To the Editor
Dermatomyositis (DM) is a heterogeneous disease with a multitude of physical findings and clinical presentations, and patients with anti-TIF1-γ (p155) antibodies have distinct cutaneous features and are also at increased risk for malignancy.1 In this letter, we describe a novel, stereotypical patch on the hard palate, which is associated with anti-TIF1-γ antibodies that may identify patients at higher risk of cancer.
Methods
After Stanford Institutional Review Board approval, we commenced a prospective study of all consecutive DM patients seen in the Stanford dermatology clinics between November, 2014 and March, 2015. All patients met Bohan and Peter criteria or those of Sontheimer (amyopathic patients) for DM.2,3 We recorded demographic and historical clinical data, a Cutaneous Disease Activity Score Index Activity (CDASI-a) score and oral findings Clinically amyopathic and cancer-associated DM were defined as previously described.1 A two-tailed fisher exact test or Mann Whitney test was used to determine p values for dichotomous variables or continuous variables, respectively.
Results
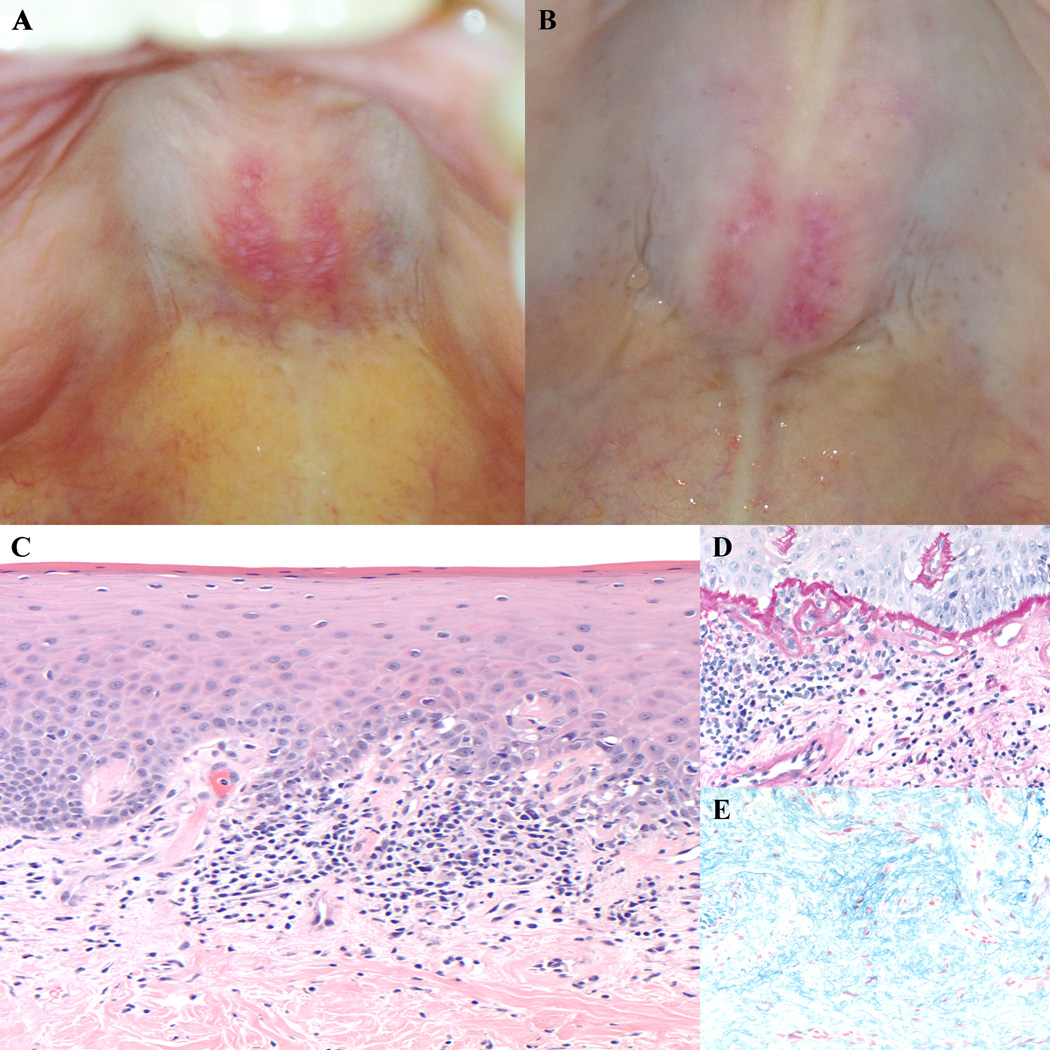
Data were recorded for 52 consecutive patients, of which 45 were included in the analysis. Six were excluded due to a lack of antibody data and one due to a prior diagnosis of oral lichen planus. Eighteen of forty-five (40%) patients had a well-demarcated, erythematous patch on the posterior hard palate. This patch did not ulcerate, often contained white macular markings and a symmetric arcuate configuration across the midline, and was asymptomatic (Fig 1a,b). Biopsy of one lesion revealed an interface dermatitis with a thickened basement membrane and increased dermal mucin (Figure 1c,d,e). We next tested if this finding was associated with any clinical or laboratory features (Table 1). The patch was significantly associated with the presence of an anti-TIF1-γ antibody (p=0.00066). None of the 16 patients with any of the other defined antibodies had this oral lesion. The oral lesion was associated with female gender (p=0.01) and clinically amyopathic disease (p = 0.03). Surprisingly, the ovoid patch was also highly associated with internal malignancy (p = 0.004); in fact, of the six anti-TIF1-γ antibody positive patients with malignancy, all six had this oral lesion. Of the seven patients with an ovoid patch and malignancy, six were anti-TIF1-g antibody positive. There was no association with the presence of anti-nuclear antibody, interstitial lung disease, CDASI-a score, ethnicity, age, or disease duration.
Figure 1.
Clinical photos of the ovoid palatal patch, with typical arcuate symmetric erythema on the hard palate intermixed with white macules (A,B). Biopsy of an ovoid palatal patch showed an interface dermatitis with dyskeratotic keratinocytes (C, 20× original magnification), a markedly thickened basement membrane (D, PASd, 40× original magnification), and increased dermal mucin (E, colloidal iron, 40× original magnification).
Table 1.
Patient Characteristics
| Oral Patch positive n (%) |
Oral Patch negative n (%) |
P value | |
|---|---|---|---|
| n | 18 (100) | 27 (100) | |
| male | 1 (6) | 11 (41) | 0.01 |
| Ethnicity | |||
| Caucasian | 13 (72) | 16 (60) | 0.53 |
| Latino | 3 (17) | 4 (15) | 1 |
| African American | 1 (6) | 1 (4) | 1 |
| Asian | 1 (6) | 5 (19) | .38 |
| Unknown | 0 (0) | 1 (4) | 1 |
| Cancer-associated DM | 7 (39) | 1 (4) | .004 |
| ILD | 1 (6) | 7 (26) | .12 |
| Clinically Amyopathic | 5 (28) | 1 (4) | .03 |
| median (SD) | median (SD) | ||
| Age, years | 59 (15) | 56 (18) | .51 |
| Length of disease, years | 8 (5) | 5 (4) | .99 |
| CDASI-a | 7.5 (11) | 5 (7) | .99 |
| Antibody Subtype | n (%) | n (%) | |
| MDA5 | 0 (0) | 4 (15) | .14 |
| Jo1 | 0 (0) | 4 (15) | .14 |
| Mi-2 | 0 (0) | 3 (11) | .26 |
| NXP2 | 0 (0) | 4 (15) | .14 |
| SAE1/2 | 0 (0) | 1 (4) | 1 |
| TIF1-γ | 15 (83) | 8 (30) | <.001 |
| no antibody | 3 (17) | 5 (19) | 1 |
| ANA | 8 (44) | 11 (40) | 1 |
DM, Dermatomyositis; ILD, interstitial lung disease; CDASI-a, Cutaneous Disease Activity Score Index Activity Score; SD, standard deviation; MDA-5, melanoma differentiation-associated gene 5; NXP-2, nuclear matrix protein 2; SAE 1/2, small ubiquitin-like modifier activating enzyme 1/2; ANA, anti-nuclear antibody
Discussion
Knowledge of the oral manifestations of DM has been limited to small series or case reports. Associated findings include lichen planus, ulcerations, gingival telangiectasia and erythema, gingival vasculopathy and desquamative gingivitis.4,5 Here, we describe a novel hard palate lesion in DM that we term the “ovoid palatal patch” that is highly associated with the presence of anti-TIF1-γ antibodies. Interestingly, this patch may identify patients with malignancy in the anti-TIF1-γ antibody positive population. The small sample size and the enrichment of anti-TIF1-γ antibody positive patients in our cohort may have limited our sensitivity for detecting this finding in the other antibody subtypes, as well as our ability to determine if the association with cancer may be due to the known association between malignancy and the anti-TIF1-γ antibody. However, it is interesting that, in our cohort, the association between the anti-TIF1-γ antibody alone and cancer does not meet statistical significance.1 Despite these limitations, we call attention to a novel, easily identifiable clinical finding in DM that is found in 40% of patients. It will be interesting to test if this finding and its characteristic location and shape distinguishes DM from other clinical mimickers (such as lupus erythematosus). Larger studies are needed to confirm the specificity of this finding and its sensitivity for predicting other outcomes such as malignancy or amyopathic disease.
Acknowledgments
Funding/Support: The Johns Hopkins Rheumatic Disease Research Core Center, where the antibody assays were performed, is supported by the NIH (grant P30-AR-053503).
| Finding/Sponsor was involved? | ||
|---|---|---|
| Design and conduct of the study | Yes____ | No__X__ |
| Collection, management, analysis and interpretation of data |
Yes____ | No__X__ |
| Preparation, review, or approval of the manuscript |
Yes____ | No__X__ |
| Decision to submit the manuscript for publication |
Yes____ | No__X__ |
Footnotes
Author Contributions: Dr(s) Bernet and Fiorentino, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bernet, Fiorentino, Lewis.
Acquisition, analysis, and interpretation of data: Bernet, Fiorentino, Lewis, Rieger, Casciola-Rosen. Drafting of the manuscript: Bernet, Fiorentino. Critical revision of the manuscript for important intellectual content: Bernet, Fiorentino, Lewis, Rieger, Casciola-Rosen. Statistical analysis: Bernet, Fiorentino. Obtained funding: Not Applicable. Administrative, technical, or material support: Not Applicable. Study supervision: Fiorentino
Financial Disclosure: None Reported
References
- 1.Fiorentino DF, Kuo K, Chung L, Zaba L, Li S, Casciola-Rosen L. Distinctive cutaneous and systemic features associated with antitranscriptional intermediary factor-1γ antibodies in adults with dermatomyositis. J Am Acad Dermatol. 2015;72(3):449–455. doi: 10.1016/j.jaad.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts) N Engl J Med. 1975;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- 3.Sontheimer RD. Dermatomyositis: an overview of recent progress with emphasis on dermatologic aspects. Dermatol Clin. 2002;((20)3):387–408. doi: 10.1016/s0733-8635(02)00021-9. [DOI] [PubMed] [Google Scholar]
- 4.Geist SM, Tanaka TI. Oral lichen planus in a dermatomyositis patient that resolved after intravenous immunoglobulin therapy. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;((118)4):111–114. doi: 10.1016/j.oooo.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka TI, Geist SM. Dermatomyositis: a contemporary review for oral health care providers. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;(114):1–8. doi: 10.1016/j.oooo.2012.07.434. [DOI] [PubMed] [Google Scholar]