Abstract
Objective:
To report prospective repeated measures data detailing the perceived benefit of deep brain stimulation (DBS) on the most commonly cited symptom and activity goals identified by patients with Parkinson disease.
Methods:
Fifty-two participants were recruited from a consecutive series. Participants completed a semi-structured interview soliciting their symptom and behavioral goals and corresponding visual analog scales measuring perceived symptom severity and limits to goal attainment. Severity ratings were completed prior to and at 2 times following DBS. Changes in severity over time were assessed using a mixed effects linear model. The pattern of relationships between the severity ratings and standard clinical research (SCR) measures routinely administered were examined using Pearson correlations.
Results:
The most common symptom goals were improvements in tremor, gait, and nonmotor symptoms, whereas the most frequent behavioral goals related to interpersonal relationships, work, and avocational pursuits. Most severity ratings were significantly correlated with each other but not with the SCR measures. Significant improvements were evident on all SCR measures after DBS. Participants' severity ratings for their symptom and behavioral goals improved significantly over time although not all severity ratings changed in the same manner.
Conclusions:
These data illustrate that improvements in participants' individually defined goals were evident over time and that some of these improvements occurred in areas in which the benefits associated with DBS are not as well-documented. The participants' severity ratings were not redundant with SCR measures, suggesting that novel and potentially important information can be gleaned by systematically assessing patients' goals.
The importance of patients' values and perspectives is central to the Patient-Centered Outcomes Research Institute.1 We have argued that this concept is especially important in the context of deep brain stimulation (DBS), in which patients decide to undergo an elective neurosurgical procedure to improve quality of life, a construct that is inherently subjective and value-laden.2 Although there is consensus among some DBS teams that patients' goals for the upcoming neurosurgery should be assessed routinely,3–6 there has been relatively little investigation of how successful DBS is in addressing patients' goals for surgery or the relationship between patients' stated goals for surgery and other standard clinical research (SCR) metrics.7,8
Despite studies demonstrating the positive benefits of DBS on motor symptoms and quality of life,9–11 some have provocatively argued that patients may not be as pleased with the outcomes of DBS as the treating physicians.12,13 This satisfaction gap suggests that SCR measures may not fully capture patients' goals with respect to outcome and argues for the need for a more systematic assessment of patients' individually defined goals for DBS and patients' perceived satisfaction with DBS in addressing those goals.
We systematically assessed the goals a consecutive series of patients with Parkinson disease (PD) articulated as most important in their decision to undergo DBS. We measured patients' severity ratings for their individually defined goals prior to and following surgery and examined the relationships between the patients' goal ratings and SCR measures. We hypothesized that individually defined patients' goals prior to surgery would include unique factors not fully assessed by standard disease-specific ratings.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by our institutional review board. All patients provided fully informed written consent. The study was funded by the National Institute of Neurologic Disorders and Stroke (RC1NS068086).
Participants.
The published literature documents large effect sizes associated with DBS in the treatment of motor symptoms of PD. A conservative approach was adopted to calculate power for this study. We assumed a moderate effect size (0.50) and a conservative test-retest reliability coefficient for our severity rating scales of 0.30. Based on these assumptions, a sample size of 50 will result in power of 0.91 for a repeated-measures design that incorporates 3 assessments.
We approached a consecutive series of 59 patients scheduled to undergo DBS for the treatment of PD from July 2009 to June 2011 to participate in our institutional review board–approved study examining patients' goals for DBS. The majority of patients approached (n = 52, 88%) agreed to participate in the study. Of those who chose not to participate, 3 provided no reason, 1 thought that the length of the interview was too burdensome, 1 was too frail, and the remaining 2 were interested in participating but were unable to due to scheduling constraints. Inclusion in the study was limited to patients who had not undergone a previous neurosurgical procedure for PD, were native English speakers, and were over 18 years of age. No patients were excluded based on the above inclusion criteria. All patients were recruited from a single large academic medical center.
Per our standard clinical practice, all patients were approved for DBS surgery on the basis of a multidisciplinary team assessment that included neurologic, neurosurgical, neuropsychological, and psychiatric (if needed) evaluations as well as multiple explicit discussions regarding patients' goals for surgery and review of the known benefits associated with DBS. The neurologists and neurosurgeons rely on the literature and response to levodopa to inform patients of the expected outcome following DBS. Our center does not have a standard text regarding expected outcome that is shared with all patients; however, in the context of working together over several years, it is likely that similar language may be used. Data from our center collected at the same time as the current study indicate that cognitive concerns were the most common reason patients were not approved for DBS (32.7%), followed by additional opportunities for medication management (29.5%) and neurobehavioral concerns (21.3%). Importantly, a smaller percentage (9.8%) of our patients were not approved due to unrealistic goals. Other contraindications included atypical parkinsonism, poor levodopa response, predominant axial symptoms, medical comorbidities, abnormal brain imaging, and, rarely, lack of family support.14
Measures.
The study employed a mixed methodology including quantitative scales and qualitative methods. All participants completed a semi-structured interview prior to surgery probing their expectations regarding DBS with respect to symptom and behavioral goals. The interviews were audiotaped and transcribed. Participants were asked to provide their top 3 symptom and behavioral goals. Embedded within the structured interview were visual analog rating scales. The participants rank-ordered their symptom and behavioral goals separately and were then instructed to rate the present severity of each goal on a visual analog rating scale with 10 representing the greatest severity (or greatest limits to participation). The research protocol, including the semi-structured interview as well as the patient-rated severity scales, was completed prior to surgery and at approximately 3 and 6 months following DBS surgery. Research assistants (with no clinical responsibilities) conducted all but 2 of the research interviews. The SCR measures were collected by either nurse practitioners (Unified Parkinson's Disease Rating Scale [UPDRS]) or neuropsychology technicians (Parkinson's Disease Questionnaire–39 [PDQ]).
Preliminary symptom and behavioral goal categories were developed by soliciting input from an experienced DBS multidisciplinary team including surgeons, neurologists, psychiatrists, neuropsychologists, a bioethicist, and nursing staff. Following data collection, these categories were evaluated and revised based on participants' verbatim responses and naturally occurring sets consistent with a grounded theory approach. Final categories were established based on consensus discussion using an inductive, recursive process common in qualitative research and participants' verbatim responses coded accordingly (C.S.K., P.J.F.).
The standard outcome metrics included those measures collected as part of routine SCR. These included the PDQ15 (collected during the participants' standard preoperative neuropsychological and 6-month postoperative evaluations), the baseline UPDRS16 motor subscale (UPDRS-III) off medication scores, the 1 month post-DBS UPDRS-III off medications/on stimulation scores, and finally, UPDRS-II16 scores (reflecting patients' self-ratings of activities of daily living) most recently available at the time of the participants' baseline and 3- and 6-month research appointments.
Analyses.
Changes in SCR measures and participant-defined severity ratings following DBS surgery for the various symptom and behavioral goal categories were assessed using separate mixed effects linear regression models, which can be considered intent-to-treat models and provide more accurate estimates when data are missing.17 Time of assessment, symptom or behavioral goal categories, and their interaction were independent variables for all participant-defined outcome ratings. For SCR measures, only time of assessment was included as the independent variable. Age and sex of the participant were included as covariates in all models. Model fit across alternative covariance structures and random effects was evaluated using both the Akaike18 and Bayesian Information Criteria,17 which showed the same pattern of results; ultimately, an unstructured residual covariance structure and random intercept fit best.
The relationships between the participants' severity ratings of their individual goals and SCR measures at baseline were examined using Pearson correlations.
RESULTS
Fifty-two participants completed the baseline assessments. Data were available on 47 of the participants at month 3 and 45 at month 6 (3 participants withdrew for personal reasons and the remaining 4 did not complete the study because they did not have surgery at our center within the study timeframe). The majority of our participants were men (n = 39, 75%) with an average age of 61.3 years (SD = 9.3 years) and 9.1 years (SD = 4.1 years) of PD. All were white and non-Hispanic/Latino. The subthalamic nucleus was the surgical target in all but one of the participants.
Symptom and behavioral goals.
Based on verbatim quotes, participants' symptoms were sorted into the following categories: tremor, gait, rigidity, dyskinesias, other medication side effects, nonmotor, and other motor. The medication side effects category included any physical symptom that participants directly attributed to their medications (e.g., drowsiness). We recognize that patients may misattribute symptoms of PD to their medications and that there is considerable overlap between these categories. Consistent with our desire to better understand the patient's experience and goals for DBS, we decided to code any symptom that patients directly attributed to their medications in the medication side effects category. Nonmotor symptoms included complaints regarding cognitive, psychiatric, sleep, fatigue, and pain symptoms. Other motor symptoms represented a mix of participants' motor symptom concerns not addressed in the previous symptom categories, such as on/off fluctuations, off state (other than specifically related to gait), bradykinesia, and dystonia.
Similarly, participants' verbatim responses to the behavioral goals questions were divided into the following categories: social, avocational pursuits, activities of daily living (ADL), work, driving, and other. The social category was comprised of goals with a clear and explicit social component (e.g., attend church) and often included the expressed desire to re-engage in previously enjoyable activities with specific family members. Avocational pursuits included all hobbies (e.g., woodworking), exercise, and sports-related activities. The ADL category consisted of specific behavioral activities important for basic daily activities such as drinking, dressing, and eating. Paid employment, volunteer work, and household chores comprised the work category. Finally, the other category included an amalgam of activities most often related to medication use, such as reducing medication costs or need for frequent dosing. Relatively vague responses, such as “improve my quality of life,” were also included in the other category.
Figure 1 illustrates the total number of symptom and behavioral goals with their relative rankings reported in each category. The blue bars represent the top-ranked goal whereas the yellow and green bars represent the second- and third-ranked goals, respectively. Control of tremor followed by improvements in gait and nonmotor symptoms were the top-ranked symptom goals, whereas social, avocational, and ADL goals were the top behavioral goals.
Figure 1. Top 3 endorsed symptom and activity goals.
ADL = activities of daily living; Med SE = medication side effects.
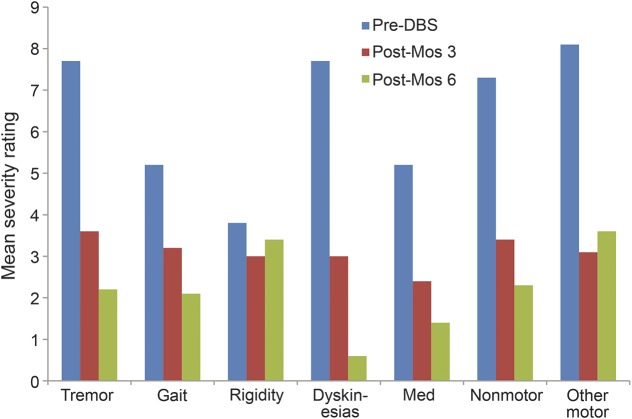
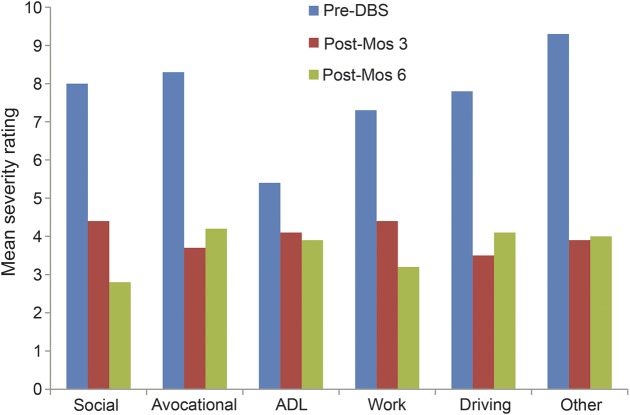
Significant improvements following DBS surgery were observed for both the SCR measures and the participant-identified symptom and behavioral goals (table 1). Examination of the effect size data indicates that large effect sizes (i.e., Cohen d ≥0.8)19 were evident on all of the outcome measures with particularly strong effect sizes evident for the UPDRS-III and the participant-identified primary symptom and behavioral goals (representing a variety of different symptom and behavioral goal categories). More detailed examination of the impact of DBS surgery on different participant-identified symptom goal categories revealed evidence of greater improvements in tremor, gait, dyskinesias, medication side effects, nonmotor, and other motor symptoms following DBS surgery vs the much smaller improvements evident in rigidity from the participants' perspective (time × symptom category interaction F(12,41) = 2.78, p = 0.007; figure 2). There were no significant differences in the magnitude of improvements in participants' behavioral goal severity ratings across specific activity categories (time × activity category interaction F(10,41) = 0.33, p = 0.966), suggesting that similar improvements were evident in all behavioral goal categories over time (figure 3).
Table 1.
Change over time in standard and participant-defined outcome measures
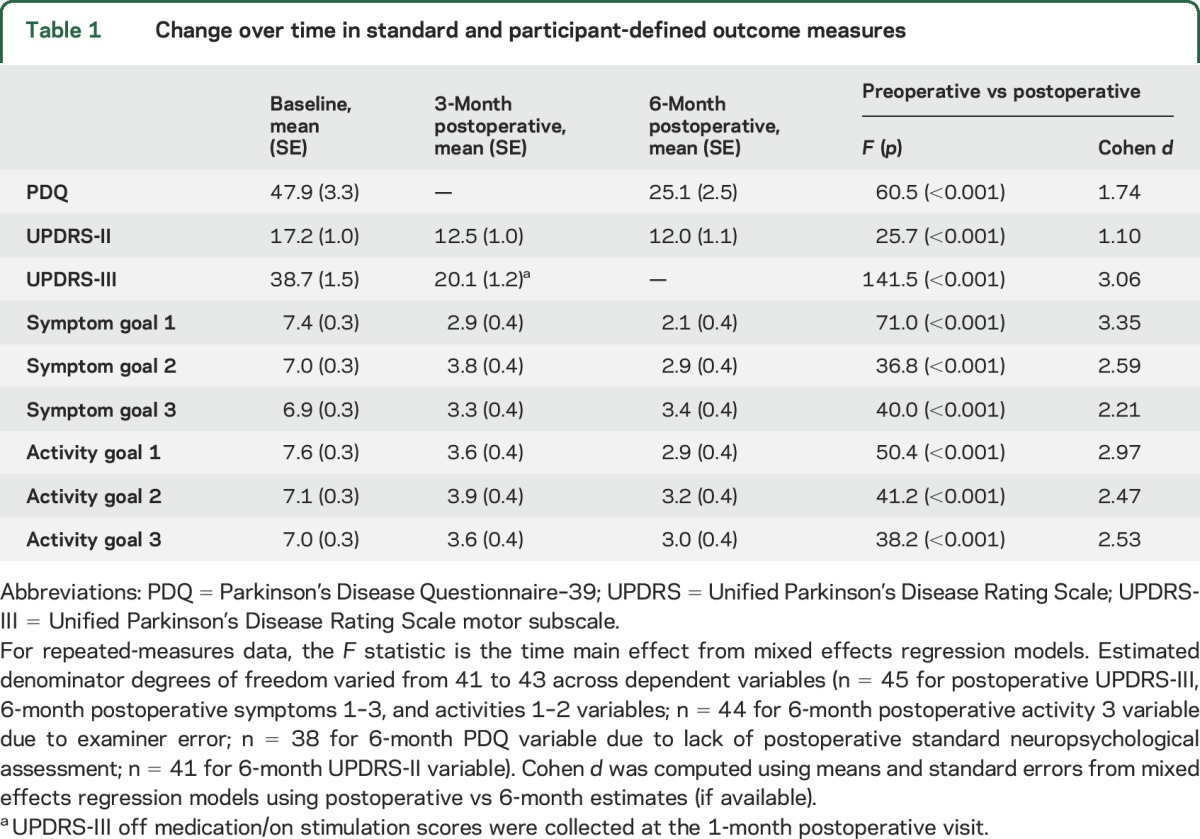
Figure 2. Change in symptom severity ratings over time by category.
DBS = deep brain stimulation.
Figure 3. Change in activity severity ratings over time by category.
ADL = activities of daily living; DBS = deep brain stimulation.
Correlations with standard clinical research metrics.
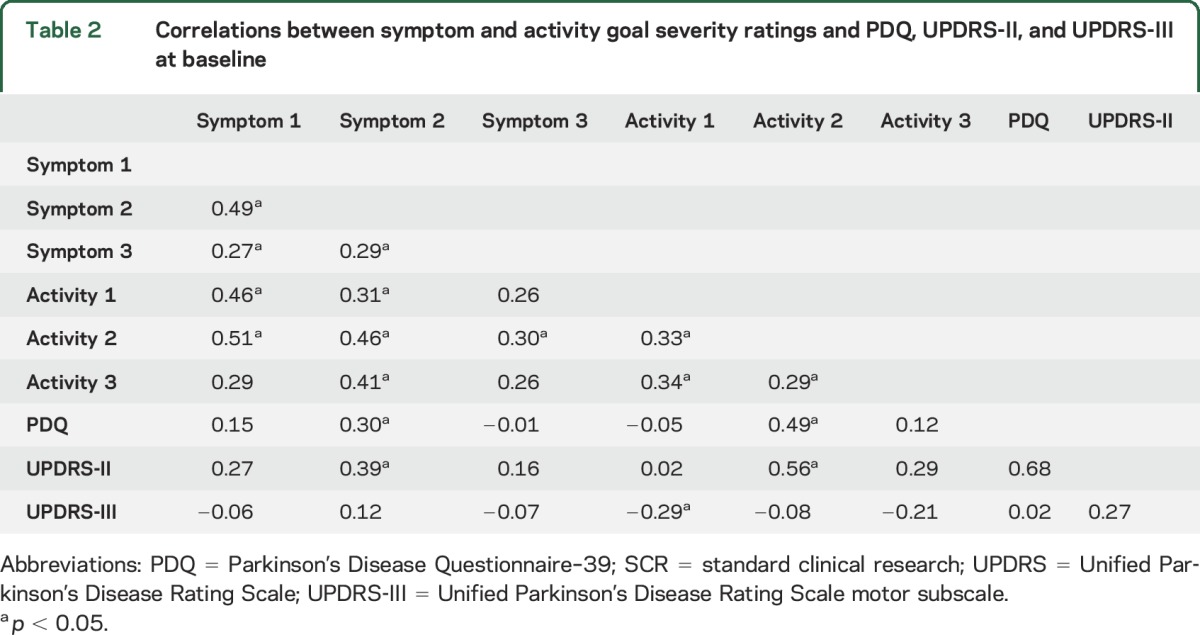
The majority of the symptom and behavioral goal severity measures were significantly correlated with each other prior to DBS surgery (i.e., 10 out of 12 possible correlations were significant; all r range = 0.27–0.51, all p < 0.05). In contrast, the participant-identified severity measures were generally not consistently significantly correlated with the SCR outcome metrics (i.e., 13 out of 18 correlations were nonsignificant; r range 0.02–0.29, p > 0.05) (table 2).
Table 2.
Correlations between symptom and activity goal severity ratings and PDQ, UPDRS-II, and UPDRS-III at baseline
DISCUSSION
Our data provide a systematic qualitative and quantitative assessment of patients' individually defined goals for DBS with respect to symptoms of PD and participation in behavioral goals. Patients most often identified improvements in tremor or fine motor control as their top symptom goal. Patients' goals also encompassed a number of other symptoms, including nonmotor symptoms. In addition to specific symptom goals, we queried patients regarding their behavioral goals. Avocational pursuits, work, and social relationships are among the primary behavioral goals patients expressed. These findings mirror similar data from studies examining epilepsy surgery patients' goals for surgery, highlighting that patients' expectations extend beyond seizure control and include cognitive, psychiatric, work, social, and driving goals.20 On average, our patients described limitations to their current ability to participate in valued activities with respect to both their symptom and behavioral goals prior to DBS. Following DBS surgery, improvements were apparent over time in patients' symptom and behavioral goals, with the exception of less perceived benefits in rigidity following DBS relative to other symptom goals.
Our study differs in important ways from other studies, which have examined patients' goals and expectations in the treatment of PD.7,8 Similar to our study, a prior study7 incorporated a semi-structured interview into their methods to better understand patients' expectations and postoperative subjective perceived benefit; however, this study did not incorporate patient-reported severity ratings that would have allowed more detailed quantitative assessment of perceived benefit. In contrast, another report8 provided detailed quantitative assessment of patients' perceptions of benefit following treatment for PD symptoms as measured using an adaptation of the Patient Center Outcomes Questionnaire, but the items included in the measure were derived from clinicians' experiences and did not specifically reflect patient-identified goals, as did our study. We sought to better understand patients' individually defined goals for DBS from both qualitative and quantitative perspectives.
The general absence of significant correlations between most of the participant-identified goal severity ratings and SCR metrics suggests that novel and nonredundant information may be gleaned by systematically assessing patients' goals. Some of the symptoms our patients described are evaluated using the UPDRS-III, but others are captured with the less commonly reported UPDRS parts I, II, and IV. Neither these additional UPDRS measures nor the PDQ fully assess the richness of the behavioral goals our patients expressed; for example, the PDQ does not tap into work-related goals or driving. Social and avocational pursuits, as expressed by our patients, are only assessed using a global vague question in the PDQ regarding the impact of PD in limiting a patient's ability to engage in preferred leisure activities. We recommend that other DBS teams systematically assess patients' goals for DBS and consider quantifying them using visual analogue scales. We have argued elsewhere for the need for a more comprehensive assessment of outcome that includes traditional outcome measures as well as functional and patient-specific metrics.21
Importantly, not all patients with DBS experience a satisfaction gap. Our patients clearly reported improvements in their individually defined symptom and behavioral goals after DBS surgery. In fact, the effect size data suggest that DBS may be even more efficacious in addressing the things that patients care most about, particularly patients' primary symptom and behavioral goals, relative to many other SCR outcome measures. Importantly, these data hold true regardless of the category of goals, suggesting that DBS was highly effective in addressing a variety of symptom and behavioral goals that individual patients identified as most important. This observation raises the intriguing possibility that patients may be more attentive to and expect greater benefit in those goals they value most highly. This observation merits further investigation in future studies. Interestingly, the improvements in patients' defined goals extended to PD symptoms (e.g., nonmotor), whose responsiveness to DBS is not as well-established as that of motor symptoms. Whether these differences reflect varying definitions of benefit (or the perception of benefit) between patients' vs clinicians' evaluations is uncertain. Regardless of whose definition of benefit over time is the most accurate, we argue that the patients' perspectives are critical particularly in the context of elective neurosurgery. These findings challenge us to continue to consider what perspectives we are not measuring in the consideration of evaluating outcome following DBS surgery. Further, our data illustrate how patient-completed outcome metrics, such as the UPDRS-II and PDQ, do not always reflect patients' goals. Truly patient-centered care should include direct assessment of patients' stated goals and values and not simply patient-completed measures.
These findings might not be generalizable onto other patient groups with PD. It is possible that patients who pursue DBS may differ in important ways from patients who do not pursue DBS. For example, patients who pursue DBS may have a stronger family support system and advocacy, which might affect the patients' stated goals (e.g., it is possible that due to the strong social support to pursue DBS, our sample may not have rated social activities as highly as another sample with PD). In addition, as part of the selection process, we screen out patients with clinically meaningful cognitive symptoms and ensure that any psychiatric symptoms are well-controlled prior to moving forward14; thus, these nonmotor symptom goals may be relatively minimized in our sample and potentially even more prominent in other PD groups. Our team also is attentive to patients' stated goals for DBS and, in some cases, patients have not been offered surgery due to unrealistic expectations14; thus, our sample may not be representative of other DBS patients. Our small sample size precluded our ability to examine the potential effect of PD phenotype or site of surgery, which are potentially important considerations. Finally, this was an open study. It is possible that participation in a study whose design implies the value the clinical care team places on patients' goals and satisfaction may have resulted in a positive response bias. These are empirical questions that require additional study in addition to more long-term follow-up to determine if the findings evident in the short-term following DBS surgery persist.
Despite these potential limitations, our data provide valuable insights into the goals, values, and preferences voiced by patients with PD regarding their decisions to seek out DBS. Systematic assessment of patients' goals prior to and following DBS surgery provides another window, or way of knowing, into PD that may not only benefit our patients but also inform future basic science and ethics research by yielding unique insights into living a life with PD that are not available to most clinical researchers.
GLOSSARY
- ADL
activities of daily living
- DBS
deep brain stimulation
- PD
Parkinson disease
- PDQ
Parkinson's Disease Questionnaire–39
- SCR
standard clinical research
- UPDRS
Unified Parkinson's Disease Rating Scale
- UPDRS-III
Unified Parkinson's Disease Rating Scale motor subscale
AUTHOR CONTRIBUTIONS
Cynthia S. Kubu: Literature search, hypothesis generation, study design, data collection, data analyses, data interpretation, writing, figures. Scott E. Cooper: hypothesis generation, study design, data interpretation, writing. Andre Machado: hypothesis generation, study design, data interpretation, writing. Thomas Frazier: data analyses, data interpretation, writing, figures. Jerrold Vitek: hypothesis generation, study design, data interpretation, writing. Paul Ford: literature search, hypothesis generation, study design, data collection, data interpretation, writing.
STUDY FUNDING
The study was funded by the National Institute of Neurologic Disorders and Stroke, RC1NS068086. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurologic Disorders and Stroke or the National Institutes of Health.
DISCLOSURE
C. Kubu received grant funding from the NIH during this study. S. Cooper and A. Machado report no disclosures relevant to the manuscript. T. Frazier reports federal funding or research support from, acted as a consultant to, received travel support from, and/or received a speaker's honorarium from the Cole Family Research Fund, Simons Foundation, Ingalls Foundation, Forest Laboratories, Ecoeos, IntegraGen, Kugona LLC, Shire Development, Bristol-Myers Squibb, National Institutes of health, and the Brain and Behavior Research Foundation. J. Vitek reports personal fees from Medtronic, Boston Scientific, and Surgical Information Systems outside of the submitted work. P. Ford received grant funding from the NIH during this study. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Institute of Medicine. Cross the Quality Chasm: A New Health System for the 21st Century. IOM, PDF Report of Brief Summary. 2001. Available at: iom.nationalacademies.org/∼/media/Files/Report%20Files/2001/Crossing-the-Quality-Chasm/Quality%20Chasm%202001%20%20report%20brief.pdf. Accessed February 16, 2016. [Google Scholar]
- 2.Kubu C, Frazier T, Machado A, Cooper S, Ford P. More than tremor: goals and benefits associated with DBS from the patient's perspective. Am J Bioeth Neurosci 2016;7:W12–W13. [Google Scholar]
- 3.Ford PJ, Kubu CS. Stimulating debate: ethics in a multidisciplinary functional neurosurgery committee. J Med Ethics 2006;32:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kubu CS, Ford PJ. Ethics in the clinical application of neural implants. Camb Q Healthc Ethics 2007;16:317–321. [DOI] [PubMed] [Google Scholar]
- 5.Okun MS, Foote KD. A mnemonic for Parkinson disease patients considering DBS: a tool to improve perceived outcome of surgery. Neurologist 2004;10:290. [DOI] [PubMed] [Google Scholar]
- 6.Okun MS, Rodriguez RL, Mikos A, et al. Deep brain stimulation and the role of the neuropsychologist. Clin Neuropsychol 2007;21:162–189. [DOI] [PubMed] [Google Scholar]
- 7.Maier F, Lewis CJ, Horstkoetter N, et al. Patients' expectations of deep brain stimulation, and subjective perceived outcome related to clinical measures in Parkinson's disease: a mixed-method approach. J Neurol Neurosurg Psychiatry 2013;84:1273–1281. [DOI] [PubMed] [Google Scholar]
- 8.Nisenzon AN, Robinson ME, Bowers D, et al. Measurement of patient-centered outcomes in Parkinson disease: what do patients really want from their treatment? Parkinsonism Relat Disord 2011;2011:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson's disease. N Engl J Med 2006;355:896–908. [DOI] [PubMed] [Google Scholar]
- 10.Benabid AL, Chabardes S, Mitrofanis J, et al. Deep brain stimulation of the subthalamic nucleus for the treatment of Parkinson's disease. Lancet Neurol 2009;8:67–81. [DOI] [PubMed] [Google Scholar]
- 11.Weaver FM, Follett I, Stern M, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA 2009;301:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schupbach M, Gargiulo M, Welter ML, et al. Neurosurgery in Parkinson disease: a distressed mind in a repaired body? Neurology 2006;66:1811–1816. [DOI] [PubMed] [Google Scholar]
- 13.Agid Y, Schupbach M, Gargiulo M, et al. Neurosurgery in Parkinson's disease: the doctor is happy, the patient less so? J Neural Transm Suppl 2009;70:409–414. [DOI] [PubMed] [Google Scholar]
- 14.Abboud H, Mehanna R, Machado A, et al. Comprehensive, multidisciplinary deep brain stimulation screening for Parkinson patients: no room for “short cuts.” Mov Disord 2014;1:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkinson C, Fitzpatrick R, Peto V, Greenhall R, Hyman N. The Parkinson’s Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing 1997;26:353–357. [DOI] [PubMed] [Google Scholar]
- 16.Fahn S, Elton RL; UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, eds. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan; 1987:153–163. [Google Scholar]
- 17.Schwarz G. Estimating the dimension of a model. Ann Statistic 1978;6:461–464. [Google Scholar]
- 18.Akaike H. Factor analysis and AIC. Psychometrika 1987;52:317–332. [Google Scholar]
- 19.Cohen JA. Power primer. Psychol Bull 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 20.Taylor DS, McMackin D, Staunton H, Delanty N, Phillips J. Patients' aims for epilepsy surgery: desires beyond seizure freedom. Epilepsia 2001;42:629–633. [DOI] [PubMed] [Google Scholar]
- 21.Kubu CS, Ford PJ. Beyond mere symptom relief in deep brain stimulation: an ethical obligation for multi-faceted assessment of outcome. AJOB Neurosci 2012;3:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]