Key Clinical Message
A 64‐year‐old female was diagnosed with adult T‐cell leukemia/lymphoma. She then underwent an unrelated allogeneic bone marrow transplantation with a reduced‐intensity regimen. She achieved engraftment followed by HHV‐6 encephalopathy. This was complicated by Chryseobacterium indologenes pneumonia. Chryseobacterium indologenes is now a possible emergent organism resistant to carbapenem after transplantation.
Keywords: Adult T‐cell leukemia/lymphoma, carbapenem, Chryseobacterium indologenes, pneumonia, stem cell transplantation
Introduction
Chryseobacterium indologenes (previously classified as Flavobacterium indologenes) is a Gram‐negative rod organism found in soil and plants. Although this bacterium only rarely causes human disease, it is sometimes found in food and water sources, usually in hospitals as a nosocomial transinfection 1, 2, 3. Understandably, an immunocompromised host may be affected by C. indologenes. In approximately half of C. indologenes infection cases, the organism is present on an indwelling device, and severe illness is the result in half of cases 4, 5, 6. An even more troublesome fact is that C. indologenes presents a high rate of natural resistance against broad‐spectrum cefem compounds including carbapenem 7. Worldwide reports of C. indologenes infections in humans have been increasing, although reports of stem cell transplantation (SCT) recipients infected with C. indologenes are still rare. Here, we describe the first case of a patient with C. indologenes pneumonia after an SCT for adult T‐cell leukemia/lymphoma (ATLL), which illustrates the increasing risk of this emergent organism during chemotherapy for hematologic malignancies.
Case Report
A 64‐year‐old female was diagnosed with adult T‐cell leukemia/lymphoma (ATLL), which became clinically overt as acute and lymphoma type 4 months prior to a bone marrow transplantation (BMT). She first received a modified LSG15 regimen 8, with three intensified and alternating regimens of VCAP (vincristine, cyclophosphamide, doxorubicin, and prednisolone), AMP (adriamycin, MCNU, and prednisolone), and VECP (vindesine, etoposide, carboplatin, and prednisolone), followed by anti‐CCR4 antibody, which led to a good partial response. The patient had undergone an unrelated BMT following a reduced‐intensity preparation regimen (RIST) consisting of fludarabine 25 mg/m2 for 5 days and busulfan 0.8 mg/kg ×4/day for 4 days, and 2 Gy total body irradiation (TBI). With combined tacrolimus and short‐term methotrexate (MTX) as graft‐versus‐host disease (GVHD) prophylaxis, she achieved engraftment uneventfully on day 19.
On day 50, a routine cytomegalovirus (CMV) antigenemia testing converted to positive, and we initiated treatment with valganciclovir (valGCV) 180 mg/day orally. This preemptive therapy 9 was effective, and the CMV antigenemia disappeared on day 70. On day 61, the patient's course was complicated with HHV‐6 encephalopathy, with a sudden‐onset consciousness disturbance progressing over a 12‐h span. The diagnosis of HHV‐6 encephalitis was made based on the detection of HHV‐6 DNA 2.0 × 105 copies/mL in the patient's cerebrospinal fluid (CSF) the following day (day 62). As soon as the encephalitis was diagnosed, we switched the valGCV to foscarnet 60 mg/kg, twice a day. On day 84, a cranial MRI scan showing diffuse high intensity on the limbic system confirmed the diagnosis of HHV‐6 encephalitis.
Despite the antiviral therapy, systemic convulsions impaired the patient's pulmonary condition, and she required mechanical ventilator support on day 65. She was intermittently febrile beginning 1 day after intubation (day 66). Her neutrophil count was 6895/μL at the onset of the febrile episode, indicating the fever was not neutropenic. We started meropenem (0.5 g, every 8 h) empirically. A tracheotomy was performed on day 79. On day 82, a chest X‐ray showed an infiltration shadow on bilateral lungs, and the patient's condition deteriorated to extensive acute respiratory distress syndrome (ARDS) within 3 days (day 85) (Fig. 1). Sputum collected from the intratracheal tube on day 78 revealed C. indologenes. Sputum was suppurative (Miller & Jones classification P3), and Geckler classification was 5. No other antimicrobial‐resistant organisms were recovered from her sputum. She underwent tazobactam/piperacillin as antimicrobial target therapy and meropenem as an empirical treatment, but died of pneumonia (respiratory failure) due to C. indologenes on day 91. The antimicrobial susceptibilities of the patient's isolate are listed in Table 1.
Figure 1.
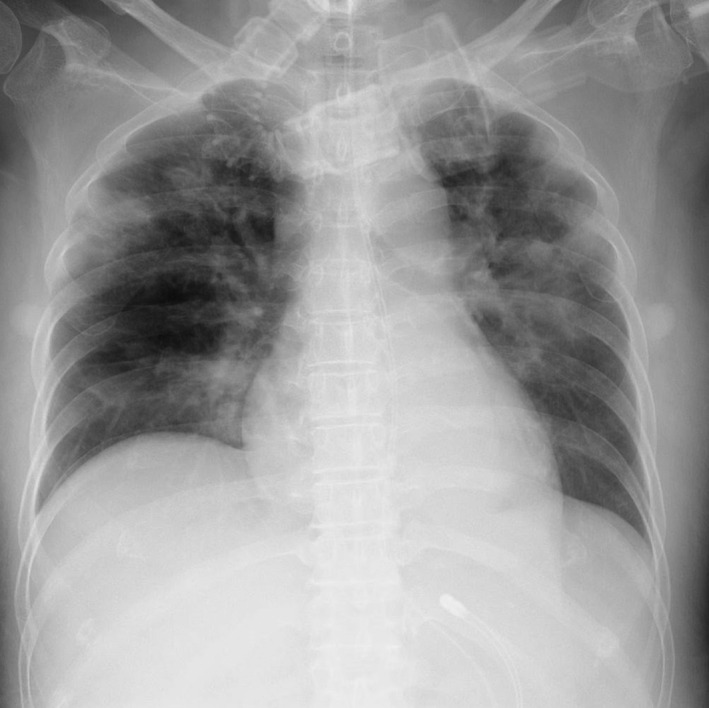
On day 85, diffuse infiltration shadow in bilateral lungs was revealed by a chest X‐ray of the patient, a 64‐year‐old female.
Table 1.
Antimicrobial susceptibilities of isolated Chryseobacterium indologenes from the patient's sputum culture
| Antimicrobial | MIC | Sensitivity |
|---|---|---|
| TAZ/PIPC | 4 | S |
| CTM | >64 | a |
| CAZ | 8 | S |
| CPZ/SBT | 8 | S |
| CPR | 2 | S |
| AZT | >64 | R |
| MINO | 1 | S |
| IPM/CS | 32 | R |
| MEPM | 32 | R |
| CPFX | 4 | R |
| LVFX | 4 | I |
| AMK | 16 | S |
MIC, minimum inhibitory concentration; S, sensitive; I, intermediate; R, resistant; TAZ/PIPC, tazobactam/piperacillin; CTM, cefotiam; CAZ, ceftazidime; CPZ/SBT, sulbactam/cefoperazone; CPR, cefpirome; AZT, azactam; MINO, minocycline; IPM/CS, imipenem/cilastatin; MEPM, meropenem; CPFX, ciprofloxacin; LVFX, levofloxacin; AMK, amikacin.
Breakpoints were adapted according to Clinical and Laboratory Standards Institute (CLSI) criteria. Susceptibility was determined by disk diffusion, following the CLSI recommendations (CLSI 2010, Performance Standards for Antimicrobial Susceptibility Testing; Twentieth Informational Supplement, M100‐S20, Jan. 2010).
Not applicable.
Discussion
The pathogenicity of C. indologenes has not been well defined, but some individuals infected with this bacterium were reported to have had an invasive infection with a fatal outcome. The highly active protease of C. indologenes is in part responsible for its virulence 10. Reports of C. indologenes infections in humans have been increasing worldwide, but reports of this infection in SCT recipients remain rare. Some recent case reports recommend early detection of C. indologenes for an early determination of appropriate antimicrobials to eliminate it. For example, the case of a critically ill patient with ventilator‐associated pneumonia (VAP) was anecdotally described as having been diagnosed by a quantitative culture of the patient's bronchoalveolar lavage 4. One case report of a patient with peritonitis caused by C. indologenes complicated by multi‐organ failure describes treatment with an intraperitoneal injection of antibiotics 6. The proposed source of the Chryseobacterium is the environment, including the air, water, or perhaps food. Although C. indologenes is an environmental organism, it is not a typical, hospital‐acquired pneumonia pathogen. We propose that for SCT recipients, careful monitoring for colonization and infection should be conducted by routine weekly culture.
In a recent analysis of a vast number of isolates of C. indologenes collected worldwide, the majority of local hospital antibiograms for this organism remained susceptible to trimethoprim–sulfamethoxazole (TMP‐SMZ) 4, 11. Interestingly, isolates from outside of Asia were relatively sensitive to beta‐lactams and fluoroquinolones, whereas isolates from the Asia–Pacific region were not. With the development of broad‐spectrum cephalosporins, the majority of Gram‐negative rods (other than Stenotrophomonas maltophilia and multidrug‐resistant nosocomial pathogens) affecting immunocompromised patients were eliminated within a few decades 12. C. indologenes, we believe, should be added to the list of emerging pathogens to watch for during SCT.
Of note, the resistance of C. indologenes to broad‐spectrum cephalosporins is thought to be increasing rapidly in Asia. Given the resistance of such an organism to broad‐spectrum cephalosporin, we propose that a prudent selection of antimicrobials should be required as empirical therapy for SCT recipients. The emergence of highly resistant organisms is of particular concern in the especially vulnerable immunosuppressed population of patients after chemotherapy for hematologic malignancy and after stem cell transplantation. Major guidelines and local protocols may not yet take these less‐common organisms into account in empiric antibiotic recommendations. As the experts point out, selection of empiric antibiotics in such seriously ill patients may need to be reconsidered, as carbapenems may no longer be the “biggest gun” for some populations. The broader problem of multidrug‐resistant Gram‐negative organisms such as Acinetobacter and extended spectrum beta‐lactamase (ESBL)‐producing Enterobacteriaceae in the Middle East and Asia–Pacific regions may be influenced by local patterns of antibiotic use. Although the lack of management for these organisms makes the treatment outcome unfavorable, empiric therapy with carbapenem as a single agent for febrile neutropenia might no longer be the gold standard.
Conflict of Interest
The authors declare that they have no conflict of interest.
Authorship
OI and MU: managed the patient's case, contributed to the literature search, and wrote the manuscript. OI: also made substantial contributions to the concept and design of this report. MU: qualified the patient's data, suggested important intellectual content, and reviewed the manuscript. MU: managed the research. Both authors read and approved the final version of the manuscript.
References
- 1. Hsueh, P. R. , Hsiue T. R., Wu J. J., Teng L. J., Ho S. W., Hsieh W. C., et al. 1996. Flavobacterium indologenes bacteremia: clinical and microbiological characteristics. Clin. Infect. Dis. 23:550–555. [DOI] [PubMed] [Google Scholar]
- 2. Hsueh, P. R. , Teng L. J., Yang P. C., Ho S. W., Hsieh W. C., and Luh K. T.. 1997. Increasing incidence of nosocomial Chryseobacterium indologenes infections in Taiwan. Eur. J. Clin. Microbiol. Infect. Dis. 16:568–574. [DOI] [PubMed] [Google Scholar]
- 3. Chen, F. L. , Wang G. C., Teng S. O., Ou T. Y., Yu F. L., and Lee W. S.. 2013. Clinical and epidemiological features of Chryseobacterium indologenes infections: analysis of 215 cases. J. Microbiol. Immunol. Infect. 46:425–432. [DOI] [PubMed] [Google Scholar]
- 4. Monteen, M. R. , Ponnapula S., Wood G. C., Croce M. A., Swanson J. M., Boucher B. A., et al. 2013. Treatment of chryseobacterium indologenes ventilator‐associated pneumonia in a critically Ill Trauma patient: a case report. Ann. Pharmacother. 47:1736–1739. [DOI] [PubMed] [Google Scholar]
- 5. Olbrich, P. , Rivero‐Garvía M., Falcón‐Neyra M. D., Lepe J. A., Cisneros J. M., Marquez‐Rivas J., et al. 2014. Chryseobacterium indologenes central nervous system infection in infancy: an emergent pathogen? Infection 42:179–183. [DOI] [PubMed] [Google Scholar]
- 6. Afshar, M. , Nobakht E., and Lew S. Q.. 2013. Chryseobacterium indologenes peritonitis in peritoneal dialysis. BMJ Case Rep. 2013:pii: bcr2013009410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang, J. C. , Hsueh P. R., Wu J. J., Ho S. W., Hsieh W. C., and Luh K. T.. 1997. Antimicrobial susceptibility of flavobacteria as determined by agar dilution and disk diffusion methods. Antimicrob. Agents Chemother. 41:1301–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsukasaki, K. , Utsunomiya A., Fukuda H., Shibata T., Fukushima T., Takatsuka Y., et al. 2007. VCAP‐AMP‐VECP compared with biweekly CHOP for adult T‐cell leukemia‐lymphoma: Japan Clinical Oncology Group Study JCOG9801. J. Clin. Oncol. 25:5458–5464. [DOI] [PubMed] [Google Scholar]
- 9. Kanda, Y. , Mineishi S., Saito T., Saito A., Ohnishi M., Niiya H., et al. 2002. Response‐oriented preemptive therapy against cytomegalovirus disease with low‐dose ganciclovir: a prospective evaluation. Transplantation 73:568–572. [DOI] [PubMed] [Google Scholar]
- 10. Hsueh, P. R. , Wu J. J., Hsiue T. R., and Hsieh W. C.. 1995. Bacteremic necrotizing fasciitis due to Flavobacterium odoratum. Clin. Infect. Dis. 21:1337–1338. [DOI] [PubMed] [Google Scholar]
- 11. Kirby, J. T. , Sader H. S., Walsh T. R., and Jones R. N.. 2004. Antimicrobial susceptibility and epidemiology of a worldwide collection of Chryseobacterium spp: report from the SENTRY Antimicrobial Surveillance Program (1997‐2001). J. Clin. Microbiol. 42:445–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dettenkofer, M. , Wenzler‐Röttele S., Babikir R., Bertz H., Ebner W., Meyer E., et al. 2005. Surveillance of nosocomial sepsis and pneumonia in patients with a bone marrow or peripheral blood stem cell transplant: a multicenter project. Clin. Infect. Dis. 40:926–931. [DOI] [PubMed] [Google Scholar]


