Abstract
We analyzed quantitative maps of T 1 and T 2 relaxation times and muscle fat fraction measurements in magnetic resonance imaging of the upper arm skeletal muscles and heart in ambulatory boys with Duchenne muscular dystrophy and age‐range‐matched healthy volunteer boys. The cardiac‐optimized sequences detected fatty infiltration and edema in the upper arm skeletal muscles but not the myocardium in these Duchenne muscular dystrophy boys who had normal ejection fraction. Imaging the heart and skeletal muscle using the same magnetic resonance imaging methods during a single scan may be useful in assessing relative disease status and therapeutic response in clinical trials of Duchenne muscular dystrophy.
Introduction
Duchenne muscular dystrophy (DMD) is the most common inherited myopathy (1 in 5000 live births).1 Muscles in the hips, thighs, and shoulders are first affected followed by the arms, legs, and trunk. Patients ultimately lose independence in mobility. Outcome measures in clinical trials including the 6‐min walk test target the abilities of the lower limb function in ambulatory boys. These measures may not be applicable to patients who lose their independent mobility during the duration of the clinical trial. Functional measures assessing the upper limb performance in DMD are being developed.2, 3 Magnetic resonance imaging (MRI) allows a reliable and repeatable assessment of individual muscles. Few studies have focused on the MRI of forearm muscles in DMD.4, 5, 6 Quantitative MRI of the upper arm muscles has not been previously reported in this disease.
Individuals with DMD develop cardiomyopathy by age 18 years.7 Whereas corticosteroids and supportive measures have improved overall prognosis and lifespan, cardiomyopathy continues to be relentlessly progressive and has become a major cause of death.8 Recent guidelines emphasize the need for early diagnosis and aggressive treatment for cardiomyopathy in DMD.9 MRI is increasingly used to evaluate myocardial damage and ventricular function.10, 11 Pathological changes in progressive degeneration such as interstitial edema, fatty transformation, and fibrosis are shared between skeletal and cardiac muscle.12 The timing of disease in myocardium in relation to skeletal muscle of younger boys with DMD has not yet been delineated.
Methods
Participants and study design
In this cross‐sectional study, MRI was acquired in ambulatory DMD participants with mutations amenable to exon 51 skipping during first screening visit for a clinical trial evaluating an oligonucleotide therapy (NCT01462292) (Data S1). All DMD participants were on oral corticosteroids with a dose equivalent to prednisone 0.75 mg/kg per day, 11 patients were treated with prednisone only, and 1 patient with deflazacort only. The DMD participants were not on any cardiac‐protective therapy. The subjects did not report symptoms of cardiomyopathy such as shortness of breath, chest pain, fainting, dizziness, or swelling in the ankles and legs. The echocardiograms showed normal left ventricular ejection fraction (>55%), end‐diastolic and end‐systolic volume, stroke volume, and mass normalized to body surface area. The DMD participants had no demonstrable weakness of the elbow flexion and extension by manual muscle strength testing. Age‐range‐matched healthy volunteer boys were recruited from the NIH Clinical Research Volunteer Program registry. All were asked to avoid any excessive physical activity beyond their normal levels for a week before the study visit. The study was registered on clinicaltrials.gov (NCT01451281) and was in compliance with the NIH Privacy Act and approved by an NIH Institutional Review Board. Informed written assent and consent were obtained from each subject and parent or guardian.
MRI acquisition and analysis
Cardiac and right upper extremity MRI was acquired on 1.5T scanners (Siemens Avanto or Espree, Erlangen, Germany). An 18‐element torso‐phased array coil was used for both cardiac and arm imaging. The upper arm was kept in an abducted position at the subject's side by the coil, which was partially wrapped around the torso and secured by the straps used to hold the coil in position. Imaging of the upper extremity was performed at the mid‐upper arm, defined on the humerus as the midpoint between the inferior edge of the trochlea and the humerus head. Myocardial and upper arm T 1 and T 2 were acquired with an ECG‐gated‐modified Look‐Locker method13 and a T 2‐prepared, steady‐state‐free precession method (no fat suppression).14 The fat and water fractions were acquired with a four‐point cardiac‐optimized Dixon method.15 Regions of interest (ROI) drawn for the biceps and triceps muscles in the six slices of the mid‐upper arm covering a distance of 36 mm. Myocardial ROIs were drawn to include the entire myocardium, but excluded the papillary muscles in mid‐ventricular short‐axis slices of the left ventricle (LV), which were identified based on the end‐diastolic image. All ROIs drawn manually as illustrated in Figure 1 on Dixon images were aligned to T 1 and T 2 images so that the same regions were analyzed for quantitative measurements. The upper arm cross‐sectional area (CSA) was measured by manual planimetry. The LV size and function was assessed with cine MRI using manual planimetry by a cardiologist with expertise in cardiac MRI. All analyses were done by investigators blinded to other clinical information.
Figure 1.
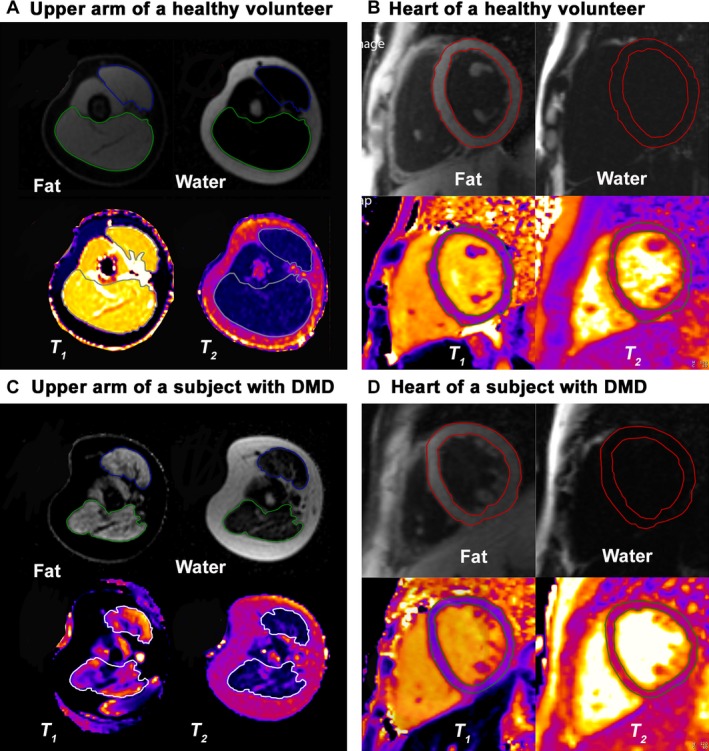
Representative fat water images, and T 1‐ and T 2 ‐parametric maps of the upper arm muscles and heart in a healthy volunteer (A and B) and a subject with Duchenne muscular dystrophy (DMD) (C and D). The biceps and triceps muscles are outlined on the fat and water images with regions of interest colored blue and green, respectively, but with white regions of interest on the T 1 ‐ and T 2‐parametric maps. There is increased fat signal from both muscles in a subject with DMD (C) than in a healthy volunteer (A). The red regions of interest delineate the location of the epicardial and endocardial borders of the left ventricular myocardium on the fat and water separation images. The green regions of interest delineate the location of the epicardial and endocardial borders of the left ventricular myocardium on the T 1 and T 2 maps. There is very little fat signal from the myocardium of a subject with DMD (D), which is similar to that in a healthy volunteer (B).
Statistical analysis
The Mann–Whitney test was used in cross‐sectional comparisons. The level of significance was set at P < 0.05. Pearson's correlation was used for continuous variables. Spearman's rank correlation was used for ordinal variables. Data analyses were done using Prism 6.0 (Graphpad Software, La Jolla, CA).
Results
The right upper arm and cardiac MRI were obtained in 12 ambulatory DMD participants (age range: 6–14 years) and 20 healthy volunteers (age range: 5–14 years). The median age of the DMD participants was younger than that of the healthy volunteers by 2 years (P = 0.06) (Table 1). The DMD participants were shorter, weighed less, and consequently had smaller body surface area (P < 0.001) relative to the healthy volunteers (Table 1).
Table 1.
Demographics and MRI measures of the upper arm muscle and nonmuscle CSA and LV systolic function in DMD participants and the healthy volunteers
| DMD (N = 12) Median (IQR) | Healthy volunteer (N = 20) Median (IQR) | Mann–Whitney P value | |
|---|---|---|---|
| Age (years) | 8.0 (2.75) | 10.0 (2.75) | 0.06 |
| Height (cm) | 123.4 (12.6) | 145.2 (12.5) | <0.0001 |
| Weight (kg) | 30.7 (13.6) | 40.3 (13.7) | <0.05 |
| Body mass index (kg/m2) | 20.3 (6.6) | 17.3 (4.6) | 0.06 |
| Body surface area (m2) | 1.0 (0.26) | 1.3 (0.25) | <0.001 |
| Right upper arm CSA (cm2)a | 36.0 (16) | 33.0 (18) | 0.3 |
| Muscle (% of CSA)a | 43.0 (16) | 50.0 (12) | 0.1 |
| Biceps CSA (cm2)a | 4.0 (1.6) | 4.5 (1.9) | 0.2 |
| Triceps CSA (cm2)a | 10.0 (5) | 10.0 (4) | 0.3 |
| Nonmuscle soft tissue (% of CSA)a | 53.0 (17) | 45.0 (10) | <0.05 |
| Bone (% of CSA)a | 3.0 (1.6) | 6.0 (1.6) | <0.0001 |
| Heart rate (beats/min) | 97.0 (15) | 79.0 (21) | <0.001 |
| LV EF (%) | 60.0 (14.2) | 62.3 (7.2) | 0.6 |
| LV EDVi (mL/m2) | 75.4 (14.2) | 75.3 (13.6) | 0.6 |
| LV ESVi (mL/m2) | 30.3 (9.6) | 30.2 (5.8) | 0.9 |
| LV ED mass (g/m2) | 44.4 (10) | 43.8 (16.5) | 0.8 |
| SV index (mL/m2) | 44.0 (9) | 47.5 (10.2) | 0.5 |
| Cardiac index (L/min per m2) | 4.1 (1.1) | 3.8 (1.1) | <0.05 |
MRI, magnetic resonance imaging; LV, left ventricular; DMD, Duchenne muscular dystrophy; IQR, interquartile range; CSA, cross‐sectional area; EF, ejection fraction; EDVi, end‐diastolic volume index; ESVi, end‐systolic volume index; ED, end diastolic; SV, stroke volume.
N = 19 healthy volunteers.
The upper arm skeletal muscle MRI
Upper arm skeletal muscle MRI was evaluated in 12 DMD participants and 19 healthy volunteers. The upper arm MRI in one 7‐year‐old healthy volunteer was not suitable for quantitative analysis due to motion artifact. Upper arm and muscle CSA were similar between the DMD participants and the healthy volunteers. The fraction of the arm CSA comprising subcutaneous fat or connective tissue was larger and that of bone was smaller in DMD participants than in the healthy volunteers (Table 1). We found that median muscle fat fractions were higher in the biceps and triceps muscles of DMD participants than the healthy volunteers (20% vs. 9%, P < 0.001, and 13% vs. 7%, P < 0.0001, respectively; Fig. 2A); data from individual subjects are shown in Table S1. There was an age‐associated increase in muscle fat in the biceps muscle of DMD participants (Fig. 2B). The T 1 relaxation times were not significantly different in the biceps and triceps muscles between the groups (882 msec vs. 849 msec, P = 0.7, and 864 msec vs. 846 msec, P = 0.8, respectively). The median T 2 relaxation times were increased in both muscles of DMD participants compared to the healthy volunteers (37 msec vs. 31 msec, P < 0.0001; Fig. 2C). The T 2 correlated with muscle fat (%) in DMD participants (r = 0.6, P < 0.01).
Figure 2.
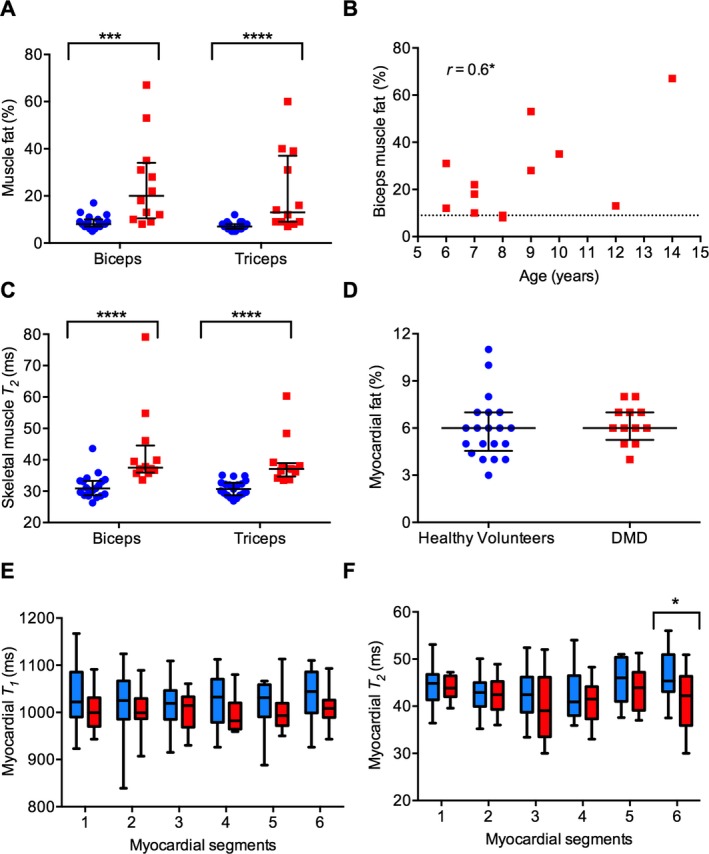
MRI quantification of muscle fat (%) and T 2 (msec) in the biceps and triceps muscles and the myocardium of the healthy volunteers (blue) and subjects with Duchenne muscular dystrophy (DMD) (red). (A) Median muscle fat (%) of the biceps and triceps muscles is significantly higher in DMD participants (n = 12) than in the healthy volunteers (n = 19). (B) Age correlation of the biceps muscle fat (%) in DMD participants. Horizontal dotted line represents the median value of the age‐range‐matched healthy volunteers. (C) Median muscle T 2 of the biceps and triceps muscles is significantly higher in DMD participants (n = 12) than in the healthy volunteers (n = 19). (D) The myocardial fat (%) in DMD participants (n = 12) is similar to the healthy volunteers (n = 20). (E) Median myocardial T 1 (msec) of DMD participants (n = 12) tended to be lower than that of the healthy volunteers (n = 18), although the difference is not statistically significant. The myocardial segments are numbered as in (F). (F) Median myocardial T 2 of the anteroseptal segment (6) is significantly lower in DMD participants (n = 10) than in the healthy volunteers (n = 16). The T 2 values in the anterior (1), anterolateral (2), inferolateral (3), inferior (4), and inferoseptal (5) myocardial segments of DMD participants are similar to that in healthy volunteers. Box and whiskers represent 5th–95th percentile values. Error bars in scatter plots represent median with interquartile range. *P < 0.05, ***P < 0.001, ****P < 0.0001. Also, see Table S1.
Cardiac MRI
Subjects with DMD had higher heart rates (P < 0.001) than healthy volunteers (Table 1). The MRI measures of LV systolic function and myocardial fat percentage were obtained in 12 DMD participants and 20 healthy volunteers. The LV mass, ejection fraction, and end‐diastolic and end‐systolic volumes adjusted for body surface area were not different between the groups. Cardiac index was normal for age16 in both groups, although higher in subjects with DMD (P = 0.03; Table 1), which may be partly related to tachycardia. Myocardial fat fractions were similar between the groups (Fig. 2D). Myocardial T 1 relaxation times in two healthy volunteers and myocardial T 2 relaxation times in four healthy volunteers and two DMD participants were not measured due to poor signal‐to‐noise ratio of the MR images. We found that median myocardial T 1 values were lower in DMD participants than healthy volunteers, but with overlapping range of values in both groups (Fig. 2E). Myocardial T 2 relaxation times of the anteroseptal segment were lower in DMD participants than healthy volunteers (42 msec vs. 45 msec, P = 0.04; Fig. 2F, Table S1).
Discussion
We simultaneously assessed and analyzed MRI biomarkers of muscle fat percentage and muscle T 1 and T 2 relaxation times in the upper arm skeletal muscles and heart of ambulatory boys with DMD at a stage when they did not yet have symptomatic heart disease. We found that the MRI abnormalities were readily apparent in the biceps and triceps muscles but not heart of subjects with DMD.
Our DMD participants did have sinus tachycardia which can be an independent risk factor for cardiac failure.17 In patients with DMD, dilated cardiomyopathy is the most common cardiac manifestation and is associated with high risk of heart failure and premature death once the patient becomes symptomatic. Recognition of cardiac symptoms may occur at a later stage in the disease course of DMD due to physical inactivity and other respiratory symptoms that may obscure the diagnosis.18 Early noninvasive diagnosis could promote early treatment and prevention of heart failure in patients.19
MRI allows accurate assessment of composition of the myocardium and correlation with LV function in DMD.18 We found that MRI measures of LV systolic function were normal in the relatively young DMD participants (75% of patients <10 years old). A recent study reported decreased myocardial T 2 in relatively older patients (age range 8–27 years) with LV ejection fraction lower than controls.20 Whether evidence of decreased in myocardial T 1 and T 2 relaxation times in our younger DMD cohort represent early changes in fatty infiltration or fibrosis remains to be confirmed in future studies.
Gadolinium assessment of myocardial fibrosis and extracellular volume fraction may be useful in predicting heart failure from LV diastolic dysfunction.21, 22 In this study gadolinium exposure was specifically excluded to minimize chances of introducing adverse renal complications not attributable to the oligonucleotide therapy in DMD participants who were participating in a clinical trial.
In DMD, MRI assessment of the upper limb muscles and heart is applicable across different stages of the disease, from young boys to nonambulant individuals, because upper limbs and heart are affected at a later stage compared to lower limbs. Natural history studies are needed to better understand the relation between the disease progression in skeletal muscles of the upper and lower limbs and in heart of patients, which is not yet clearly understood. MRI biomarkers of muscle fat percentage are responsive to disease progression in the lower limb and forearm muscles of patients with DMD.4, 23, 24 Moreover, muscle fat percentage changes were detected in the lower limb muscles of younger patients who were functionally stable or showed improvement in the functional outcome measures over 1 year.23, 25 Imaging fatty replacement in the myocardium may be a noncontrast method of assessing the disease state of the myocardium in individuals with DMD. In this study, the cardiac‐optimized MRI methods detected increased muscle fat fraction and edema in the upper arm muscles but not in the myocardium of DMD participants. Thus, our young DMD participants had limited abnormalities in the myocardium compared to the upper arm muscles.
Ancillary findings of this study are lower humeral CSA and higher nonmuscle soft tissue CSA in the DMD participants than in the healthy volunteers. Lower bone mass and higher fat mass have been reported in patients with DMD. The compromised state of the muscle–bone unit, defined as the bone mineral content per CSA of local muscle, likely influences the bone health and increases fracture risk in DMD.26 MRI has the potential to monitor the bone health in addition to the state of skeletal muscle in DMD.27
This study was done at a single site, although the participants were recruited from across the country. In addition, specific eligibility criteria of the clinical trial affected the sample size of the DMD participants. We were limited by having patients with dystrophin mutations potentially correctable by exon 51 skipping, which was an absolute requirement for their participation in the clinical trial of an oligonucleotide therapy. This may have introduced a bias as specific dystrophin mutations likely affect the severity of cardiac disease in patients with DMD.28 Our study design as a part of the clinical trial did not include functional assessments of upper limb muscles. Future studies are needed to investigate the relation between the imaging biomarkers and their value in predicting clinically meaningful milestones such as time to loss of self‐care, hospitalization for heart failure, and survival.
An objective of this study was to detect subclinical abnormalities of the heart using MRI techniques amenable to routine clinical application. Fat fraction was measured with cardiac optimized Dixon methods,15 the fat image and the water image were created from the same raw data and the same ROI was used to make both measurements simultaneously. Whereas these methods should minimize measurement variability, errors could still occur related to where the edges of the ROI are drawn, partial volume errors, and image artifacts. The percent myocardial fat in healthy volunteers was considerably higher than the ~0.5% measured by MR spectroscopy.29 The cardiac‐optimized fat water sequence had to be feasible within a breath hold, which limited the amount of data that can be acquired thus compromising signal‐to‐noise ratio. The technique may be modified to include signal averaging and motion correction or navigator technology to allow higher fidelity measurements.
In conclusion, we show the feasibility of imaging the upper arm skeletal muscles and heart of young boys with DMD and age‐range‐matched healthy volunteers using the same MR sequences applicable in clinical setting. Simultaneous assessments provide clinically useful tests for monitoring disease progression and therapeutic response in the heart and upper arm muscles in patients with DMD. These tests are also pertinent to other muscular dystrophies affecting heart.
Author Contributions
Conception and design of the study (A. M., K. H. F., W. P. B., A. E. A.), data analysis and interpretation (L. G., A. H., A. E. A., A. M.), and drafting the manuscript and revising the manuscript for intellectual content (A. M., A. E. A., K. F.). A. M. had access to all the data and takes responsibility for the data, accuracy of the data analysis, and the conduct of the research.
Conflict of Interest
Dr. Arai reports nonfinancial support from Siemens (Erlangen, Germany), outside the submitted work; all other authors have nothing to disclose.
Supporting information
Table S1. MRI measures of the upper arm skeletal muscle and heart in DMD participants and the healthy volunteers. The data from Figure 1 in each participant are shown.
Data S1. The subject eligibility and recruitment, and the inclusion and exclusion criteria for the NIH DMD Imaging Study
Acknowledgments
The study was supported by NINDS intramural research funds. The authors thank the study participants and their families; Donovan Stock, Elizabeth Hartnett, and Alice Schindler (NINDS) for help with coordinating study visits and scheduling MRI studies; Hirity Shimellis for assistance with database audit, Christine Mancini and Laura Olivieri, and Sujata Shanbhag (NHLBI) for help with acquiring MRI data; and the Principal Investigators of the referring sites participating in the DMD114876 trial (Barry Russman, Portland, OR; Benjamin Renfroe, Gulf Breeze, FL; Brenda Wong, Cincinnati, OH; Douglas Sproule, New York, NY; Edward Smith, Durham, NC; and Kathryn Wagner, Baltimore, MD) for sharing study participants. We also thank GlaxoSmithKline and Prosensa/BioMarin, study sponsors of the DMD114876 trial, for sharing the echocardiography data.
References
- 1. Mendell JR, Lloyd‐Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve 2013;48:21–26. [DOI] [PubMed] [Google Scholar]
- 2. Mayhew A, Mazzone ES, Eagle M, et al. Development of the performance of the upper limb module for Duchenne muscular dystrophy. Dev Med Child Neurol 2013;55:1038–1045. [DOI] [PubMed] [Google Scholar]
- 3. Mercuri E, McDonald C, Mayhew A, et al. International workshop on assessment of upper limb function in Duchenne muscular dystrophy: Rome, 15‐16 February 2012. Neuromuscul Disord 2012;22:1025–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hogrel JY, Wary C, Moraux A, et al. Longitudinal functional and NMR assessment of upper limbs in Duchenne muscular dystrophy. Neurology 2016;86:1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ricotti V, Evans RB, Sinclair CDJ, et al. Upper limb muscle MRI fat‐water quantification and clinical functional correlation in non‐ambulant Duchenne muscular dystrophy. Neuromuscul Disord 2014;24:839. [Google Scholar]
- 6. Wary C, Azzabou N, Giraudeau C, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR Biomed 2015;28:1150–1162. [DOI] [PubMed] [Google Scholar]
- 7. Nigro G, Comi LI, Politano L, Bain RJ. The incidence and evolution of cardiomyopathy in Duchenne muscular dystrophy. Int J Cardiol 1990;26:271–277. [DOI] [PubMed] [Google Scholar]
- 8. Gloss D, Moxley RT III, Ashwal S, Oskoui M. Practice guideline update summary: corticosteroid treatment of Duchenne muscular dystrophy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016;86:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9. McNally EM, Kaltman JR, Benson DW, et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 2015;131:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagenbuch SC, Gottliebson WM, Wansapura J, et al. Detection of progressive cardiac dysfunction by serial evaluation of circumferential strain in patients with Duchenne muscular dystrophy. Am J Cardiol 2010;105:1451–1455. [DOI] [PubMed] [Google Scholar]
- 11. Hor KN, Taylor MD, Al‐Khalidi HR, et al. Prevalence and distribution of late gadolinium enhancement in a large population of patients with Duchenne muscular dystrophy: effect of age and left ventricular systolic function. J Cardiovasc Magn Reson 2013;15:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishimura T, Yanagisawa A, Sakata H, et al. Thallium‐201 single photon emission computed tomography (SPECT) in patients with Duchenne's progressive muscular dystrophy: a histopathologic correlation study. Jpn Circ J 2001;65:99–105. [DOI] [PubMed] [Google Scholar]
- 13. Kellman P, Arai AE, Xue H. T1 and extracellular volume mapping in the heart: estimation of error maps and the influence of noise on precision. J Cardiovasc Magn Reson 2013;15:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giri S, Chung YC, Merchant A, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kellman P, Hernando D, Shah S, et al. Multiecho dixon fat and water separation method for detecting fibrofatty infiltration in the myocardium. Magn Reson Med 2009;61:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keanne JF, Lock JE. Hemodynamic evaluation of congenital heart disease In: Lock JE, Keane JF, Perry SB, eds. Diagnostic and interventional catheterization in congenital heart disease. 2nd ed Norwell, MA: Kluwar Academic Publishers, 2000:37–72. [Google Scholar]
- 17. Thomas TO, Morgan TM, Burnette WB, Markham LW. Correlation of heart rate and cardiac dysfunction in Duchenne muscular dystrophy. Pediatr Cardiol 2012;33:1175–1179. [DOI] [PubMed] [Google Scholar]
- 18. Kamdar F, Garry DJ. Dystrophin‐deficient cardiomyopathy. J Am Coll Cardiol 2016;67:2533–2546. [DOI] [PubMed] [Google Scholar]
- 19. Jefferies JL, Eidem BW, Belmont JW, et al. Genetic predictors and remodeling of dilated cardiomyopathy in muscular dystrophy. Circulation 2005;112:2799–2804. [DOI] [PubMed] [Google Scholar]
- 20. Soslow JH, Damon SM, Crum K, et al. Increased myocardial native T1 and extracellular volume in patients with Duchenne muscular dystrophy. J Cardiovasc Magn Reson 2016;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Florian A, Ludwig A, Rosch S, et al. Myocardial fibrosis imaging based on T1‐mapping and extracellular volume fraction (ECV) measurement in muscular dystrophy patients: diagnostic value compared with conventional late gadolinium enhancement (LGE) imaging. Eur Heart J Cardiovasc Imaging 2014;15:1004–1012. [DOI] [PubMed] [Google Scholar]
- 22. Florian A, Ludwig A, Engelen M, et al. Left ventricular systolic function and the pattern of late‐gadolinium‐enhancement independently and additively predict adverse cardiac events in muscular dystrophy patients. J Cardiovasc Magn Reson 2014;16:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Willcocks RJ, Rooney WD, Triplett WT, et al. Multicenter prospective longitudinal study of magnetic resonance biomarkers in a large Duchenne muscular dystrophy cohort. Ann Neurol 2016;79:535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hollingsworth KG, Garrood P, Eagle M, et al. Magnetic resonance imaging in Duchenne muscular dystrophy: longitudinal assessment of natural history over 18 months. Muscle Nerve 2013;48:586–588. [DOI] [PubMed] [Google Scholar]
- 25. Mankodi A, Bishop CA, Auh S, et al. Quantifying disease activity in fatty‐infiltrated skeletal muscle by IDEAL‐CPMG in Duchenne muscular dystrophy. Neuromuscul Disord 2016;26:650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayo AL, Craven BC, McAdam LC, Biggar WD. Bone health in boys with Duchenne muscular dystrophy on long‐term daily deflazacort therapy. Neuromuscul Disord 2012;22:1040–1045. [DOI] [PubMed] [Google Scholar]
- 27. Seifert AC, Li C, Rajapakse CS, et al. Bone mineral (31)P and matrix‐bound water densities measured by solid‐state (31)P and (1)H MRI. NMR Biomed 2014;27:739–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tandon A, Jefferies JL, Villa CR, et al. Dystrophin genotype‐cardiac phenotype correlations in Duchenne and Becker muscular dystrophies using cardiac magnetic resonance imaging. Am J Cardiol 2015;115:967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van der Meer RW, Hammer S, Smit JW, et al. Short‐term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes 2007;56:2849–2853. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. MRI measures of the upper arm skeletal muscle and heart in DMD participants and the healthy volunteers. The data from Figure 1 in each participant are shown.
Data S1. The subject eligibility and recruitment, and the inclusion and exclusion criteria for the NIH DMD Imaging Study


