Abstract
Background & objectives
In the past, patients with haemoglobinopathies were at high risk of acquiring hepatitis C virus (HCV) due to multiple transfusions before HCV screening. In these patients, the coexistence of haemochromatosis and chronic hepatitis C (CHC) often leads to more severe liver disease. We assessed the HCV prevalence, clinical characteristics and outcome in this setting with particular attention to the response to treatment including therapies with the new direct acting antivirals (DAAs).
Methods
The medical records of 81 consecutive patients followed the last 15 years were reviewed retrospectively.
Results
43/81 (53%) patients were anti-HCV positive including 31/43 (72.1%) with CHC (HCV-RNA positive; age 25±7 years; 45.2% with genotype 1b; 19.4% cirrhotics; baseline ferritin 887 ng/ml; range: 81–10.820). Thirty patients received IFN-based therapy with or without ribavirin with sustained virological response (SVR) in 14/30 (46.7%). Eleven patients (9 non-responders to IFN-based therapies, one in relapse and one naïve) received treatment with DAAs (SVR: 100%). 3/11 patients increased their transfusion needs while 1/11 reported mild arthralgias. No drug-drug interactions between DAAs and chelation agents were observed as attested by the stability of ferritin levels during treatment.
Conclusions
More than 1/3 of patients with haemoglobinopathies suffered from CHC. Response rates to IFN-based treatment seem to be similar to other patients with CHC, while most importantly, treatment with DAAs was excellent and safe even in difficult to treat patients (most null responders with severe fibrosis) suggesting that this group of HCV patients should no longer be regarded as a difficult to treat.
Keywords: Thalassemic Syndromes, HCV, Treatment, DAAs
Introduction
Hepatitis C virus (HCV) infection is one of the leading causes of chronic liver disease worldwide. Indeed, the most recent estimates of disease burden show an increase in seroprevalence over the last 15 years to 2.8%, resulting in >185 million infections worldwide.1,2 In the past, patients with haemoglobinopathies represented a population at high risk of acquiring HCV as before 1992 there was no blood screening for HCV. As a result, the prevalence of antibodies to HCV (anti-HCV) in patients with thalassemia varies depending on the population studied between 12% and 85%.3
As chelation therapy with new drugs seems to prevent cardiac damage and improve survival, the chronic liver disease has emerged as a critical clinical issue in this setting. The ‘hit’ to the liver is at least dual: high frequency of chronic viral infections, especially HCV, and secondary hemochromatosis of the liver due to multiple transfusions and dyserythropoiesis.4 Furthermore, chronic hepatitis C (CHC) and iron overload were proved to be independent risk factors for liver fibrosis progression and their concomitant presence results in a striking increase of the risk.5 So, it is essential for patients with haemoglobinopathies to have a multidisciplinary approach, in order to achieve both effective chelation therapy and treatment of CHC, with a view to preventing liver complications and improve morbidity and mortality.3,4,6
Clinical care for patients with the HCV-related liver disease has improved considerably during the last two decades, thanks to the enhanced understanding of the pathophysiology of the disease, and the therapeutic advances.7 Until 2011, interferon-α (IFN-α) with or without ribavirin was the approved treatment for patients with thalassemia, resulting in sustained virological response (SVR) rates of 40–60%.8–10 The recent license of Direct Acting Antivirals (DAAs) opened new venues in the treatment of CHC regarding extremely high SVR rates and better compliance due to their oral route of administration and the rarity of significant side effects.7 In addition, the new treatment modalities for HCV infection permit avoiding ribavirin use in the majority of cases, which is well-known to increase the transfusion needs by a median of 30–40% as well as side effects in these patients.9,11–17 The recent EASL treatment recommendations on hepatitis C,7 suggest that patients with haemoglobinopathies should be treated with an IFN-free regimen, without ribavirin. However, patients with haemoglobinopathies and CHC have been excluded from the major clinical trials that led to the approval of DAAs. Indeed, so far, only some case reports have been published showing favourable results after DAAs administration in CHC patients with concurrent haemoglobinopathies.18,19 Hence, at present, no experience is available regarding the safety and efficacy of DAAs in this population which is traditionally considered difficult to treat, because of the coexistence of liver hemochromatosis, the more advanced liver disease and the usual nonresponse or relapse to previous IFN-based therapies.
Accordingly, the aims of our study were:
° to assess the prevalence of CHC in patients with haemoglobinopathies in Central Greece a region with low anti-HCV prevalence (0.34%) in the general population;20
° to report the clinical characteristics and the outcome in this setting giving particular attention to the response to treatment after utilizing the new DAAs and a strong emphasis on the safety and efficacy of use in this specific population.
Patients and Methods
The medical records of 81 consecutive patients with haemoglobinopathies (70 with β-thalassemia major and 11 with sickle cell anaemia/β-thalassemia) followed by our Department between 2000 and 2015 were retrospectively analysed. For patients who had received IFN-based treatment, sustained virological response (SVR) was defined according to the European Association for the Study of the Liver (EASL) clinical practice guidelines published in 2011 (serum HCV-RNA<50 IU/ml 24 weeks after treatment withdrawal).8 For patients who received DAAs, SVR was defined according to the EASL clinical practice guidelines published in 2015 (undetectable serum HCV-RNA 12 weeks after stopping treatment).7
Anti-HCV was determined using a second- or third-generation enzyme immunoassay at least twice within six months while active virus replication was defined by the detection of HCV-RNA using a sensitive commercially available quantitative real-time PCR kit (COBAS Taqman HCV Test; cut-off of detection: 25 IU/ml).
The histological evaluation was assessed using the Knodell histologic/activity index score.21 According to previous publications of our group,22–24 for statistical reasons patients were divided into two groups according to inflammation: minimal-mild (score:0–8) and moderate-severe (score:9–18) and according to fibrosis: none-mild-moderate (score:0–2) and severe fibrosis-cirrhosis (score:3–4).
In addition, fibrosis was assessed in 11 patients who received DAAs by transient elastography (FibroScan device powered by VCTE Echosens equipped with the standard M probe). Liver stiffness measurements (LMS) were expressed as median (kPa) of all valid measurements with associated interquartile range (IQR) and success rate. LSM was considered valid if there were ten successful acquisitions and an IQR/LSM <0.3. Patients were divided into four groups; F0–F1, F1–F2, F2–F3 and F3–F4 according to Metavir score.
The study was approved by the Ethics Committee of Thessaly University, Medical School.
Statistical analysis
Results are expressed as median (range) and mean ± SD where appropriate. Data were analysed by Mann-Whitney U-test (MWU), x2 (two-by-two with Yate’s correction) and Fisher’s exact test. Two-sided p values less than 0.05 were considered as statistically significant. 95%CI were calculated with the Wilson procedure with a correction for continuity.
Results
Forty-three out of 81 patients (53%; 95%CI: 42–63.8%) tested anti-HCV positive. Thirty-one patients had CHC (31/81; 38%; 95%CI: 27.4–48.6%) as they were HCV-RNA positive. The baseline epidemiological, clinical, laboratory and histologic characteristics of CHC patients with haemoglobinopathies are shown in Table 1. All patients but one with sickle cell anaemia/β-thalassemia, were receiving chelation therapy to avoid iron overload, however, ferritin levels varied (Table 1). Liver histology before starting therapy was available in 22 patients. In total, clinical and/or histological evidence of cirrhosis had 6/31 (19.4%; 95%CI: 5–33%) patients.
Table 1.
Characteristics of patients with haemoglobinopathies and chronic hepatitis C (CHC) before starting treatment for CHC (n=31).
| Age (years, at diagnosis) | 25 (14–45) |
|
| |
| Male/Female | 17/14 |
|
| |
| β-thalassemia major | 30 |
| Sickle cell anaemia/β-thalassemia | 1 |
|
| |
| Spleenectomy (yes/no) | 20/11 |
|
| |
| Disease duration (years) | 23 (5–25) |
|
| |
| AST (IU/L) | 66 ± 56 |
| ALT (IU/L) | 79 ± 62 |
| γ-GT ( IU/L) | 38 ± 30 |
| ALP (IU/L) | 128 ± 117 |
| Albumin (g/dL) | 4.5 ± 0.5 |
| Bilirubin (mg/dl) | 2 ± 1.6 |
| PLT (×103/μl) | 369 ± 146 |
| Ferritin (ng/ml) | 887 (81–10820) |
|
| |
| HCV-RNA (IU/ml) | 1.98 × 106 (1180-2.76 × 106) |
|
| |
| Genotypes 1a/ 1b/ 2/ 3/ 4/ UD | 2/ 14 / 2/ 5/ 2/6 |
|
| |
| Cirrhosis (%) | 6 (19.4%) |
|
| |
| Histology (n=22) | |
| Grade (mild/ moderate-severe) | 8/ 14 |
| Stage (mild-moderate/ severe-cirrhosis) | 6/ 16 |
| Siderosis (I/ II/ III/ IV) | 4/ 6/ 4/ 8 |
|
| |
| Follow-up (months) | 41.5 (3–186) |
Abbreviations are same as in text. UD, undetermined
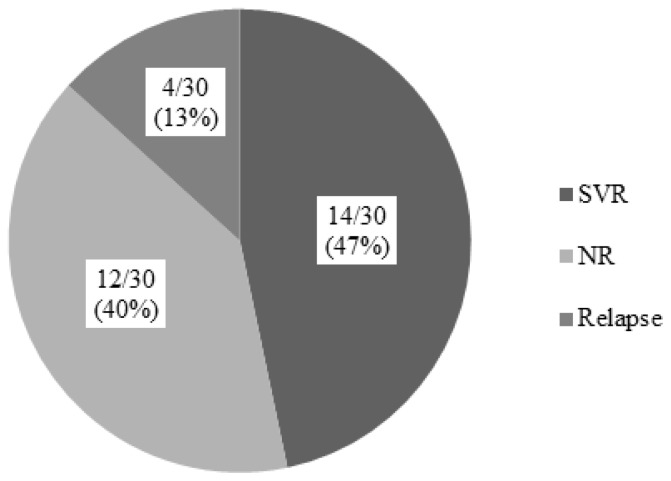
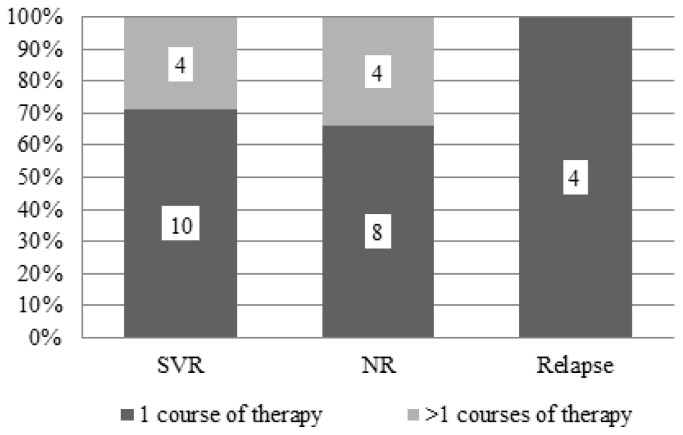
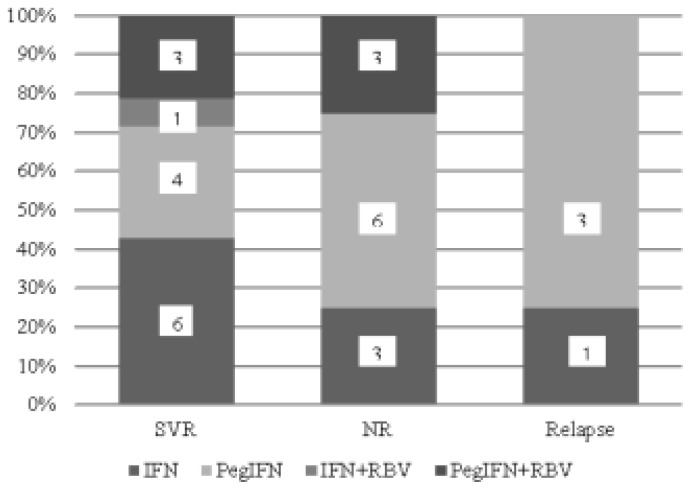
Thirty out of 31 CHC patients received IFN-based therapy with or without ribavirin. The SVR rate independently of HCV-genotype, in IFN-based treated patients, was 46.7% (14/30; 95%CI: 29–64%) (Figure 1). In fact, 22 patients had received one treatment course with IFN-based therapy, while the remaining (8/30; 26.6%; 95%CI: 11–42%) had received more than one course. In Figure 2, the type of treatment response to IFN-based therapy in respect to the number of treatment courses is shown. In Figure 3, the type of treatment response according to the IFN-based treatment schedule is also shown.
Figure 1.
Response to IFN-based therapy in 30 patients with haemoglobinopathies and chronic hepatitis C. SVR=sustained virological response, NR=no response.
Figure 2.
Response to IFN-based therapy in 30 patients with haemoglobinopathies and chronic hepatitis C according to the number of treatment courses. SVR=sustained virological response, NR=no response.
Figure 3.
Response to IFN-based therapy in 30 patients with haemoglobinopathies and chronic hepatitis C according to the kind of treatment schedule. SVR=sustained virological response, NR=no response, IFN=interferon alpha, PegIFN=pegylated interferon alpha, RBV=ribavirin.
The SVR rate in patients who received monotherapy with IFN or PegIFN (10/23; 43%; 95%CI: 23–63%) did not significantly differ compared to that observed in patients who received combination therapy with ribavirin (4/7; 57%; 95%CI: 20–94%). Two out of 7 patients who received ribavirin increased their transfusion needs. In total, 4/30 (13.3%; 95%CI: 1–25%) developed neutropenia during treatment but treatment was not discontinued while one patient presented anxiety and severe myalgias which led to treatment discontinuation.
Regarding outcome (except three patients who were lost to follow-up), 6 out of 22 (27%; 95%CI: 8–45%) patients who were not cirrhotic at initial presentation, developed cirrhosis. Two out of 6 patients who developed cirrhosis achieved an SVR with IFN-based therapies whereas 10/16 patients, who did not develop cirrhosis achieved SVR during follow-up (p>0.05). Of note, no patient died during the follow-up of the study.
Eleven patients (9 non-responders to previous treatment with IFN-based therapies, one in relapse and one naïve patient) received treatment with DAAs according to the recent EASL guidelines7 (Tables 2 and 3). The baseline epidemiological, clinical, laboratory and histologic characteristics of these 11 patients are shown in Table 2. Seven patients had MRI T2* measurements during the last six months and had mild (n=5) to moderate liver hemochromatosis (n=2; Table 2). Regarding chelation therapy, three patients were receiving deferoxamine, two deferasirox, one deferiprone and four a combination of deferoxamine with deferiprone. The estimation of liver disease stage was based on transient elastography and 7/11 (63.6%; 95%CI: 35–92%) patients had severe fibrosis (F3–F4) (Table 2).
Table 2.
Characteristics of patients with haemoglobinopathies and chronic hepatitis C before starting treatment with direct acting antivirals (n=11).
| Age (years) | 40 (33–59) |
|
| |
| Male/Female | 7/4 |
|
| |
| β-thalassemia major | 10 |
| Sickle cell anaemia/β-thalassemia | 1 |
|
| |
| AST (IU/L) | 51 ± 43 |
| ALT (IU/L) | 41 ± 33 |
| γ-GT ( IU/L) | 34 ± 21 |
| ALP (IU/L) | 76 ± 15 |
| Albumin (g/dl) | 4.2 ± 0.5 |
| Bilirubin (mg/dl) | 1.4 ± 2.1 |
| PLT (×103/μl) | 374 ± 132 |
| Ferritin (ng/ml) | 1331 (82–6222) |
|
| |
| HCV-RNA (IU/ml) | 3.9 ×106 (1.5 ×106–9.85 ×106) IU/ml |
|
| |
| Genotypes | |
| 1a/ 1b/ 2/ 3/4 | 2/ 4/ 1/ 3/1 |
|
| |
| Cirrhosis (%) | 6 (54.5%) |
|
| |
| Elastography (kPa) | 15.5 (9.2–34.3) |
| F0–F1 | 0 |
| F1–F2 | 1 |
| F2–F3 | 3 |
| F3–F4 | 7 |
|
| |
| MRI T2* (msec) (n=7) | 13.2 (3.6–31) |
Abbreviations are same as in text.
Table 3.
Genotypes, previous treatment courses and treatment response of 11 patients with haemoglobinopathies and chronic hepatitis C who received direct acting antivirals.
| Patient | Genotype | Previous treatment | Response |
|---|---|---|---|
| 1 | 1b | PegIFN 12 months | NR |
| 2 | 1b | IFN 12 months | NR |
| 3 | 1b | IFN 18 months/ PegIFN 6 months | Relapse/ NR |
| 4 | 1b | PegIFN 8 months/ PegIFN + RBV 6 months | NR/ NR |
| 5 | 3 | PegIFN 6 months | NR |
| 6 | 3 | IFN 12 months | NR |
| 7 | 3 | IFN 12 months | NR |
| 8 | 1a | PegIFN 8 months/ PegIFN + RBV 2 months | NR/ side effects |
| 9 | 1a | PegIFN + RBV 18 months | NR |
| 10 | 4 | IFN 12 months | Relapse |
| 11 | 2 | naive | NA |
IFN=interferon alpha, PegIFN=pegylated interferon alpha, RBV=ribavirin, NR=no response, NA=not applicable.
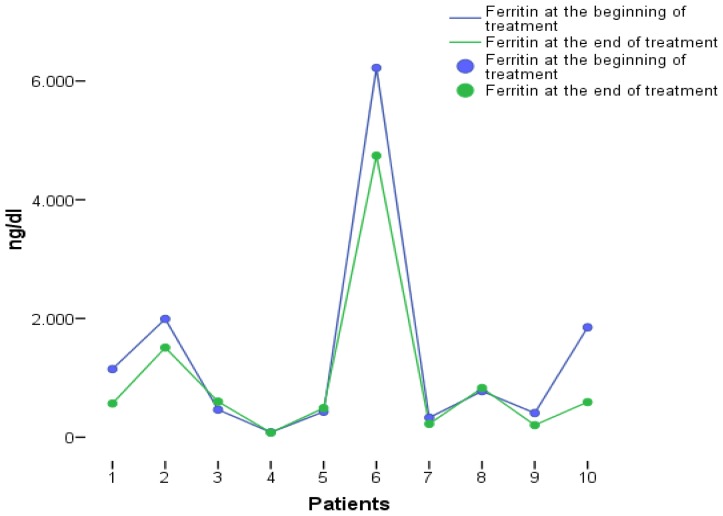
The treatment schedules with DAAs are shown in Table 4. Regarding safety, only one patient mentioned arthralgias which, however, did not lead to treatment discontinuation. Three patients increased the transfusion needs (two of them were receiving ribavirin). As far as ferritin levels are concerned, there was no significant elevation during treatment with DAAs (Figure 4). All 11 patients treated with DAAs achieved SVR at week 12 post treatment.
Table 4.
Treatment schedules with direct acting antivirals, treatment duration and side effects during treatment of 11 patients with hemoglobinopathies and chronic hepatitis C.
| Patient | Geno-type | Treatment schedule | Duration (weeks) | Increase in transfusion needs (yes/no) | Side effects |
|---|---|---|---|---|---|
| 1 | 1b | Ombitasvir/paritaprevir/rito-navir+dasabuvir+RBV | 12 | No | No |
| 2 | 1b | Sofosbuvir+Simeprevir | 12 | No | No |
| 3 | 1b | Sofosbuvir+Simeprevir+RBV | 12 | Yes | No |
| 4 | 1b | Sofosbuvir+Daclatasvir | 12 | No | Arthral-gias |
| 5 | 3 | Sofosbuvir+Daclatasvir | 24 | No | No |
| 6 | 3 | Sofosbuvir+Daclatasvir | 24 | Yes | No |
| 7 | 3 | Sofosbuvir+Daclatasvir | 24 | No | No |
| 8 | 1a | Ombitasvir/paritaprevir/rito-navir+dasabuvir+RBV | 12 | No | No |
| 9 | 1a | Sofosbuvir+Daclatasvir+RBV | 12 | No | No |
| 10 | 4 | Sofosbuvir+Ledipasvir | 12 | No | No |
| 11 | 2 | Sofosbuvir+RBV | 12 | Yes | No |
RBV=ribavirin
Figure 4.
Serum ferritin levels before and at the end of treatment with direct acting antivirals.
Discussion
The current study assessed the prevalence of anti-HCV positivity and that of CHC among patients with haemoglobinopathies, the characteristics of the patients with CHC and haemoglobinopathies, and their response to treatment with emphasis to the preliminary results of the treatment responses to DAAs in this patient group. The following three major points have risen:
the prevalence of anti-HCV and CHC in patients with haemoglobinopathies is still increased;
HCV infection is diagnosed in young age, however 20% of the patients had already cirrhosis before starting treatment and almost 50% were infected with HCV genotype 1b;
the response rates to IFN-based therapies were the same as in other patient groups, while most importantly, treatment with DAAs was very effective (SVR 100%) and safe even in difficult to treat HCV patients (most null responders with severe fibrosis/cirrhosis).
In our retrospective study, we found a prevalence anti-HCV positivity of 53% in patients with haemoglobinopathies, while more than one-third of the patients (38%) had CHC. This datum is in accordance with previous studies from Greece25,26 and other European countries.27 As far as HCV genotype is concerned, almost half of our patients were infected with genotype 1b, which is the most prevalent genotype among multitransfused patients [3,28]. In addition, nearly 20% of the patients had cirrhosis before treatment initiation which is in accordance with previous studies in patients with CHC and thalassemia,3,26,29 while the rate of progression of liver disease to cirrhosis was 27% with a median disease duration of 23 years. The progression to cirrhosis seems to be higher than that expected in other patients with CHC without haemoglobinopathies.30,31 Of note, the achievement of SVR did not influence the progression to cirrhosis which is rather dependent on the degree of liver iron overload.29
In general, the SVR rate among patients treated with IFN-based therapies (47%) was similar to previous reports in this population,3,14,15 while the SVR rate among those who received IFN/Peg-IFN monotherapy was 43%, similar as in past studies.9,12,16,17 In a small subset of patients (n=7) who received IFN/PegIFN in combination with ribavirin, the SVR rate was 57%, while only the minority of them increased their transfusion needs.3,11,13,32 However, haemolysis was reversible after drug discontinuation, and no significant iron overload worsening was noticed. According to our study, the SVR rates both in patients treated with IFN monotherapy and those treated with IFN in combination with ribavirin were similar to other CHC patients.33–35
The main and most important finding of our study, however, was the high SVR rate (100%) achieved among HCV treatment-experienced difficult (null-responders) patients with haemoglobinopathies who were treated with DAAs. All patients received the appropriate treatment according to the HCV genotype and fibrosis severity, but they considered to be difficult to treat population, as all but one, were treatment-experienced and had advanced liver fibrosis (stage F3–4 according to liver elastography). Even though the number of patients was small to drawn safe conclusions, this is one of the first studies showing quite convincingly that the SVR rate to DAAs in multitransfused difficult-to-treat HCV patients is similar to that observed in nonmulti-transfused naïve individuals with CHC.36–40
As far as safety is concerned, none of the 11 patients who received DAAs withdrew treatment because of side effects while transfusion rate was elevated in 3 patients, 2 of whom also received ribavirin. However, no significant elevation of serum ferritin levels was observed during and after treatment. In addition, all patients were under treatment with chelation agents. There are no data so far about the drug-drug interactions of chelation agents and DAAs. Although the present study was not designed to investigate pharmacodynamics, chelation treatment does not seem to affect treatment with DAAs.
Conclusion
More than one-third of patients with haemoglobinopathies are still chronically infected with CHC. Patients with haemoglobinopathies and HCV infection seem to have similar SVR rates after IFN-based treatment compared to other CHC patients. However, the most important and fascinating finding of our study was the excellent virological response rates (SVR 100%) after treatment with DAAs even in difficult to treat HCV patients (null responders with advanced fibrosis/cirrhosis). Side-effects related to DAAs treatment were minimal, while transfusions rate seems to increase in some patients treated with ribavirin, without however an elevation of ferritin levels. Therefore, our results further support the statement of EASL and AASLD guidelines that IFN-α free treatment with DAAs should be considered as first-line therapy in treatment-naïve or treatment experienced patients with or without cirrhosis due to CHC and concurrent haemoglobinopathies. Finally, chelation therapy does not seem to affect treatment with DAAs, however, current literature lacks sufficient evidence to give definitive support to these preliminary results. Thus, larger studies are warranted to ensure the safety and efficacy of DAAs in patients with haemoglobinopathies and CHC. However, our initial results strongly suggest that this group of HCV patients should no longer be regarded as a “difficult” to treat when the new DAAs are used. Our study support the need to make available the DAAs also for south-east Mediterranean and Asian countries considering the large number of patients with haemoglobinopathies and HCV in these areas.41–45
Footnotes
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–42. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Papatheodoridis G, Thomas HC, Golna C, Bernardi M, Carballo M, Cornberg M, Dalekos G, Degertekin B, Dourakis S, Flisiak R, Goldberg D, Gore C, Goulis I, Hadziyannis S, Kalamitsis G, Kanavos P, Kautz A, Koskinas I, Leite BR, Malliori M, Manolakopoulos S, Maticic M, Papaevangelou V, Pirona A, Prati D, Raptopoulou-Gigi M, Reic T, Robaeys G, Schatz E, Souliotis K, Tountas Y, Wiktor S, Wilson D, Yfantopoulos J, Hatzakis A. Addressing barriers to the prevention, diagnosis and treatment of hepatitis B and C in the face of persisting fiscal constraints in Europe: report from a high level conference. J Viral Hepat. 2016;23(Suppl 1):1–12. doi: 10.1111/jvh.12493. [DOI] [PubMed] [Google Scholar]
- 3.Di Marco V, Capra M, Angelucci E, Borgna-Pignatti C, Telfer P, Harmatz P, Kattamis A, Prossamariti L, Filosa A, Rund D, Gamberini MR, Cianciulli P, De Montalembert M, Gagliardotto F, Foster G, Grangè JD, Cassarà F, Iacono A, Cappellini MD, Brittenham GM, Prati D, Pietrangelo A, Craxì A, Maggio A Italian Society for the Study of Thalassemia and Haemoglobinopathies; Italian Association for the Study of the Liver. Management of chronic viral hepatitis in patients with thalassemia: recommendations from an international panel. Blood. 2010;116:2875–83. doi: 10.1182/blood-2009-11-248724. [DOI] [PubMed] [Google Scholar]
- 4.Hershko C. Pathogenesis and management of iron toxicity in thalassemia. Ann N Y Acad Sci. 2010;1202:1–9. doi: 10.1111/j.1749-6632.2010.05544.x. [DOI] [PubMed] [Google Scholar]
- 5.Angelucci E, Muretto P, Nicolucci A, Baronciani D, Erer B, Gaziev J, Ripalti M, Sodani P, Tomassoni S, Visani G, Lucarelli G. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100:17–21. doi: 10.1182/blood.V100.1.17. [DOI] [PubMed] [Google Scholar]
- 6.Di Marco V, Capra M, Gagliardotto F, Borsellino Z, Cabibi D, Barbaria F, Ferraro D, Cuccia L, Ruffo GB, Bronte F, Di Stefano R, Almasio PL, Craxì A. Liver disease in chelated transfusiondependent thalassemics: the role of iron overload and chronic hepatitis C. Haematologica. 2008;93:1243–6. doi: 10.3324/haematol.12554. [DOI] [PubMed] [Google Scholar]
- 7.EASL recommendations on treatment of Hepatitis C 2015. J Hepatol. 2015;63:199–236. doi: 10.1016/j.jhep.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 8.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245–264. doi: 10.1016/j.jhep.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Donohue SM, Wonke B, Hoffbrand AV, Reittie J, Ganeshaguru K, Scheuer PJ, Brown D, Dusheiko G. Alpha interferon in the treatment of chronic hepatitis C infection in thalassaemia major. Br J Haematol. 1993;83:491–497. doi: 10.1111/j.1365-2141.1993.tb04676.x. [DOI] [PubMed] [Google Scholar]
- 10.Hussein NR, Tunjel I, Basharat Z, Taha A, Irving W. The treatment of HCV in patients with haemoglobinopathy in Kurdistan Region, Iraq: a single centre experience. Epidemiol Infect. 2016;144:1634–40. doi: 10.1017/S0950268815003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Telfer PT, Garson JA, Whitby K, et al. Combination therapy with interferon alpha and ribavirin for chronic hepatitis C virus infection in thalassaemic patients. Br J Haematol. 1997;98:850–855. doi: 10.1046/j.1365-2141.1997.2953112.x. [DOI] [PubMed] [Google Scholar]
- 12.Di Marco V, Lo Iacono O, Almasio P, Ciaccio C, Capra M, Rizzo M, Malizia R, Maggio A, Fabiano C, Barbaria F, Craxi A. Long-term efficacy of alpha interferon in beta-thalassemics with chronic hepatitis C. Blood. 1997;90:2207–12. [PubMed] [Google Scholar]
- 13.Li CK, Chan PK, Ling SC, Ha SY. Interferon and ribavirin as frontline treatment for chronic hepatitis C infection in thalassaemia major. Br J Haematol. 2002;117:755–8. doi: 10.1046/j.1365-2141.2002.03491.x. [DOI] [PubMed] [Google Scholar]
- 14.Butensky E, Pakbaz Z, Foote D, Walters MC, Vichinsky EP, Harmatz P. Treatment of hepatitis C virus infection in thalassemia. Ann N Y Acad Sci. 2005;1054:290–299. doi: 10.1196/annals.1345.038. [DOI] [PubMed] [Google Scholar]
- 15.Alavian SM, Tabatabaei SV. Treatment of chronic hepatitis C in polytransfused thalassaemic patients: a meta-analysis. J Viral Hepat. 2010;17:236–244. doi: 10.1111/j.1365-2893.2009.01170.x. [DOI] [PubMed] [Google Scholar]
- 16.Vafiadis I, Trilianos P, Vlachogiannakos J, Karagiorga M, Hatziliami A, Voskaridou E, Ladas SD. Efficacy and safety of interferon-based therapy in the treatment of adult thalassemic patients with chronic hepatitis C: a 12 years audit. Ann Hepatol. 2013;12:532–8. [PubMed] [Google Scholar]
- 17.Kalafateli M, Kourakli A, Gatselis N, Lambropoulou P, Thomopoulos K, Tsamandas A, Christofidou M, Zachou K, Jelastopoulou E, Nikolopoulou V, Symeonidis A, Dalekos GN, Lambropoulou-Karatza C, Triantos C. Efficacy of interferon a-2b monotherapy in b-thalassemics with chronic hepatitis C. J Gastrointestin Liver Dis. 2015;24:189–96. doi: 10.15403/jgld.2014.1121.242.a2b. [DOI] [PubMed] [Google Scholar]
- 18.Papadopoulos N, Deutsch M, Georgalas A, Poulakidas H, Karnesis L. Simeprevir and sofosbuvir combination treatment in a patient with HCV cirrhosis and HbS beta 0-thalassemia: Efficacy and safety despite baseline hyperbilirubinemia. Case Rep Hematol. 2016;2016:7635128. doi: 10.1155/2016/7635128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hussein NR. Sofosbuvir-containing regimen for the treatment of hepatitis C virus in a patient with sickle-thalassemia: The first case report. Int J Infect. 2016 doi: 10.17795/iji-38077. doi: 10.17795/iji-38077. (In press) [DOI] [Google Scholar]
- 20.Gatselis NK, Rigopoulou E, Stefos A, Kardasi M, Dalekos GN. Risk factors associated with HCV infection in semi-rural areas of central Greece. Eur J Intern Med. 2007;18:48–55. doi: 10.1016/j.ejim.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431–35. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 22.Gatselis NK, Zachou K, Norman GL, Tzellas G, Speletas M, Gabeta S, Germenis A, Koukoulis GK, Dalekos GN. IgA antibodies against deamidated gliadin peptides in patients with chronic liver diseases. Clin Chim Acta. 2012;413:1683–8. doi: 10.1016/j.cca.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Kaffe ET, Rigopoulou EI, Koukoulis GK, Dalekos GN, Moulas AN. Oxidative stress and antioxidant status in patients with autoimmune liver diseases. Redox Rep. 2015;20:33–41. doi: 10.1179/1351000214Y.0000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norman GL, Gatselis NK, Shums Z, Liaskos C, Bogdanos DP, Koukoulis GK, Dalekos GN. Cartilage oligomeric matrix protein: A novel non-invasive marker for assessing cirrhosis and risk of hepatocellular carcinoma. World J Hepatol. 2015;7:1875–83. doi: 10.4254/wjh.v7.i14.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kountouras D, Tsagarakis NJ, Fatourou E, Dalagiorgos E, Chrysanthos N, Berdoussi H, Vgontza N, Karagiorga M, Lagiandreou A, Kaligeros K, Voskaridou E, Roussou P, Diamanti-Kandarakis E, Koskinas J. Liver disease in adult transfusion dependent beta-thalassaemia patients: investigating the role of iron overload and chronic HCV infection. Liver Int. 2013;33:420–7. doi: 10.1111/liv.12095. [DOI] [PubMed] [Google Scholar]
- 26.Triantos C, Kourakli A, Kalafateli M, Giannakopoulou D, Koukias N, Thomopoulos K, Lampropoulou P, Bartzavali C, Fragopanagou H, Kagadis GC, Christofidou M, Tsamandas A, Nikolopoulou V, Karakantza M, Labropoulou-Karatza C. Hepatitis C in patients with ß-thalassemia major. A single-centre experience. Ann Hematol. 2013;92:739–46. doi: 10.1007/s00277-013-1692-6. [DOI] [PubMed] [Google Scholar]
- 27.Prati D, Poli F, Farma E, Picone A, Porta E, Mattei C, Zanella A, Scalamogna A, Gamba A, Gronda E, Faggian G, Livi U, Puricelli C, Viganò M, Sirchia G. A multicenter prospective study on the risk of acquiring liver disease in anti-hepatitis C virus negative patients affected from homozygous beta-thalassemia. Blood. 1998;92:3460–64. [PubMed] [Google Scholar]
- 28.Christofidou M, Lambropoulou-Karatza C, Dimitracopoulos G, Spiliopoulou I. Distribution of hepatitis C virus genotypes in viremic patients with beta-thalassemia. Eur J Clin Microbiol Infect Dis. 2000;19:728–30. doi: 10.1007/s100960000342. [DOI] [PubMed] [Google Scholar]
- 29.Lai ME, Origa R, Daniou F, Leoni GB, Vacquer S, Anni F, Corrias C, Farci P, Conqiu G, Galanello R. Natural history of hepatitis C in thalassemia major: a long-term prospective study. Eur J Hematol. 2013;90:501–7. doi: 10.1111/ejh.12086. [DOI] [PubMed] [Google Scholar]
- 30.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–31. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 31.Lingala S, Ghany MG. Natural history of hepatitis C. Gastroenterol Clin North Am. 2015;44:717–34. doi: 10.1016/j.gtc.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sherker AH, Senosier M, Kermack D. Treatment of transfusion-dependent thalassemic patients infected with hepatitis C virus with interferon alpha-2b and ribavirin. Hepatology. 2003;37:223. doi: 10.1053/jhep.2003.50037. [DOI] [PubMed] [Google Scholar]
- 33.Poynard T, Marcellin P, Lee SS, Niederau C, Minuk GS, Ideo G, Bain V, Heathcote J, Zeuzem S, Trepo C, Albrecht J. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352:1426–32. doi: 10.1016/S0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 34.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Haussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 35.Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–59. doi: 10.1136/gut.2005.076646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sulkowski MS, Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, Nelson DR, Everson GT, Eley T, Wind-Rotolo M, Huang SP, Gao M, Hernandez D, McPhee F, Sherman D, Hindes R, Symonds W, Pasquinelli C, Grasela DM AI444040 Study Group. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–21. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 37.Afdhal N, Reddy KR, Nelson DR, Lawitz E, Gordon SC, Schiff E, Nahass R, Ghalib R, Gitlin N, Herring R, Lalezari J, Younes ZH, Pockros PJ, Di Bisceglie AM, Arora S, Subramanian GM, Zhu Y, Dvory-Sobol H, Yang JC, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Sulkowski M, Kwo P ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370:1483–93. doi: 10.1056/NEJMoa1316366. [DOI] [PubMed] [Google Scholar]
- 38.Kowdley KV, Gordon SC, Reddy KR, Rossaro L, Bernstein DE, Lawitz E, Shiffman ML, Schiff E, Ghalib R, Ryan M, Rustgi V, Chojkier M, Herring R, Di Bisceglie AM, Pockros PJ, Subramanian GM, An D, Svarovskaia E, Hyland RH, Pang PS, Symonds WT, McHutchison JG, Muir AJ, Pound D, Fried MW ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370:1879–88. doi: 10.1056/NEJMoa1402355. [DOI] [PubMed] [Google Scholar]
- 39.Feld JJ, Kowdley KV, Coakley E, Sigal S, Nelson DR, Crawford D, Weiland O, Aguilar H, Xiong J, Pilot-Matias T, DaSilva-Tillmann B, Larsen L, Podsadecki T, Bernstein B. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370:1594–1603. doi: 10.1056/NEJMoa1315722. [DOI] [PubMed] [Google Scholar]
- 40.Ferenci P, Bernstein D, Lalezari J, Cohen D, Luo Y, Cooper C, Tam E, Marinho RT, Tsai N, Nyberg A, Box TD, Younes Z, Enayati P, Green S, Baruch Y, Bhandari BR, Caruntu FA, Sepe T, Chulanov V, Janczewska E, Rizzardini G, Gervain J, Planas R, Moreno C, Hassanein T, Xie W, King M, Podsadecki T, Reddy KR PEARL-III Study; PEARL-IV Study. ABT-450/rombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370:1983–92. doi: 10.1056/NEJMoa1402338. [DOI] [PubMed] [Google Scholar]
- 41.Din G, Malik S, Ali I, Ahmed S, Dasti JI. Prevalence of hepatitis C virus infection among thalassemia patients: a perspective from a multi-ethnic population of Pakistan. Asian Pac J Trop Med. 2014;7S1:S127–33. doi: 10.1016/S1995-7645(14)60218-2. [DOI] [PubMed] [Google Scholar]
- 42.Rafiei A, Darzyani AM, Taheri S, Haghshenas MR, Hosseinian A, Makhlough A. Genetic diversity of HCV among various high risk populations (IDAs, thalassemia, hemophilia, HD patients) in Iran. Asian Pac J Trop Med. 2013;6:556–60. doi: 10.1016/S1995-7645(13)60096-6. [DOI] [PubMed] [Google Scholar]
- 43.Shah N, Mishra A, Chauhan D, Vora C, Shah NR. Study on effectiveness of transfusion program in thalassemia major patients receiving multiple blood transfusions at a transfusion centre in Western India. 4. Asian J Transfus Sci. 2010;4:94–8. doi: 10.4103/0973-6247.67029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yazaji W, Habbal W, Monem F. Seropositivity of hepatitis b and c among Syrian multi-transfused patients. Mediterr J Hematol Infect Dis. 2016;8(1):e2016046. doi: 10.4084/MJHID.2016.046. doi: 10.4084/MJHID.2016.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hussein E. Evaluation of infectious disease markers in multitransfused Egyptian children with thalassemia. Ann Clin Lab Sci. 2014 Winter;44(1):62–6 P. Mid:24695476. [PubMed] [Google Scholar]