National Kidney Foundation. Am J Kidney Dis 1999;33:1004–1010. [DOI] [PubMed] [Google Scholar]
- 25.Mussap M, Plebani M. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci 2004;41:467–550. [DOI] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 27.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 28.Román GC, Tatemichi TK, Erkinjuntti T, et al. Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 1993;43:250. [DOI] [PubMed] [Google Scholar]
- 29.Buchman AS, Tanne D, Boyle PA, Shah RC, Leurgans SE, Bennett DA. Kidney function is associated with the rate of cognitive decline in the elderly. Neurology 2009;73:920–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darsie B, Shlipak MG, Sarnak MJ, Katz R, Fitzpatrick AL, Odden MC. Kidney function and cognitive health in older adults: the Cardiovascular Health Study. Am J Epidemiol 2014;180:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feng L, Yap KB, Yeoh LY, Ng TP. Kidney function and cognitive and functional decline in elderly adults: findings from the Singapore longitudinal aging study. J Am Geriatr Soc 2012;60:1208–1214. [DOI] [PubMed] [Google Scholar]
- 32.Khatri M, Nickolas T, Moon YP, et al. CKD associates with cognitive decline. J Am Soc Nephrol 2009;20:2427–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang F, Zhang L, Liu L, Wang H. Level of kidney function correlates with cognitive decline. Am J Nephrol 2010;32:117–121. [DOI] [PubMed] [Google Scholar]
- 34.Davey A, Elias MF, Robbins MA, Seliger SL, Dore GA. Decline in renal functioning is associated with longitudinal decline in global cognitive functioning, abstract reasoning and verbal memory. Nephrol Dial Transplant 2013;28:1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helmer C, Stengel B, Metzger M, et al. Chronic kidney disease, cognitive decline, and incident dementia: the 3C Study. Neurology 2011;77:2043–2051. [DOI] [PubMed] [Google Scholar]
- 36.Jassal SK, Kritz-Silverstein D, Barrett-Connor E. A prospective study of albuminuria and cognitive function in older adults: the Rancho Bernardo study. Am J Epidemiol 2010;171:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lipnicki DM, Sachdev PS, Crawford J, et al. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS One 2013;8:e65841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sajjad I, Grodstein F, Kang JH, Curhan GC, Lin J. Kidney dysfunction and cognitive decline in women. Clin J Am Soc Nephrol 2012;7:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seliger SL, Wendell CR, Waldstein SR, Ferrucci L, Zonderman AB. Renal function and long-term decline in cognitive function: the Baltimore Longitudinal Study of Aging. Am J Nephrol 2015;41:305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol 2005;16:2127–2133. [DOI] [PubMed] [Google Scholar]
- 41.Etgen T, Sander D, Chonchol M, et al. Chronic kidney disease is associated with incident cognitive impairment in the elderly: the INVADE study. Nephrol Dial Transplant 2009;24:3144–3150. [DOI] [PubMed] [Google Scholar]
- 42.Kurella Tamura M, Muntner P, Wadley V, et al. Albuminuria, kidney function, and the incidence of cognitive impairment among adults in the United States. Am J Kidney Dis 2011;58:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slinin Y, Paudel ML, Ishani A, et al. Kidney function and cognitive performance and decline in older men. J Am Geriatr Soc 2008;56:2082–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Slinin Y, Peters KW, Ishani A, et al. Cystatin C and cognitive impairment 10 years later in older women. J Gerontol A Biol Sci Med Sci 2015;70:771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Hare AM, Walker R, Haneuse S, et al. Relationship between longitudinal measures of renal function and onset of dementia in a community cohort of older adults. J Am Geriatr Soc 2012;60:2215–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki Y, Marioni R, Kasai M, Ishii H, Yamaguchi S, Meguro K. Chronic kidney disease: a risk factor for dementia onset: a population-based study: The Osaki-Tajiri Project. J Am Geriatr Soc 2011;59:1175–1181. [DOI] [PubMed] [Google Scholar]
- 47.Higuchi M, Chen R, Abbott RD, et al. Mid-life proteinuria and late-life cognitive function and dementia in elderly men: the Honolulu-Asia Aging Study. Alzheimer Dis Assoc Disord 2015;29:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Seliger SL, Siscovick DS, Stehman-Breen CO, et al. Moderate renal impairment and risk of dementia among older adults: the Cardiovascular Health Cognition Study. J Am Soc Nephrol 2004;15:1904–1911. [DOI] [PubMed] [Google Scholar]
- 49.Yaffe K, Lindquist K, Shlipak MG, et al. Cystatin C as a marker of cognitive function in elders: findings from the Health ABC Study. Ann Neurol 2008;63:798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sundelöf J, Arnlov J, Ingelsson E, et al. Serum cystatin C and the risk of Alzheimer disease in elderly men. Neurology 2008;71:1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis 2007;49:A6–A7, S1–S296. [DOI] [PubMed] [Google Scholar]
- 52.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease: a possible marker for increased risk of stroke. Stroke 1997;28:557–563. [DOI] [PubMed] [Google Scholar]
- 53.Ishimura E, Shoji T, Emoto M, et al. Renal insufficiency accelerates atherosclerosis in patients with type 2 diabetes mellitus. Am J Kidney Dis 2001;38:S186–S190. [DOI] [PubMed] [Google Scholar]
- 54.Miwa K, Tanaka M, Okazaki S, et al. Chronic kidney disease is associated with dementia independent of cerebral small-vessel disease. Neurology 2014;82:1051–1057. [DOI] [PubMed] [Google Scholar]
- 55.Sastre M, Calero M, Pawlik M, et al. Binding of cystatin C to Alzheimer's amyloid beta inhibits in vitro amyloid fibril formation. Neurobiol Aging 2004;25:1033–1043. [DOI] [PubMed] [Google Scholar]
- 56.Ghiso J, Jensson O, Frangione B. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc Natl Acad Sci USA 1986;83:2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Riverol M, Becker JT, Lopez OL, et al. Relationship between systemic and cerebral vascular disease and brain structure integrity in normal elderly individuals. J Alzheimers Dis 2015;44:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhong XM, Hou L, Luo XN, et al. Alterations of CSF cystatin C levels and their correlations with CSF Alphabeta40 and Alphabeta42 levels in patients with Alzheimer's disease, dementia with Lewy bodies and the atrophic form of general paresis. PLoS One 2013;8:e55328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Deyn PP, Vanholder R, Eloot S, Glorieux G. Guanidino compounds as uremic (neuro)toxins. Semin Dial 2009;22:340–345. [DOI] [PubMed] [Google Scholar]
- 60.Nagai A, Ryu JK, Terashima M, et al. Neuronal cell death induced by cystatin C in vivo and in cultured human CNS neurons is inhibited with cathepsin B. Brain Res 2005;1066:120–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.