Abstract
Objective
The aim of this study was to examine the relative contributions of body mass index (BMI) and pubertal measures for risk and age of onset of pediatric MS.
Methods
Case–control study of 254 (63% female) MS cases (onset<18 years of age) and 420 (49% female) controls conducted at 14 U.S. Pediatric MS Centers. Sex‐ and age‐stratified BMI percentiles were calculated using CDC growth charts from height and weight measured at enrollment for controls, and within 1 year of onset for MS cases. Sex‐stratified associations between MS risk and age at symptom onset with both BMI and pubertal factors were estimated controlling for race and ethnicity.
Results
Only 11% of girls and 15% of boys were prepubertal (Tanner stage I) at MS onset. 80% of girls had onset of MS after menarche. BMI percentiles were higher in MS cases versus controls (girls: P < 0.001; boys: P = 0.018). BMI was associated with odds of MS in multivariate models in postpubertal girls (OR = 1.60, 95% confidence interval [CI]: 1.12, 2.27, P = 0.009) and boys (OR = 1.43, 95% CI: 1.08, 1.88, P = 0.011). In girls with MS onset after menarche, higher BMI was associated with younger age at first symptoms (P = 0.031). Younger menarche was associated with stronger effects of BMI through mediation and interaction analysis. In pubertal/postpubertal boys, 89% of whom were obese/overweight, earlier sexual maturity was associated with earlier onset of MS (P < 0.001).
Interpretation
Higher BMI in early adolescence is a risk factor for MS in girls and boys. Earlier age at sexual maturity contributes to earlier age at MS onset, particularly in association with obesity.
Introduction
Several lines of evidence suggest that puberty and elevated body mass index (BMI) may contribute to the risk of MS. An elevated female:male sex ratio in pediatric MS appears with onset around the age of 11,1 suggesting a role for puberty as a turning point for the initiation of MS. Earlier menarche (onset of menses) has been observed in women with adult‐onset MS compared to healthy females,2 suggesting that hormonal changes at puberty may influence early MS pathogenetic mechanisms. There is increasing evidence suggesting that the peri‐pubertal period is critical period for exposure to environmental risk factors for MS, such as exposure to EBV/infectious mononucleosis, place of residence, sunlight exposure, and hypovitaminosis D (reviewed3).
Prior studies have suggested that attainment of menarche increases the risk of MS in girls with acute demyelinating syndrome (ADS),4 and that relapse rate increases in girls in the peri‐menarcheal phase.5 One study has demonstrated a marginally earlier age at menarche in a large cohort of females with MS.2 A study in adult MS revealed a slight but significant difference in age at menarche in MS patients versus healthy individuals (12.4 vs. 12.6 years, P < 0.001). The relative risk reduction was 0.9 per each year of increased age at menarche. Another study reported similar findings: with each one‐year increase in the age at menarche, the age at first symptoms was postponed by 1.16 years.6
In parallel, there is increasing evidence that obesity during adolescence is associated with a higher risk of MS in adults.7, 8, 9, 10, 11 In children, one study found an effect of obesity in girls but not in boys;12 however, a smaller number of male cases may have limited the ability to detect an effect. A higher BMI during adolescence may be associated with younger age at MS onset in women.13 Attainment of sufficient body weight, and particularly body fat, is believed to be necessary for menarche to occur in girls,3, 14, 15, 16 and higher body weight is associated with earlier pubertal onset in girls.
Puberty and obesity hormonal pathways impact the immune system, with potentially differing effects on MS triggers.17, 18, 19 Thus, there is need to further clarify the specific effects of sex hormones and obesity‐related factors on the risk and age of MS onset. Pediatric MS is an ideal setting to answer these questions, since symptoms appear close to puberty allowing for better recall and measurement of pubertal milestones.
In this study, we explored the effects of menarcheal age, pubertal staging and BMI on risk of MS and age of onset in a multicenter U.S. cohort of children with or without MS recruited from the same centers.
Methods
Study setting
This case–control study was carried out as part of a larger investigation on pediatric MS risk factors conducted at 14 pediatric MS centers (R01NS071463, PI Waubant). Participating centers: Ann & Robert H. Lurie Children's Hospital of Chicago, Boston Children's Hospital, Children's Hospital of Colorado, Children's Hospital of Philadelphia, Loma Linda University, Massachusetts General Hospital for Children, Mayo Clinic Rochester, State University of New York at Buffalo, Stony Brook University Medical Center, Texas Children's Hospital Baylor, University of Texas Southwestern/Children's Medical Center Dallas, University of Alabama, University of California San Francisco, and Washington University School of Medicine in St. Louis. Participants were recruited between November 2011 and June 2014. This study was approved by the institutional review board at each participating institution, and consent was obtained as per individual institutional guidelines.
Clinical data were prospectively collected from pediatric MS patients using standardized case report forms, including demographic features, developmental history, medical history information, and neurological examinations at visits.20 Data were entered into an OpenClinica database, housed at the University of Utah, Data Coordinating and Analysis Center.
Study participants
Cases included children with a diagnosis of relapsing‐remitting MS (RRMS) with onset before 18 years of age and less than 4 years of disease duration.17 Eligibility of all cases was ascertained by a review committee (LK, TL, EW). Eligibility for controls included age less than 22 years, absence of autoimmune disorders (except for asthma or eczema), past treatment with immunosuppressive therapy, and severe health conditions. Parents of controls could not have MS. Eligible controls were invited to participate by recruitment in general or specialty pediatric clinics at the same participating institutions. Controls were selected in order to have similar distributions of sex, age, and race/ethnicity as cases in a larger study. Race and ethnicity were self‐reported according to NIH categories.
BMI measurements: We used the 2000 CDC Growth Charts (http://www.cdc.gov/growthcharts/cdc_charts.htm) to calculate BMI z‐scores (number of standard deviations from average) and BMI percentiles as well as height and weight‐for‐age z‐scores and percentiles. We considered children overweight or obese if their BMI was above the 85th or 95th percentiles for age, respectively. BMI for MS cases was calculated using heights and weights recorded within 1 year of disease onset. Measurements for controls were taken at the enrollment visit. Children with missing height, weight, or age values were excluded from these analyses, as were implausible values (BMI z‐score beyond ±4).
Sexual maturity measurements: Puberty is characterized by thelarche (breast development) and pubarche (onset of pubic hair) followed by menarche in girls; and by gonadarche (enlargement of testicles) and pubarche in boys. These transitions follow characteristic changes in the hypothalamic‐pituitary‐gonadal axis including increasing pulsatile secretion of the gonadotropins and production of sex hormones by the gonads. Adrenal maturation (adrenarche), which is associated with increasing levels of the adrenal hormones, precedes gonadarche by a couple of years. Sexual maturity was assessed using Tanner staging21, 22 at the first clinic visit following initial symptoms for MS cases, and at the enrollment visit for controls.
Tanner staging: Tanner staging describes breast development in girls, genital development in boys, and pubic hair in both, and ranges from prepubertal (Tanner stage I) to fully mature (Tanner stage V). Tanner staging for pubic hair (2) is as follows: Stage I: no hairs; Stage II: sparse, lightly pigmented hairs along the labia majora in girls and at the base of the phallus in boys; Stage III: increasing hairs that are more pigmented and beginning to curl spreading to the mons; Stage IV: pigmented coarser hairs that cover the mons but have not spread to the medial thighs; Stage V: spread of pubic hairs to the medial thighs. Tanner staging was obtained by showing parents and child (if adolescent) Tanner staging pictures (1 and 2), and asking them to circle the most appropriate match. Because Tanner stage 2 (hair growth) has been shown to demonstrate high interrater reliability,23 we used this as our primary marker of sexual maturity in boys. Additionally, we categorized boys as having advanced maturity for their age when the child's age was more than one standard deviation below the average age of controls with the same Tanner stage 2 (for pubic hair). Boys whose age was above that threshold, and all boys at Tanner stage I, were considered as not having advanced maturity.
We stratified analyses of girls by menarcheal status. Girls, who had experienced menarche at or before the age of onset of first MS symptoms, were considered postmenarcheal for our analyses. We excluded girls for whom data on menarche was not documented (17% of cases, 6% of controls) or for whom age at menarche was not documented (2% of postpubertal cases, 3% of postpubertal controls). Pubertal boys were defined as those who had reached at least Tanner stage II for pubic hair at the clinic visit following first symptoms. We excluded boys who did not have Tanner stage documented within a year of first symptoms in cases (about 34%) or at enrollment in controls (12%).
Statistical analysis
We compared pediatric onset MS cases to concurrently enrolled non‐MS controls for demographics, pubertal characteristics, height, weight, and BMI using frequencies, percentages and means and standard deviations (SD). We tested for differences between cases and controls using Fisher's exact tests for discrete characteristics, and Wilcoxon rank‐sum tests for continuous characteristics. We also tested for differences among boys and girls separately. We tested for a difference in age at menarche between cases and controls, adjusting for race and ethnicity, using a Van Elteren's test.
Among MS cases, the association between being overweight or obese and prepubertal onset was tested with Fisher's exact tests for boys and girls separately. Associations between age at menarche and BMI in female cases and controls were estimated using Spearman correlation coefficients (ρ). The association between being overweight or obese and having advanced maturity in male cases and controls was tested using the Fisher's exact test.
We compared age at menarche (females), and the percent of MS cases who were overweight or obese using estimates obtained from children enrolled in the 2011–2012 National Health and Nutrition Examination Survey (NHANES) (http://www.cdc.gov/nchs/nhanes.htm). We compared cases to NHANES estimates by age and gender, race and ethnicity. Confidence intervals and P values for differences were calculated by combining standard error estimates from the two data sources.
We used logistic regression models to estimate associations between obesity, puberty, and the risk of MS adjusting for race/ethnicity. We modeled pre and postmenarcheal onset girls, pre and pubertal/postpubertal boys separately, and estimated both unadjusted and adjusted ORs from logistic regression models, with 95% confidence intervals (CIs) and P‐values testing for associations. Model fit was evaluated using Hosmer and Lemeshow goodness of fit tests.
Linear regression models were used to estimate associations between obesity, puberty, race/ethnicity, and age of symptom onset among MS patients. We modeled pre and postmenarcheal girls, and pre and pubertal/postpubertal boys separately. We estimated unadjusted and adjusted regression coefficients with 95% CIs and P‐values testing for linear associations.
Mediation analysis
Due to the relationship between obesity and puberty among postmenarcheal girls, we utilized methods explained by VanderWeele24, 25 to estimate the mediating and interacting effects of menarcheal age, and the effect of BMI, on both the risk of MS, and the age at onset of MS. These models assume that BMI affects menarche and the outcome (either risk of MS or age at onset); menarche affects the outcome; the outcome affects neither predictor; and menarche does not affect BMI. This method also assumes that confounders are sufficiently controlled (these models control for race and ethnicity) and requires assumptions about consistency and composition, which assumptions are discussed elsewhere.24 Models result in estimates of the total combined effect of BMI and menarcheal age on the outcome. That effect is also decomposed into four components including a controlled direct effect of BMI (due neither to mediation or interaction), a reference interaction effect (due to interaction only), a mediated interaction effect (due to mediation and interaction), and a pure indirect effect (due to mediation of menarcheal age alone).
Results
Participant characteristics
We report on 254 MS cases and 420 controls (Table 1 Table S4). There were more female cases (63%) compared to controls (49%) (P < 0.001). Race and ethnicity differed between cases and controls (P = 0.004) with more Whites in the control group.
Table 1.
Demographic characteristics of girls and boys with MS versus non‐MS controls. Means and standard deviations given unless otherwise noted
| Overall | Boys | Girls | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control N = 420 | Case N = 254 | P | Control N = 213 | Case N = 93 | P | Control N = 207 | Case N = 161 | P | |
| Female N(%) | 207 (49%) | 161 (63%) | <0.001a | ||||||
| Race/Ethnicity N(%) | 0.004a | 0.082a | 0.004a | ||||||
| White/non‐HL | 219 (52%) | 110 (43%) | 100 (47%) | 43 (46%) | 119 (57%) | 67 (42%) | |||
| Hispanic‐Latino | 72 (17%) | 74 (29%) | 44 (21%) | 30 (32%) | 28 (14%) | 44 (27%) | |||
| Black/non‐HL | 65 (15%) | 36 (14%) | 32 (15%) | 8 (9%) | 33 (16%) | 28 (17%) | |||
| Other race/non‐HL | 40 (10%) | 21 (8%) | 23 (11%) | 6 (6%) | 17 (8%) | 15 (9%) | |||
| Unknown | 24 (6%) | 13 (5%) | 14 (7%) | 6 (6%) | 10 (5%) | 7 (4%) | |||
| Aged | 14 (3.7) | 14 (3.4) | 0.9622 | 14 (3.6) | 14 (3.6) | 0.614b | 14 (3.7) | 14 (3.3) | 0.966b |
| Postmenarcheal (girls) or postpubertal MS (boys) onset N(%)f | N/A | 159 (82%) | N/A | 52 (85%) | N/A | 107 (80%) | |||
| Age at Menarchef | N/A | N/A | N/A | N/A | 11.9 (1.2) | 11.6 (1.3) | 0.025b | ||
| First Available EDSSf | N/A | 1.7 (1.3) | N/A | 1.6 (1.2) | N/A | 1.7 (1.4) | |||
| Tanner Stage 1 N(%)c , f | 0.010a | 0.263a | 0.061a | ||||||
| I | 53 (14%) | 19 (12%) | 25 (13%) | 8 (13%) | 28 (15%) | 11 (11%) | |||
| II | 53 (14%) | 12 (8%) | 26 (14%) | 6 (10%) | 27 (15%) | 6 (6%) | |||
| III | 87 (24%) | 26 (16%) | 48 (26%) | 9 (15%) | 39 (22%) | 17 (18%) | |||
| IV | 72 (20%) | 39 (25%) | 35 (19%) | 15 (25%) | 37 (20%) | 24 (25%) | |||
| V | 102 (28%) | 62 (39%) | 52 (28%) | 23 (38%) | 50 (28%) | 39 (40%) | |||
| Tanner Stage 2 N(%)c , f | 0.031a | 0.118a | 0.135a | ||||||
| I | 69 (19%) | 20 (13%) | 37 (20%) | 9 (15%) | 32 (18%) | 11 (11%) | |||
| II | 49 (13%) | 14 (9%) | 25 (13%) | 8 (13%) | 24 (13%) | 6 (6%) | |||
| III | 66 (18%) | 24 (15%) | 32 (17%) | 5 (8%) | 34 (19%) | 19 (20%) | |||
| IV | 91 (25%) | 40 (25%) | 45 (24%) | 13 (21%) | 46 (25%) | 27 (28%) | |||
| V | 93 (25%) | 60 (38%) | 48 (26%) | 26 (43%) | 45 (25%) | 34 (35%) | |||
| BMI‐for‐age percentilef | 63 (29.9) | 76 (25.4) | <0.001b | 63 (30.6) | 72 (29.3) | 0.018b | 64 (29.1) | 79 (22.6) | <0.001b |
| BMI‐for‐age Z‐scoref | 0.5 (1.2) | 1.0 (1.1) | <0.001b | 0.5 (1.2) | 0.9 (1.3) | 0.018b | 0.5 (1.2) | 1.1 (0.9) | <0.001b |
| Height‐for‐age percentilef | 58 (30.8) | 55 (29.6) | 0.201b | 59 (32.3) | 53 (29.6) | 0.185b | 58 (29.2) | 57 (29.7) | 0.656b |
| Height‐for‐age Z‐scoref | 0.3 (1.3) | 0.2 (1.1) | 0.201b | 0.3 (1.4) | 0.2 (1.1) | 0.185b | 0.4 (1.2) | 0.2 (1.1) | 0.656b |
| Weight‐for‐age percentilef | 65 (30.0) | 75 (25.5) | <0.001b | 65 (30.8) | 71 (29.0) | 0.125b | 64 (29.2) | 78 (22.9) | <0.001b |
| Weight‐for‐age Z‐scoref | 0.4 (2.0) | 1.0 (1.1) | <0.001b | 0.6 (1.3) | 0.9 (1.3) | 0.125b | 0.2 (2.6) | 1.0 (1.0) | <0.001b |
| Overweight or obesee , f | 112 (34%) | 98 (52%) | <0.001a | 61 (34%) | 33 (48%) | 0.057a | 51 (33%) | 65 (54%) | <0.001a |
BMI, body mass index
Fishers exact test comparing MS cases to non‐MS controls.
Two‐sided Wilcoxon rank‐sum with normal approximation and continuity correction comparing MS cases to non‐MS controls.
Nearest the time of the first event, within 1 year.
Age at onset for cases, and age at enrollment for controls.
Overweight or obese if greater‐than 85th percentile BMI for age.
Missing or invalid data for the following overall (girls): menarcheal/pubertal status at onset: 59 (27) cases; menstrual age: 78 (78) controls, 49 (49) cases; first available EDSS: 9 (7) cases; Tanner stage 1: 53 (26) controls, 96 (64) cases; Tanner stage 2: 52 (26) controls, 96 (64) cases; BMI missing or Z>± 4: 92 (57) controls, 65 (41) cases; Height for age missing or Z >± 4: 89 (51) controls, 66 (42) cases; Weight for age missing or Z>±4: 59 (37) controls, 66 (41) cases.
BMI in MS compared to controls
BMI percentiles and z‐scores were higher in girls (P < 0.001) and boys (P = 0.018) with MS compared to controls (Table 1 and Fig. 1A and B and Figure S1). Height did not differ in MS cases stratified by sex versus controls. However, weight percentiles and z‐scores were higher in female cases versus controls (P < 0.001) but not in male case versus controls. The proportion of overweight or obese was higher in MS cases in girls (54% of cases, 33% of controls, P < 0.001) and boys (48% of cases, 34% of controls, P = 0.057).
Figure 1.
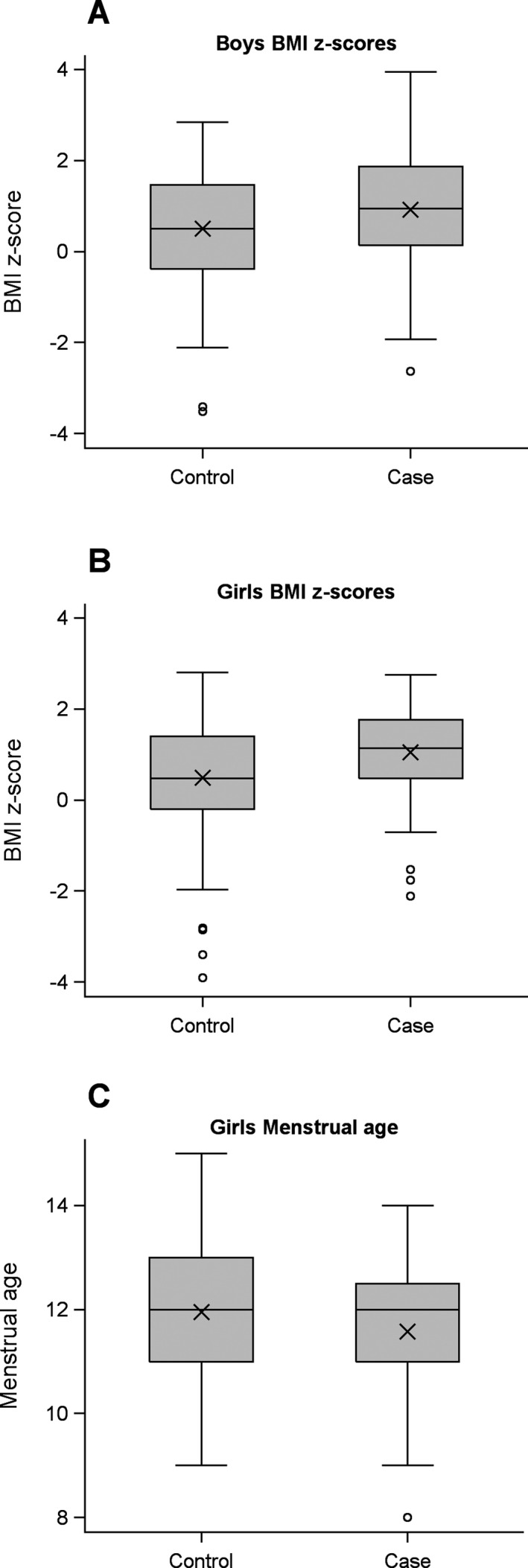
(A and B) Boxplots of Body Mass Index (BMI) z‐scores calculated from height and weight for cases and controls. For MS cases, measurements were taken at the clinic visit following first symptoms, excluding cases seen more than 1 year after first symptoms. Z‐scores represent the number of standard deviations above (+) or below (‐) the average BMI adjusted for age. Boys (A) and girls (B) are plotted separately. (C) Boxplots of menstrual age reported by girls. Boxplots show the 25th and 75th percentiles (box), median (center line), mean (X), max/min or 1.5 times the interquartile range (vertical lines with caps), and observations beyond 1.5 times the interquartile range (circles).
Compared to NHANES controls, MS girls aged 12–16 years old were more likely to be overweight or obese (P < 0.001) (Table S1) but differences were less conclusive in other age groups and among males. Overall, there were relatively more overweight/obese MS children in all age and gender categories, although only some comparisons reached statistical significance (Table S1b).
Puberty markers
Females
Among female MS cases, 89% of girls were Tanner stage II or higher for pubic hair during the year of first symptom onset (Table 1, Fig. 2B). Among female MS cases, 80% had experienced menarche by the year of disease onset. Age at menarche was marginally younger in MS cases compared to controls (11.6 vs. 11.9 years, Wilcoxon rank‐sum P = 0.025). However, after adjusting for race/ethnicity, there was less evidence of an association (P = 0.056). Compared to NHANES controls, pediatric‐onset MS cases were significantly younger at menarche overall, (mean age 11.6 vs. 12.7 years, P < 0.001), and when stratified by race/ethnicity groups (Table S1).
Figure 2.
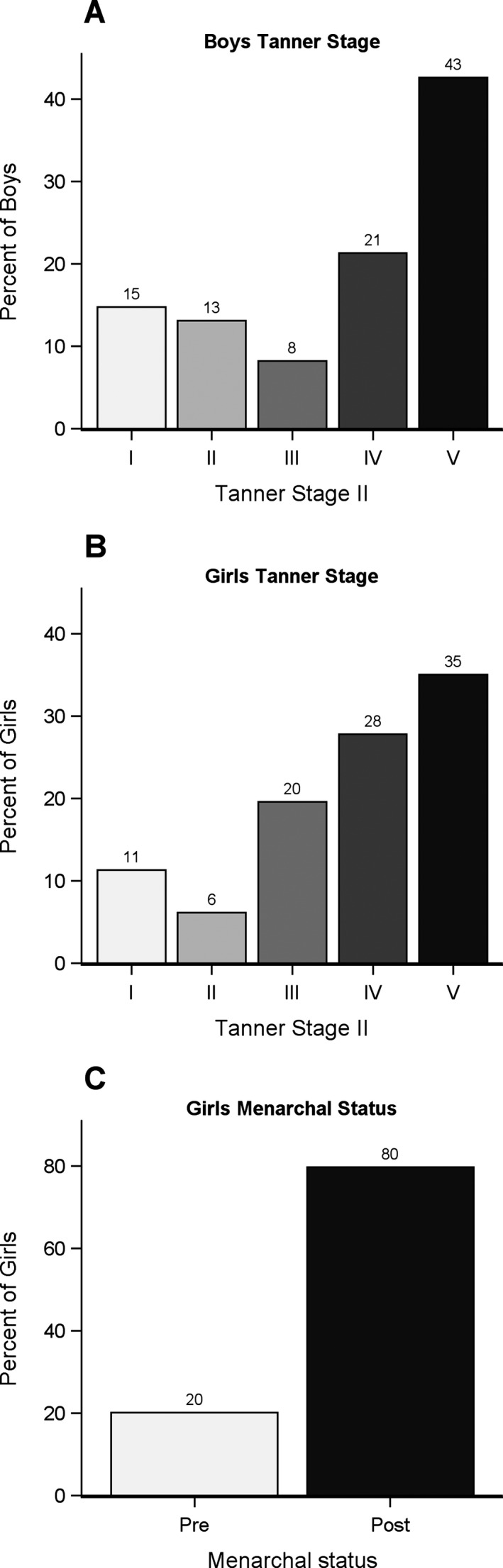
Tanner stage for boys (A) and girls (B). Tanner stage was documented at the first clinic visit following first symptoms, excluding cases seen more than 1 year after first symptoms. (C) Menarchal status for girls at the time of first symptoms. Girls with menarche at the same age (in years) as first symptoms are counted as postmenarche.
Males
85% of male MS cases reported Tanner stage II or higher for pubic hair compared to 80% of controls (P = 0.118). (Table 1).
Puberty and obesity correlations
Females
Among MS cases, 26% of premenarcheal females were overweight or obese compared to 60% of postmenarcheal females (Fisher's exact test P = 0.011 and Figure S2). Higher BMI in cases and controls was correlated with younger age at menarche (Spearman's ρ: cases −0.26 P = 0.011, controls −0.37 P < 0.001).
Males
Among males, there was no correlation between Tanner stage and age‐adjusted BMI (Spearman's ρ: cases 0.00, P = 0.996; controls 0.01, P = 0.934).
We observed that 15% (9 of 61) of male MS cases had advanced maturity for their Tanner staging for pubic hair (i.e., more than 1 standard deviation younger than the average control enrolled with the same Tanner stage). Eight of these nine (89%) boys were overweight or obese compared to less than half (42%) of the other 52 male MS cases (Fisher's exact test P = 0.013). There was no such relationship among controls (Fisher's exact P = 1.00).
Risk of MS: effect of BMI and pubertal age
BMI effect
We used a set of logistic regression models to estimate the effect of both BMI and puberty on the risk of MS (Table 2).
Table 2.
Risk of MS: Logistic regression results modeling the odds of MS (cases) compared to Non‐MS (controls). Pre and Postpubertala boys and girls modeled separately. Odds‐ratios with 95% confidence intervals (CI) are shown
| Model | Characteristic | Unadjusted Odds‐Ratio (95% CI) | P‐value | Adjustedc Odds‐Ratio (95% CI) | P‐value |
|---|---|---|---|---|---|
| Postmenarcheal Girls: (N = 181, 83 cases) | BMI‐for‐age z‐score | 1.68 (1.21, 2.34) | 0.002 | 1.60 (1.12, 2.27) | 0.009 |
| Menarcheal Age | 0.83 (0.65, 1.05) | 0.116 | 0.97 (0.75, 1.26) | 0.841 | |
| Premenarcheal Girls (N = 57, 19 cases) | BMI‐for‐age z‐score | 1.48 (0.88, 2.51) | 0.143 | 1.12 (0.61, 2.08) | 0.711 |
|
All Boys (N = 207, 59 cases) |
BMI‐for‐age z‐score | 1.42 (1.09, 1.86) | 0.009 | 1.43 (1.08, 1.88) | 0.011 |
| Advanced Maturityb | 1.09 (0.47, 2.54) | 0.844 | 0.99 (0.41, 2.38) | 0.975 |
BMI, body mass index
Postpubertal defined as postmenarche (females) or Tanner II ≥ 2 (males).
A boy has advanced maturity if his age is more than 1 standard deviation below the average age of controls with the same Tanner stage (pubic hair). Boys in stage I are not advanced. Tanner stage must be measured within 1 year of first symptoms for cases.
Adjusted for factors shown and race/ethnicity.
Bolded are statistically significant. p<0.05
Girls
In postmenarcheal girls, BMI z‐score was associated with risk of MS with an unadjusted odds ratio of 1.68 (CI: 1.21, 2.34, P = 0.002) and adjusted odds‐ratio of 1.60 (CI: 1.12, 2.27, P = 0.009). In premenarcheal onset girls, there was no significant effect of BMI on risk of MS.
Boys
Models fit to pre and pubertal/postpubertal boys were similar and limited by numbers. Therefore, we fit a model to all boys (Table 2). According to that model, the odds of developing MS were greater when BMI was higher (adjusted OR 1.43, 95% CI: 1.08, 1.88).
Mediation and interaction of menarche and the effect of BMI on the risk of MS:
Due to the correlation between BMI and age at menarche among females, we used a method explained by VanderWeele24 to estimate the possible interacting and mediating influence of menarche on the effect of age‐adjusted BMI on the risk of MS in postmenarcheal females (Table S3). This mediation and interaction model estimated the odds‐ratio of MS for overweight or obese females to be 2.18 (CI: 0.79, 3.57) compared to females with lower BMI, which is in line with the odds‐ratios for a continuous BMI predictor obtained in our adjusted and unadjusted models (1.68 and 1.60). Neither interaction nor mediation components of menarcheal age contributed significantly to the overall effect of BMI on the risk of MS (Table S2).
Effect of BMI and puberty age on age at first symptoms
Age at first symptoms in girls
In postmenarcheal‐onset girls, race/ethnicity category showed no significant effect on age at onset. Younger menarcheal age showed a nonsignificant trend with younger age at onset in univariable linear regression (Slope coefficient: 0.3, 95% CI: −0.0, 0.6; P = 0.059). Higher BMI z‐score was associated with younger age at first symptom onset in univariable linear regression (Slope coefficient: −0.5, 95% CI: −0.9, −0.0; P = 0.031), but was not significant in multivariable regression models adjusted for menstrual age. This model may be limited due to collinearity between predictors since BMI and menarcheal age are highly correlated and sufficient BMI is required in order to reach menarche (Table 3.
Table 3.
Linear regression analysis results for age at symptom onset. Pre and postpubertala boys and girls modeled separately. Regression coefficients with 95% confidence intervals (CI) are shown
| Model | Characteristic | Univariable Linear Regression Coefficient (95% CI) | P‐valuec | Multivariable Linear Regression Coefficient (95% CI) | P‐valuec |
|---|---|---|---|---|---|
| Postmenarcheal onset Girls: (N = 83) | Interceptd | 13.0 (8.9, 17.0) | <0.001 | ||
| BMI‐for‐age z‐score | −0.5 (−0.9, −0.0) | 0.031 | −0.4 (−0.8, 0.0) | 0.072 | |
| Menarcheal Age | 0.3 (−0.0, 0.6) | 0.059 | 0.3 (−0.1, 0.6) | 0.096 | |
| Black NH | −0.4 (−1.4, 0.7) | 0.181 | −0.2 (−1.3, 0.8) | 0.090 | |
| Other NH | −1.3 (−2.6, −0.0) | −1.6 (−2.9, −0.4) | |||
| Hispanic‐Latino | −0.7 (−1.6, 0.2) | −0.5 (−1.3, 0.4) | |||
| White NH | Reference | Reference | |||
| Premenarcheal onset Girls (N = 19) | Interceptd | 12.0 (9.6, 14.4) | <0.001 | ||
| BMI‐for‐age z‐score | −1.3 (−3.4, 0.8) | 0.221 | −0.0 (−2.0, 2.0) | 0.993 | |
| Black NH | −3.4 (−7.6, 0.8) | 0.012 | −3.4 (−7.8, 1.0) | 0.031 | |
| Other NH | −4.8 (−8.6, −1.1) | −4.8 (−8.8, −0.9) | |||
| Hispanic‐Latino | −6.1 (−9.6, −2.5) | −6.1 (−10.3, −1.9) | |||
| White NH | Reference | Reference | |||
| Pubertal/Postpubertal Boys (N = 50) | Interceptd | 15.4 (14.7, 16.2) | <0.001 | ||
| BMI‐for‐age z‐score | −0.1 (−0.6, 0.4) | 0.610 | 0.2 (−0.2, 0.7) | 0.329 | |
| Advanced Sexual Maturityb | −2.8 (−4.2, −1.4) | <0.001 | −3.6 (−5.2, −1.9) | <0.001 | |
| Black NH | 0.0 (−3.2, 3.2) | 0.689 | −0.4 (−3.2, 2.4) | 0.374 | |
| Other NH | −0.6 (−3.8, 2.6) | 2.4 (−0.8, 5.5) | |||
| Hispanic‐Latino | −0.8 (−2.1, 0.5) | −0.3 (−1.5, 1.0) | |||
| White NH | Reference | Reference | |||
| Prepubertal Boys (N = 9) | Interceptd | 9.5 (4.0, 15.1) | 0.009 | ||
| BMI‐for‐age z‐score | −1.5 (−3.1, 0.0) | 0.055 | −1.6 (−3.9, 0.6) | 0.117 | |
| Black NH | −1.6 (−9.6, 6.4) | 0.758 | −0.0 (−7.2, 7.2) | 0.674 | |
| Other NH | −2.3 (−12.4, 7.9) | −3.1 (−11.9, 5.6) | |||
| Hispanic‐Latino | 1.2 (−6.0, 8.3) | 0.9 (−5.2, 7.0) | |||
| White NH | Reference | Reference |
BMI, body mass index.
Postpubertal defined as postmenarche (females) or Tanner stage (pubic hair) ≥ 2 (males).
A boy has advanced maturity if his age is more than 1 standard deviation below the average age of controls with the same Tanner stage (pubic hair). Boys in stage I are not advanced. Tanner stage must be measured within 1 year of first symptoms for cases.
P‐values from Type III tests.
Univariable regression coefficients are from separate models, each with their own intercept term.
Bolded are statistically significant, p<0.05
Mediation and interaction of menarche and the effect of BMI on the age of first symptoms in postmenarcheal girls with MS
Due to the relationship and between BMI and menarche, we used a regression model detailed by VanderWeele24 to estimate how menarcheal age interacts with, and mediates, the effect of age‐adjusted BMI on age of onset for postmenarcheal female MS patients. According to this model, there was evidence that BMI influences age at onset. Age of onset occurred 0.91 (95% CI: 0.14, 1.67, P = 0.022) years earlier on average for overweight or obese females versus those with a lower BMI (categorized as being less than the 85th percentile according to BMI). The strength of that effect differed by menarcheal age, with younger menarche being associated with stronger effects of BMI. Fixing menarcheal age at 10 resulted in an estimated direct effect for BMI of 1.78 years earlier onset (CI: 0.45, 3.10, P = 0.009); fixing menarcheal age at 11 resulted in an estimated 1.18 years earlier onset (CI: 0.31, 2.04, P = 0.008); a menarcheal age of 12 resulted in 0.58 years earlier onset (CI: −0.19, 1.34, P = 0.137); and a menarcheal age 13 resulted in 0.02 years later onset (CI: −1.09, 1.14, P = 0.965).
Age at first symptoms in boys
In the pubertal/postpubertal boys, advanced sexual maturity (i.e., being more than one standard deviation in age below the average enrolled control with the same Tanner stage), showed a significant association with younger age at onset in univariable and multivariable analysis. Boys with advanced sexual maturity were 3.6 years younger at MS onset compared to those that were closer‐to, or above the average age for their Tanner stage (P < 0.001). Although BMI was itself not a predictor of age at onset, 89% (8/9) of the sexually advanced MS boys in the analysis were overweight or obese.
Discussion
We have observed distinct effects of obesity and sexual maturity on risk of pediatric MS and age at MS onset. Markers of sexual maturity, particularly in association with increased BMI were associated with younger age at onset in both boys and girls, while obesity alone was associated with increased risk of MS in both boys and girls.
Using markers of sexual development, we demonstrate that prepubertal onset of MS is rare, with only 11% of girls and 15% of boys classified as Tanner stage I at onset. Only 20% of girls were premenarcheal at MS onset. These results suggest that the onset of puberty may be a key turning point for the onset of MS. A complex relationship between menarcheal age and obesity on age at onset of MS appears to exist. Our results suggest that when menarche occurs at a younger than average age, increased BMI has an effect on age at onset. Menarcheal age itself had a marginal effect on age at onset. This interaction may be used to inform further studies assessing sex hormone and adipokine analysis on early pathogenetic mechanisms in MS.
Earlier age at pubarche, which requires a testosterone surge by the adrenals and/or testes, was associated with earlier age at MS onset in boys. Interestingly, the more sexually mature boys were also more obese. In males, higher BMI is typically associated with greater peripheral conversion of adrenal and gonadal androgens to estrogens (estrone and estradiol, respectively) in adipose tissue, consistent with a role of circulating estrogens in MS pathogenesis.26 Interestingly, in men with MS, there is evidence of decreased prenatal androgen exposure,29 and this association, with respect to our observations, requires further study in boys with MS.
Several studies have shown that obesity in the adolescent years, but not the adult years, is a risk factor for MS.7, 8, 9, 10, 11 This study further emphasizes this trend and confirms a role of obesity in development of pediatric‐onset MS. In contrast to another pediatric study which demonstrated an effect only in girls,12 we found obesity to be a risk factor in both boys and postmenarcheal‐onset girls. Obesity is associated with a change in adipokine profiles including an increase in leptin which plays a proinflammatory role, and lowered ghrelin which is anti‐inflammatory. These adipokines play varied role in thymic maturation, T‐cell proliferation, development of memory cells and cytokine production,17, 28, 29, 30, 31 all mechanisms which are purported to play a role in pediatric‐onset MS.32, 33, 34, 35
The limitations of this study are that BMI was measured after disease onset, and arguably could be the result of increased disability. However, mean EDSS in our cases was low, and only patients with body measurements within 1 year of disease onset were included. Steroid use could contribute to differences in measures; however, these are early MS cases, with relatively short exposures to these agents. The relatively lower number of prepubertal subjects could significantly limit power to detect associations in this group, and therefore current results should be replicated in a larger datasets including larger numbers of prepubertal subjects. Another significant limitation is missing data, particularly Tanner staging in 32 of the MS boys. This in particular, could influence the risk of MS analysis, where Tanner staging and sexual maturity was not found to play a role in our current analysis.
Strengths of our study include a large cohort of children with MS including boys and girls with representation from centers across the United States. The examination of BMI along with measures of sexual maturation allows us to examine the interplay of these factors, which may affect MS immunopathogenesis through differential mechanisms.
In summary, our study supports the growing data that adolescent obesity is a risk factor for MS, and confirms a role in pediatric‐onset MS in both boys and girls. This finding is of significant concern given the growing rates of pediatric obesity worldwide,36 and may contribute to the observations of an increasing female gender ratio in MS over the past five decades.37 Our study highlights an effect of sexual maturity and the pubertal transition on the age of MS onset, particularly in association with obesity. Further studies are required to elucidate the immunopathogenetic mechanisms mediated by these hormonal factors in the early stages of MS.
Author Contributions
TC, JG, MR, CC, and EW contributed to the conception and design of the study. All coauthors contributed to acquisition and analysis of the data and the drafting of the manuscript. CO and CC contributed to the statistical analysis.
Conflicts of Interest
Dr. Gorman received funding from National MS Society related to the submitted manuscript. Dr. Greenberg reports grants from Biogen, personal fees from Novartis, personal fees from EMD Serono, grants from Acorda Therapeutics, outside the submitted work. Dr. Rose reports grants from National Multiple Sclerosis Society, during the conduct of the study. Dr. Graves reports grants from Race to Erase MS, grants from NMSS, grants from Genentech, outside the submitted work.
Supporting information
Figure S1. Scatterplots of age‐adjusted BMI z‐score for MS cases (A) and controls (B). For MS cases, height and weight measurements were taken at the clinic visit following first symptoms, excluding cases seen more than 1 year after first symptoms. For controls, BMI was taken at the time of enrollment.
Figure S2. Scatterplots and boxplots of age at first symptoms (y‐axis) and factors (x‐axes) included in statistical models to determine association between obesity (BMI z‐scores), puberty (menstrual age for girls, and advanced maturity for boys), and race/ethnicity. Post and prepubertal girls and boys were modeled separately.
Table S1. Comparison of MS cases to the US population estimates from NHANES dataset.
Table S2. Mediation and Interaction of Menarcheal Age and the Effect of BMI on the Risk of MS.
Table S3. Mediation and Interaction of Menarcheal Age and the Effect of BMI on Age at Onset among pubertal/postpubertal female MS patients.
Table S4. Comparison of demographic criteria in subjects enrolled in overarching E+G study, and those eligible for this analysis.
Acknowledgments
We thank the study staff at the University of Utah and clinical sites for their contributions to this work.
References
- 1. Pohl D, Hennemuth I, von Kries R, Hanefeld F. Paediatric multiple sclerosis and acute disseminated encephalomyelitis in Germany: results of a nationwide survey. Eur J Pediatr 2007;166:405–412. [DOI] [PubMed] [Google Scholar]
- 2. Ramagopalan SV, Valdar W, Criscuoli M, et al. Age of puberty and the risk of multiple sclerosis: a population based study. Eur J Neurol 2009;16:342–347. [DOI] [PubMed] [Google Scholar]
- 3. Chitnis T. Role of puberty in multiple sclerosis risk and course. Clin Immunol 2013;149:192–200. [DOI] [PubMed] [Google Scholar]
- 4. Ahn JJ, O'Mahony J, Moshkova M, et al. Puberty in females enhances the risk of an outcome of multiple sclerosis in children and the development of central nervous system autoimmunity in mice. Mult Scler 2015;21:735–748. [DOI] [PubMed] [Google Scholar]
- 5. Lulu S, Graves J, Waubant E. Menarche increases relapse risk in pediatric multiple sclerosis. Mult Scler 2016;22:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. D'Hooghe MB, Haentjens P, Nagels G, et al. Menarche, oral contraceptives, pregnancy and progression of disability in relapsing onset and progressive onset multiple sclerosis. J Neurol 2012;259:855–861. [DOI] [PubMed] [Google Scholar]
- 7. Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009;73:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hedstrom AK, Olsson T, Alfredsson L. High body mass index before age 20 is associated with increased risk for multiple sclerosis in both men and women. Mult Scler 2012;18:1334–1336. [DOI] [PubMed] [Google Scholar]
- 9. Munger KL, Bentzen J, Laursen B, et al. Childhood body mass index and multiple sclerosis risk: a long‐term cohort study. Mult Scler 2013;19:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gianfrancesco MA, Acuna B, Shen L, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract 2014;8:e435–e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hedstrom AK, Lima Bomfim I, Barcellos L, et al. Interaction between adolescent obesity and HLA risk genes in the etiology of multiple sclerosis. Neurology 2014;82:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Langer‐Gould A, Brara SM, Beaber BE, Koebnick C. Childhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndrome. Neurology 2013;80:548–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kavak KS, Teter BE, Hagemeier J, et al. New York State Multiple Sclerosis C. Higher weight in adolescence and young adulthood is associated with an earlier age at multiple sclerosis onset. Mult Scler 2015;21:858–865. [DOI] [PubMed] [Google Scholar]
- 14. Matkovic V, Ilich JZ, Skugor M, et al. Leptin is inversely related to age at menarche in human females. J Clin Endocrinol Metab 1997;82:3239–3245. [DOI] [PubMed] [Google Scholar]
- 15. Cheung CC, Thornton JE, Kuijper JL, et al. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 1997;138:855–858. [DOI] [PubMed] [Google Scholar]
- 16. Lee JM, Appugliese D, Kaciroti N, et al. Weight status in young girls and the onset of puberty. Pediatrics 2007;119:e624–e630. [DOI] [PubMed] [Google Scholar]
- 17. Matarese G, Procaccini C, De Rosa V. The intricate interface between immune and metabolic regulation: a role for leptin in the pathogenesis of multiple sclerosis? J Leukoc Biol 2008;84:893–899. [DOI] [PubMed] [Google Scholar]
- 18. Piccio L, Stark JL, Cross AH. Chronic calorie restriction attenuates experimental autoimmune encephalomyelitis. J Leukoc Biol 2008;84:940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matarese G, Sanna V, Di Giacomo A, et al. Leptin potentiates experimental autoimmune encephalomyelitis in SJL female mice and confers susceptibility to males. Eur J Immunol 2001;31:1324–1332. [DOI] [PubMed] [Google Scholar]
- 20. Casper TC, Rose JW, Roalstad S, et al. The US Network of Pediatric Multiple Sclerosis Centers: development, Progress, and Next Steps. J Child Neurol 2015;30:1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970;45:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Slora EJ, Bocian AB, Herman‐Giddens ME, et al. Assessing inter‐rater reliability (IRR) of Tanner staging and orchidometer use with boys: a study from PROS. J Pediatr Endocrinol Metab 2009;22:291–299. [DOI] [PubMed] [Google Scholar]
- 24. VanderWeele TJ. A unification of mediation and interaction: a 4‐way decomposition. Epidemiology 2014;25:749–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baron RM, Kenny DA. The moderator‐mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol 1986;51:1173–1182. [DOI] [PubMed] [Google Scholar]
- 26. Voskuhl RR, Palaszynski K. Sex hormones in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. Neuroscientist 2001;7:258–270. [DOI] [PubMed] [Google Scholar]
- 27. Bove R, Malik MT, Diaz‐Cruz C, et al. The 2D:4D ratio, a proxy for prenatal androgen levels, differs in men with and without MS. Neurology 2015;85:1209–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res 2009;19:187–197. [DOI] [PubMed] [Google Scholar]
- 29. Dixit VD, Yang H, Sun Y, et al. Ghrelin promotes thymopoiesis during aging. J Clin Investig 2007;117:2778–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Spielmann G, Johnston CA, O'Connor DP, et al. Excess body mass is associated with T cell differentiation indicative of immune ageing in children. Clin Exp Immunol 2014;176:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matarese G, Di Giacomo A, Sanna V, et al. Requirement for leptin in the induction and progression of autoimmune encephalomyelitis. J Immunol 2001;166:5909–5916. [DOI] [PubMed] [Google Scholar]
- 32. Vargas‐Lowy D, Chitnis T. Pathogenesis of Pediatric Multiple Sclerosis. J Child Neurol 2012;27:1394–1407. [DOI] [PubMed] [Google Scholar]
- 33. Vargas‐Lowy D, Kivisakk P, Gandhi R, et al. Increased Th17 response to myelin peptides in pediatric MS. Clin Immunol 2012;146:176–184. [DOI] [PubMed] [Google Scholar]
- 34. Balint B, Haas J, Schwarz A, et al. T‐cell homeostasis in pediatric multiple sclerosis: old cells in young patients. Neurology 2013;81:784–792. [DOI] [PubMed] [Google Scholar]
- 35. Banwell B, Bar‐Or A, Cheung R, et al. Abnormal T‐cell reactivities in childhood inflammatory demyelinating disease and type 1 diabetes. Ann Neurol 2008;63:98–111. [DOI] [PubMed] [Google Scholar]
- 36. Lobstein T, Jackson‐Leach R, Moodie ML, et al. Child and adolescent obesity: part of a bigger picture. Lancet 2015;385:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bove R, Chitnis T. Sexual disparities in the incidence and course of MS. Clin Immunol 2013;149:201–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Scatterplots of age‐adjusted BMI z‐score for MS cases (A) and controls (B). For MS cases, height and weight measurements were taken at the clinic visit following first symptoms, excluding cases seen more than 1 year after first symptoms. For controls, BMI was taken at the time of enrollment.
Figure S2. Scatterplots and boxplots of age at first symptoms (y‐axis) and factors (x‐axes) included in statistical models to determine association between obesity (BMI z‐scores), puberty (menstrual age for girls, and advanced maturity for boys), and race/ethnicity. Post and prepubertal girls and boys were modeled separately.
Table S1. Comparison of MS cases to the US population estimates from NHANES dataset.
Table S2. Mediation and Interaction of Menarcheal Age and the Effect of BMI on the Risk of MS.
Table S3. Mediation and Interaction of Menarcheal Age and the Effect of BMI on Age at Onset among pubertal/postpubertal female MS patients.
Table S4. Comparison of demographic criteria in subjects enrolled in overarching E+G study, and those eligible for this analysis.


