Abstract
An orchestration of innate and adaptive immunity determines the infection outcome and whether the host achieves clearance or allows the pathogen to establish persistent infection. The robust activation of the innate immune response plays the most critical role in both limiting viral replication and halting the spread of the pathogen immediately after infection. The magnitude of innate immune activation is coupled with the efficient mounting of the adaptive immunity. Although immunity against HCV infection is known to be inadequate as most cases transitions to chronicity, approximately 25% of acute infection cases result in spontaneous clearance. The exact immune mechanisms that govern the infection outcome remain largely unknown; recent discoveries suggest that the innate immune system facilitates this event. Both infected hepatocytes and local innate immune cells trigger the front line defense program of the liver as well as the recruitment of diverse adaptive immune cells to the site of infection. Although hepatocyte is the target of HCV infection, nearly all cell types that exist in the liver are involved in the innate defense and contribute to the pathophysiology of hepatic inflammation. The main focus of this comprehensive review is to discuss the current knowledge on how each hepatic cell type contribute to the organ system level innate immunity against HCV infection as well as interplay with the viral evasion program. Furthermore, this review article also aims to synchronize the observations from both molecular biological studies and clinical studies with the ultimate goal of improving our understanding of HCV mediated hepatitis.
Keywords: HCV, Innate Immunity, Viral Hepatitis, Kupffer Cell, Hepatocyte, LSEC, Dendritic Cell
INTRODUCTION
Hepatitis C virus (HCV) is an enveloped ss(+)RNA virus that belongs to Flaviviridae that constitutes of six genotypes. HCV genome comprises 9.6kb nucleotides that encodes 10 proteins as a single polypeptide, which are processed into structural (Core, E1 and E2) and non-structural (p7, NS2, NS3, NS4A, NS4B, NS5A and NS5B) proteins[Moradpour et al., 2007].
To date, HCV infects 170 million people world population[Edlin and Carden, 2006]. Chronic HCV infection is the leading cause of liver cirrhosis and hepatocellular carcinoma, which account for 350,000 deaths annually. In the US, the numbers of HCV carriers exceed 5 million with a significant increase in newly infected cases occurring among young adults. Because HCV infection is typically asymptomatic, it is estimated that 40–85 percent of carriers are unaware of their infection[Denniston et al., 2012]. The high risk population such as illicit drug users, alcoholics, prisoners, and low socioeconomic population tend to be indifferent to their own health status or known to have limited access to healthcare. Thus, recent advances in anti-HCV drugs would have minimal impact on the global burdens of HCV despite the fact that these drugs have greatly improved the therapeutic success rate. Therefore, it is crucial to establish a definitive prevention strategy, such as a vaccine, in order to mitigate the worldwide HCV pandemic. Towards this ultimate goal, furthering our understanding of immunity against HCV is a critical step.
The virus-host interaction during acute infection shapes the clinical outcome and whether the host undergoes spontaneous clearance or transitions to chronic infection. The successful clearance of the pathogen relies on the efficient activation of the innate immunity as it is prerequisite for a robust mounting of the adaptive immunity. The innate immunity will be triggered by host Pattern Recognition Receptors (PRRs) that senses viral products, called Pathogen-Associated Molecular Patterns (PAMPs). Upon HCV infection, the hepatocytes sense the HCV genome via cytosolic PRRs, Retinoic acid Inducible Gene I (RIG-I) and Melanoma Differentiation-Associated protein 5 (MDA5) [Cao et al., 2015; Hiet et al., 2015; Israelow et al., 2014; Saito et al., 2007; Saito et al., 2008]. This event promotes the activation of RIG-I and MDA5 signaling that results in the induction of over 300 antiviral genes called Interferon Stimulated Genes (ISGs), as well as the secretion of Type I and III interferons (IFN)[Saito and Gale, 2008]. In contrast, local innate immune cells utilize a different class of PRR, Toll Like Receptors (TLRs) expressed in cell surface or phagosomes/endosomes to hunt the pathogen. Sensing PAMPs by TLRs results in a robust cytokines production, which recruits circulating immune cells to the site of infection in order to mount adaptive immune cells. Moreover, other hepatic non-parenchymal cells such as cholangiocytes, hepatic stellate cells (HSC), and liver sinusoidal endothelial cells (LSECs) (Figure 1) are also part of the orchestrated innate immune responses that cooperatively form an organ system level immunity.
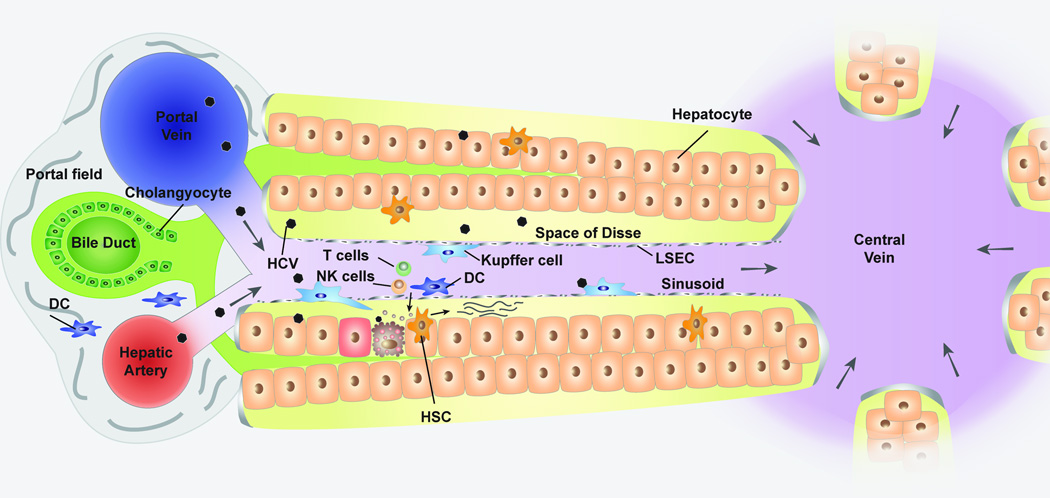
Figure 1. Diagram of hepatic lobule.
Liver receives blood supply through hepatic artery and portal vein, which forms a portal triad along with the bile duct that drains bile produced by hepatocytes. LSEC separates sinusoid and space of Disse where hepatocytes are exposed to plasma components. In response to HCV infection, the cytokines produced from infected hepatocytes recruit innate immune cells such as Kupffer cells (hepatic macrophages), dendritic cells, and NK cells to the site of infection. These innate immune cells are expected to produce substantial amount of cytokines and chemokines that play a central role in the recruitment and activation of adaptive immune cells. Hepatic stellate cells transform to myofibroblast like cells in inflamed liver, which facilitate the liver fibrosis via collagen production.
The host could fail to resolve the infection depending on the interplay between the viral evasion and the defense program. In the case of HCV infection, viral evasion program overcomes the immunity, thereby up to 70% of acute infection cases transition to chronicity. This seems that the host is outmatched in the fight; however, the remaining 30% cases that spontaneously achieve clearance of the pathogen likely provide us a lesson on how to win the battle. Single nucleotide polymorphisms (SNPs) near the IL28B and HLA class II are linked to spontaneous clearance of HCV infection[Khakoo et al., 2004; Thomas et al., 2009]. These notions suggest that the immunity has the capacity to promote the successful resolution of the infection if fine-tuned. This review will discuss our current knowledge on how individual cell types within the liver contribute to the organ system level innate immunity against HCV.
Hepatic Innate Immunity of Non-Immune cell
A. Hepatocyte
Hepatocytes, which are epithelial cells of the liver, account for 2/3 of the total liver cells and occupies 80% of the liver volume[Blouin et al., 1977]. HCV hijacks hepatocyte as a reservoir for the viral replication. The tropism to hepatocytes is partially due to the expression of cell surface proteins such as LDL-R, SBRI, CD81, Occludin and Claudin-1, which cooperatively support HCV entry[Moradpour et al., 2007]. Upon entering into a hepatocyte, HCV genome will be released into the cytoplasm where a complete viral lifecycle takes place.
RIG-I, which is a cytoplasmic DEAD-box RNA helicase protein, constantly translocates on dsRNA or structured ssRNA to hunt its potential ligands[Myong et al., 2009]. RIG-I association with 5’triphosphate (5’-ppp) and uridine/adenosine rich sequence (polyA/U) results in a conformational change and exposes its N-terminus tandem Caspase Activation and Recruitment Domain (CARD)[Saito and Gale, 2008; Saito et al., 2007; Saito et al., 2008]. Then RIG-I associates with MAVS, a mitochondrial outer membrane protein, through CARD-CARD interaction which results in the activation of transcription factors NFκB and IRF3/7[Saito and Gale, 2007]. These transcription factors translocate into nucleus and exert the transcription of ISGs and type I and III IFN. HCV genome contains both 5’-ppp and polyA/U, thereby potently activate RIG-I signaling(Figure 2).
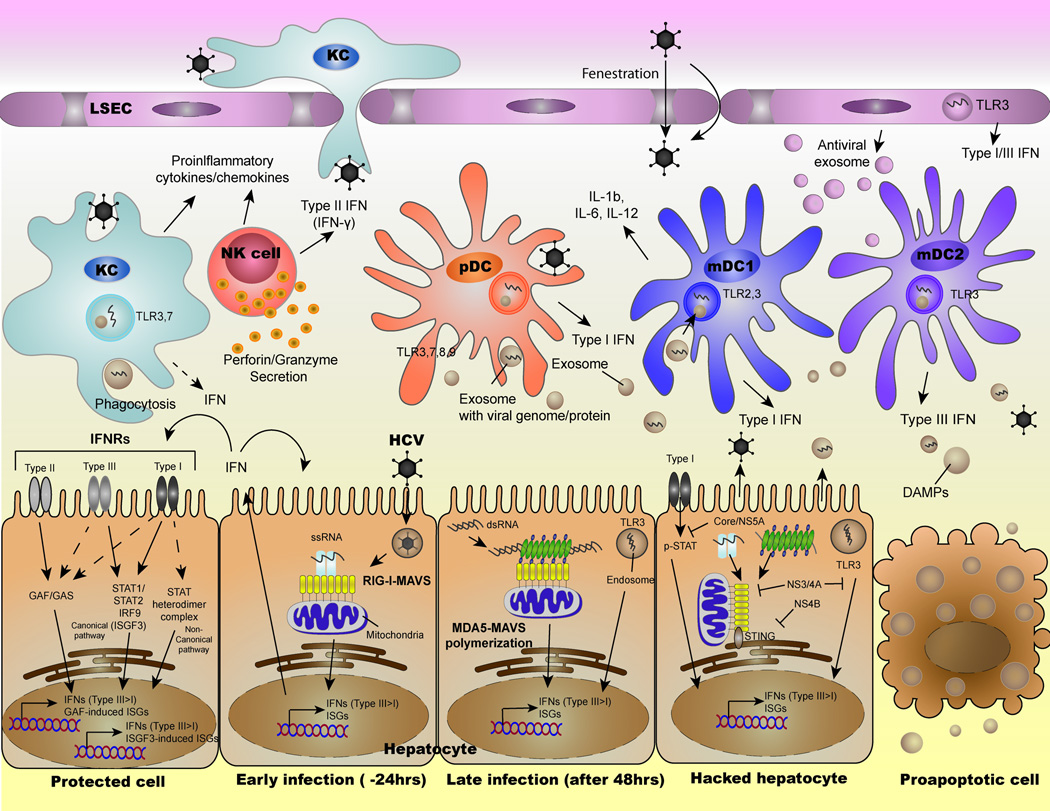
Figure 2. The current model of innate immune response against HCV and the interplay with viral evasion strategy.
RIG-I signaling induce ISGs and endogenous type I/III IFN via sensing of HCV-PAMP. Later, MDA5 also participates in the induction of ISGs and IFNs through the recognition of long dsRNA (HCV genome replication intermediate). The secreted IFN mount the expression of ISGs in neighboring cells to prevent viral spread within liver. In the hepatocytes where HCV establishes efficient replication, nearly all viral proteins abrogates antiviral innate immunity by inhibiting both RIG-I/MDA5 and IFN-Jak-STAT signaling. Also, the infected cell spread viral product in the form of extracellular vesicle to manipulate the local innate immune cells. While macrophages produce inflammatory cytokines when exposed to HCV product, DCs play predominant role in the production of type I/III IFN. NK cells contribute to the HCV suppression via secretion of type II IFN or removal of infected cells through the production of cytotoxic granules.
Another RIG-I Like Helicases (RLHs), MDA5, also plays a role in sensing HCV[Cao et al., 2015; Hiet et al., 2015]. The activation of MDA5 occur upon sensing long dsRNA (>1kb)[Kato et al., 2008]. Although MDA5 binds to short dsRNA and long ssRNA, these RNA are insufficient to confer the antiviral signaling as it requires MDA5-filament formation along the long dsRNA(Figure 2)[Wu et al., 2013]. The well-organized lining up of MDA5 promote the oligomerization of MDA5-CARD which promote the formation of MAVS filament. In response to HCV, MDA5 contributes to the induction of ISGs around 2 days after infection while RIG-I signaling kicks off at much earlier time points[Cao et al., 2015; Hiet et al., 2015]. This suggests that HCV genome forms distinctive conformations at different kinetics of infection, implying that the accumulation of replication intermediate (long dsRNA) allow MDA5 to engage with HCV RNA.
While ISGs cooperatively limit viral replication in the infected cells, the secreted IFNs prohibit the viral spread through the activation of the Jak-STAT signaling (Figure 2). The activated Jak-STAT signaling pathway promotes the formation of transcription factor, Interferon-Stimulated Gene Factor 3 (ISGF3), which contains tyrosine phosphorylated STAT1, 2 and IRF9. The ISGF3 and other STAT containing transcription factors such as STAT1:1 and STAT3:3 induces grossly redundant set of ISGs[Stark, 2007]. Moreover, IFNs activate non-canonical response pathways such as MAP kinases and PKCs. These pathways further contribute to divergence of ISGs induction by serine phosphorylation of STATs. Our recent study added an additional player, TNK1, that regulate the serine phosphorylation of STAT1 at S727 in response to type I IFN[Ooi et al., 2014]. STAT1 phosphorylation at Y701 is indispensable for the signaling activation; however, it only induces subset of ISGs. The combination of Y701 and S727 phosphorylation confer potent gene transcription activity for the comprehensive induction of ISGs.
A study with chimpanzees, which is the only other host for HCV infection, showed that acute HCV infection robustly induced type III IFN in the liver and the magnitude of the induction was much greater than that of type I IFN[Thomas et al., 2012]. The follow up in vitro studies demonstrated that hepatocytes attribute to the predominant production of type III IFN[Israelow et al., 2014; Thomas et al., 2012]. In the liver, type III IFN selectively acts on hepatocytes because the expression of the receptor subunit (IL28RA) is limited to epithelial cells[Hermant et al., 2014]. In fact, the type III IFN treatment specifically induces ISGs in hepatocytes but not in lymphocytes or monocytes[Dickensheets et al., 2013]. Interestingly, the pattern and kinetics of ISGs induction is distinctively different from that of type I IFN even through these two signaling cascade share the exact same signaling transducers besides the receptors[Marcello et al., 2006]. Indeed, a combination of type I/III IFN showed an additive effect to each other, indicating that the endogenous type I and III cooperatively suppress HCV.
In contrast to type I/III IFN, hepatocytes does not provoke the production of type II IFN (IFN-γ) in response to HCV infection. However, liver tissue of acute HCV infection expresses IFN-γ inducible genes[Bigger et al., 2001]. This suggests that other cell types in the liver produce IFN-γ in infected liver. IFN-γ binding to the receptor complex results in the formation of STAT1:1 homodimer, namely gamma-interferon activation factor (GAF). GAF induces a distinct set of ISGs from type I/III IFN with some redundancy in hepatocytes[He et al., 2010]. In fact, the combination of IFN-γ with type I IFN demonstrates superior antiviral effect than that of monotherapy[Okuse et al., 2005].
Although RLHs and IFN pathways are believed to play a central role, there might be a potential contribution of TLR3 in ISGs induction in hepatocytes[Li et al., 2012]. In general, all of the TLRs expression in hepatocytes are thought to be extremely low or irresponsive to the ligands[Seki and Brenner, 2008]. However, TLR3 itself is an ISG, therefore it is highly possible that TLR3 plays an additive role in HCV suppression once the hepatocytes are exposed to IFNs.
The ISGs are considered as the anti-viral effectors; however, for many decades it has been unclear which ISG suppresses the HCV due to the existence of a handful of genes. A recent gain of function based study shed a light on this long-standing question[Schoggins et al., 2011]. Based on this powerful study, the vast majority of individual ISGs exhibit modest anti-HCV activity with some exceptional set of ISGs that function as proviral genes. Of the antiviral ISGs, only a few of ISGs have been mechanistically studied on how it suppresses HCV. IFIT and PKR are the most well studied ISGs that inhibit viral protein translation via interaction with eIF3 and eIF2α respectively[Wang et al., 2003]. Another ISG, IFITM is localized in the tight junctions and inhibit HCV entry through its interaction with CD81[Wilkins et al., 2013]. ADAR and OAS1 exhibit antiviral effect by modulating viral genome[Malathi et al., 2010; Taylor et al., 2005]. Besides these ISGs, the mechanisms of how remaining ISGs limit HCV lifecycle require further investigations.
B. Cholangiocyte
HCV infection moderately increase the risk of intrahepatic cholangiocarcinoma (ICC)[Lee et al., 2009]. However, it has not been shown that cholangiocytes are susceptible to HCV infection. This suggests that HCV-induced ICC may be a result from cholangiocyte-like differentiation of cancer progenitor cells. In agreement with this notion, the cytoplasmic PRRs unlikely play a role in mounting the innate immune response to HCV infection. Cholangiocytes are equipped to produce inflammatory cytokines in response to a variety of TLRs ligands[Syal et al., 2012]. Thus, it is possible that cholangiocyte exhibit bystander response to HCV PAMPs or DMAPs released from infected hepatocytes; however, its significance remain elusive.
C. Hepatic Stellate Cell (HSC)
HSCs reside in the space of Disse, the perisinusoidal space[Friedman, 2008]. In resting conditions, a quiescent HSC maintains close contact with hepatocytes and primarily functions as vitamin A storage cell. In response to hepatic injury or viral infection to hepatocytes, HSC transforms to myofibroblast-like cell (MFLCs) and drives liver fibrosis through collagen production. To date, the importance of HSC in innate immune defense against HCV has not been intensively studied. Although HSC expresses HCV entry receptors such as CD81 and LDL-R, it is not permissive to HCV infection[Florimond et al., 2015]. Thus, the cytoplasmic PRRs unlikely play a role in sensing HCV PAMPs just as the cholangiocyte. HSCs express nearly all TLRs and produce significant amount of antiviral cytokines at least in response to TLR3 and 4 ligands[Wang et al., 2009a]. Therefore, HSCs likely contribute to HCV suppression upon exposure to HCV PAMPs or Danger-Associated Molecular Patterns (DAMPs); however, the degree of its significance remains elusive. Moreover, TLRs stimulation is known to promote the differentiation to MFLC, thereby the innate immune ligands serve as a mechanism of liver fibrosis if HCV infection transitions to chronicity[Watanabe et al., 2007].
Lastly, an observation from our recent work raises an additional possibility by which HSC may govern hepatic innate immunity through the supplementation of retinol[Cho et al., 2015]. The ISGs expression in hepatocytes is heavily supported by the biogenesis of retinoic acid from retinol. Thus, the quiescent HSC presumably increases the threshold of the susceptibility to HCV infection in hepatocytes while MFLC decline this mechanism. This could be one of the mechanisms on why the cirrhotic patients poorly respond to IFN therapy.
D. Liver Sinusoidal Endothelial Cell (LSEC)
LSECs comprise 10–20% of hepatic cells and 40–50% of the nonparenchymal cells volume in the liver[Blouin et al., 1977]. LSECs separate bloodstream in hepatic sinusoid and space of Disse (Figure 1). LSECs dynamically regulate transportation of nutrients and wastes between sinusoid and space of Disse using fenestrae, which allows microvilli of hepatocytes to efficiently uptake plasma components. The size of fenestrae is up to 100nm while HCV particle size ranges 40–100nm[Gastaminza et al., 2010]. Thus, LSECs fail as a physical barrier that prevents HCV to travel from sinusoid to the hepatocytes. Moreover, C-type lectins, L-/DC-SIGN expressed on the cell surface of LSEC capture and transport HCV particles from sinusoid to the space of Disse[Cormier et al., 2004]. LSECs have another proviral role via secretion of bone morphogenetic protein 4 (BMP4), which enhances HCV replication in hepatocytes[Rowe et al., 2014]. Taken together, HCV seems to exploit the function of LSEC for its own sake.
With regards to the contribution of LSEC in anti-HCV innate immunity, a recent study demonstrated that HCV is capable of transiently infecting LSECs and initiating viral protein translation[Giugliano et al., 2015]. LSECs express diverse and functional PRRs such as TLRs and RLHs[Broering et al., 2008]. Thus, LSECs can sense HCV-PAMPs with RIG-I and perhaps TLRs, which results in the substantial production of type I/III IFN[Giugliano et al., 2015]. Interestingly, type I/III IFN act on LSECs in autocrine manner and promote the production of “antiviral exosomes” that restrict HCV replication in hepatocytes. Follow up studies are warranted to further explore the contents and mechanisms of how the antiviral exosome limit the viral lifecycle.
Lastly, it has been demonstrated that LSECs can serve as antigen presenting cells (APCs) with a mouse model of Murine Cytomegalovirus infection[Kern et al., 2010]. Upon co-stimulation of TLRs signaling, their antigen presenting capacity become powerful enough to the extent that CD8+ T cells undergo full activation even in the absence of professional APCs. In accordance to the fact that transient HCV viral infection in LSEC, it is highly possible that LSECs also function as an APC in HCV infection; so that serves as a critical bridge between innate and adaptive immunity.
Hepatic professional innate immune cells
A. Kupffer cells (KCs)/Macrophages/Monocytes
KCs are the liver resident macrophages which are located in the liver sinusoid. KCs constitute 15% of the total liver cell and 80–90% of total body macrophages[Bouwens et al., 1986]. Its primarily function is to protect the liver by engulfing circulating microbial pathogens, toxin, foreign substances, and environmental debris that are delivered by portal vein[Crispe, 2011]. The engulfed materials will be catabolized in phagolysosome, wherein TLR3, 7, 8, and 9 sense PAMPs. In addition, it express a variety of PRRs on its cell surface such as Dectin-1 and TLR4, which engage with extra cellular PAMPs[Kawai and Akira, 2010].
The sensing of engulfed HCV virion is assumed to play a substantial role in hepatic inflammation due to its potent capacity in producing inflammatory mediators such as IL-1β, IL-6, and TNF-α. Recent evidences added an additional line of mechanism by which KCs sense HCV-PAMPs derived in the form of exosome or other forms of extracellular vesicles secreted from infected hepatocytes[Schorey et al., 2015]. Through this mechanism, PRRs of KCs can be exposed to broad spectrum of PAMPs and initiate innate immune response without being infected with HCV.
TLR2 have been shown to recognize Core and NS3 proteins, which result in the induction of inflammatory cytokines although the detailed mechanism that facilitate molecular interaction remain unclear[Tu et al., 2010]. TLR7/8 also contribute to the production of inflammatory mediators via the formation of NLRP3-dependent inflammasomes upon phagocytosis of HCV virion[Negash et al., 2013].
The significance of KCs in the hepatic inflammation would be underestimated if research efforts only focus on the response to HCV-PAMPs. It is important to be aware that KCs likely provoke inflammatory responses by engulfing DAMPs leaked from dying hepatocytes. The cell death seen in HCV infection is considered to be apoptosis mediated by Fas/TNF-α/TRAIL, Granzyme, or Perforin, which are the products of cytotoxic T lymphocytes (CTL) or NK cells[Luedde et al., 2014]. DAMPs leaked out of apoptotic cell could activate TLRs that do not participate in the sensing of HCV-PAMPs such as TLR4 and TLR9. Upon PAMPs or DAMPs binding to TLRs, the signal transduction takes off via shared adaptor molecules such as MyD88 or TRIF to the activation of IRFs and NF-κB. These transcription factors induce the expression of inflammatory cytokines[Medvedev, 2013].
It is important to take into account the fact that the majority of studies discussed above employed macrophage cell lines or monocytes-derived macrophages as a surrogate of KCs. However, these models are less likely relevant since emerging evidences revealed that the local environment shapes the distinctive characteristics of tissue resident macrophages[Lavin et al., 2014]. Thus, it is crucial to integrate this emerging concept when investigating the role of KCs in anti-HCV innate immunity by utilizing freshly isolated Kupffer cells and/or in vivo model system.
B. Dendritic cell (DC)
DCs are the APCs that bridge between innate and adaptive immunity. DCs exist in human peripheral blood at an extremely low frequency, approximately 0.16–0.68%[Haller Hasskamp et al., 2005]. DCs in the liver are a heterogeneous population that comprises predominantly of myeloid DCs (mDC) and to the much lesser extent plasmacytoid DCs (pDC). To date, 2 types of mDCs (mDC1 expressing BDCA-1 and mDC2 expressing BDCA-3) and 1 type of pDC (expressing BDCA-2) in human are known. Although the number is quite low, mDC2 are the most enriched in the liver than other types of DCs[Fletcher et al., 2014; Kelly et al., 2014].
DCs participate in a broad spectrum of immune response during HCV infection via diverse TLRs signaling activation. For example, mDC1 senses HCV Core and NS3 proteins via TLR2[Szabo and Dolganiuc, 2005]. TLR3 in mDC1 senses structured or HCV dsRNA, which results in the production of IL-1β, IL-6, IL-12 and, to the lesser extent, type I IFN[Zhang et al., 2013]. In contrast, mDC2, which abundantly expresses TLR3, specializes the production of type III IFN (IFN-λ) in response to synthetic ligands or HCV particle[Yoshio et al., 2013; Zhang et al., 2013].
pDC produces enormous amount of type I IFN upon exposure to TLR3, 7–9 ligands as well as HCV particle or HCV infected cells[Takahashi et al., 2010]. In addition, pDCs exposed to exosomes produced from HCV infected cells result in robust type I IFN production[Dreux et al., 2012]. These observations suggest the prominent role of both mDC and pDC in antiviral defense through the production of IFNs.
C. Natural Killer cell (NK)/Natural Killer T cell (NKT)
NKs constitute of 20–30 % of intrahepatic lymphoctyes in humans[Gao et al., 2009]. NKs are one of the innate immune cells that regulate viral infection through the production of antiviral cytokine as well as cytotoxic lytic granules[Golden-Mason and Rosen, 2013]. NKs are a heterogeneous cell population, which consist of CD56dimCD3− and CD56brightCD3−. CD56dimCD3− cells are enriched in the peripheral blood while CD56brightCD3− cells are clustered in tissues[Long et al., 2013].
NKs, especially CD56brightCD3− cells, are activated during viral infection through type I/III IFN, IL-12 and IL-18[Cooper et al., 2001]. In addition, the down regulation of MHC-I molecule in viral infected cells is the secondary mechanism of NKs activation as MHC-I serves as the inhibitory ligands of the NKs[Orange et al., 2002]. The activated NKs contribute to the suppression of viral infection through the following mechanisms: 1) type II IFN and 2) Cytotoxicity via TRAIL, Fas-L, Perforin and Granzyme.
A few studies proposed possible mechanisms of NKs activation during HCV infection as well as its antiviral implications. HCV down regulate the expression of MHC-I through the ER stress mediated impairment of proper folding of MHC-I molecules[Tardif and Siddiqui, 2003]. In addition, type I/III IFN produced from HCV infected hepatocytes or DCs results in NKs activation. Moreover, IL-18 produced from an inflammasome activated monocytes also plays important role in NKs activation[Serti et al., 2014]. The activated NKs secrete IFN-γ, which potently suppresses HCV by inducing a distinctive set of ISGs[Cheney et al., 2002]. These observations suggest that NKs indeed become activated and participate in the hepatic inflammation during HCV infection.
NKTs have two unique cell surface markers; NK1.1 and CD3. They are also one of the liver resident cells constituting 10–25% (human) and 30–40% (mice) of intrahepatic lymphocytes[Gao et al., 2009]. The characteristics of NKTs have been mostly determined by studies with animal models. To date, neither molecular biological studies nor in vitro studies have been published to assess the role of NKTs in the suppression of HCV and hepatic inflammation.
Multipronged evasion strategy of HCV against hepatic antiviral innate immunity
An evolution of evasion strategy against immunity is a key prerequisite for the efficient viral replication. HCV is one of the elite pathogens as majority of acute infection cases transition to a lifelong persistent infection. Cumulated evidences suggested that the interplay between viral evasion and host defense takes place not only in the infected hepatocytes but also in other cell types in the liver (Table 1).
Table 1.
| Antiviral Function | Viral Evasion/Proviral Function | |
|---|---|---|
| Non-Immune Cells | ||
| Hepatocyte |
|
|
| Cholangiocyte | May play a role in inflammatory cytokines production by recognizing HCV PAMPs via TLRs. |
|
| HSC |
|
|
| LSEC |
|
|
| Innate Immune Cells | ||
|
Kupffer cell /Macrophage |
|
|
| DC |
|
|
| NK cell | CD56brightCD3− cells
|
E2 inhibit the activation of NKs via binding to CD81. |
A. Hepatocytes
NS3/4A serine protease cleaves MAVS near its C-terminus end. This event leads to the mislocalization of MAVS to the cytoplasm, by which result in RLHs signaling will be abrogated[Loo et al., 2006]. Additionally, NS3/4A disrupts TLR3 signaling by cleaving the adaptor molecule, TRIF, that also contains conserved NS3/4A proteolytic cleavage Cys–(Ser/Ala) sequence[Wang et al., 2009b]. In vitro studies suggested that the disruption of RLHs and TLR3 signaling takes place within 48 hours after the infection[Loo et al., 2006]; therefore, the PRRs mediated ISGs induction might be negligible once efficient viral replication is established. Furthermore, NS4B also interfere with RIG-I signaling by targeting STING[Nitta et al., 2013]. STING is an adaptor molecule that activates IRF3 through the recruitment of IKKε and TBK1. The NS4B binding to STING has been shown to inhibit the interaction with TBK1, which subsequently compromises the activation of IRF3[Ding et al., 2013]. Moreover, another study demonstrated that NS4B of genotype 2a but not 1b promotes the loss of abundance of STING. This suggests that there is genotype-specific impairment of STING pathway[Yi et al., 2015]. However, the significance of this event requires further investigation as STING serves as an adaptor molecule for cGAS mediated cytosolic DNA sensing pathway, but not RNA virus sensing pathway[Liu et al., 2015]. Thus, the exact mechanism of how NS4B impairs RIG-I signaling remains elusive.
Moreover, HCV evolved multiple antagonizing mechanism of the IFN response pathway, in which HCV Core proteins mediated selective degradation of STAT1 as well as NS5A protein inhibit STAT1 phosphorylation[Kumthip et al., 2012; Lin et al., 2005].
B. Kupffer Cells (KCs)/Macrophage
Macrophages are potent inducer of inflammatory cytokines such as TNF-α. HCV takes advantage of TNF-α secreted from macrophages as it supports HCV particles entry to hepatocytes by upregulation of Occludin and CD81[Fletcher et al., 2014]. To date, there seem to be a controversy whether HCV can infect macrophages. A few studies demonstrated that the negative strand of HCV genome, which stands for a signature of HCV replication, was detected in monocytes/macrophages[Lerat et al., 1998]. This observation does not certainly guarantee that HCV indeed infects and replicates in macrophage as this phenomenon could be the consequence of engulfment of an apoptotic body of HCV infected hepatocytes or exosome containing viral product. In agreement with these notions, studies that demonstrated that HCV proteins impairs antiviral function of KCs/macrophages might be relevant. For example, HCV Core and NS5A protein antagonize TLR pathways in KCs by either targeting the signaling adaptor molecules or acting as an antagonizing ligand[Abe et al., 2007; Tu et al., 2010].
C. Dendritic Cells
HCV also antagonizes DCs through a numbers of evasion strategies. For example, HCV Core and E1 protein prohibit the maturation of DCs by desensitizing its activation molecules such as TNF-α and CD40L[Sarobe et al., 2003]. In addition, HCV E2 protein bind to BDCA-2, C-type lectin receptors (CLRs), and DC-immunoreceptor (DCIR) at least on pDC and inhibit its ability to produce type I IFN[Florentin et al., 2012].
D. NK Cells
A variety of viruses including HCV evolved evasion strategies against NKs. In general, the infected cells present viral antigen to CD8+ cytotoxic T lymphocytes (CTLs) using MHC-I molecules, which consequently promote viruses to develop strategies to impair the MHC-I expression in order to escape from T cell immunity. In this event, NKs play a role in viral suppression as the infected cells with the lowered MHC-I molecules expression in turn lose the inhibitory ligands of NKs. However, the elite viruses abrogate this “double security system” by selective manipulation of MHC-I molecules. For example, HIV and Kaposi’s sarcoma−associated herpesvirus (KSHV) selectively down regulate the expression of HLA-A and –B, which efficiently present viral antigen than other MHC-I In addition, these viruses preserve the expression of the HLA-C and -E, which are potent inhibitory ligands of NKs[Lodoen and Lanier, 2005]. In the case of HCV, E2 protein binds to CD81 on NK surface. CD81 is one of the inhibitory co-receptor of NKs and its ligation with E2 protein inhibits NKs to produce cytotoxic granules and type II IFN[Tseng and Klimpel, 2002].
Clinical implications of hepatic innate immunity against HCV
A. Clinical significance of the hepatic ISGs in regulation of HCV
Individuals who spontaneously clear HCV during an acute infection later become protected from reinfection[Osburn et al., 2010]. This protected population exhibit a significantly severe disease presentation upon initial infection compare to the individual who transitions to chronicity. Importantly, the degree of the disease severity of the protected population become weaker each time clearing HCV upon reinfection. Moreover, these protected individuals mount a strong and broad HCV specific adaptive immune cells response during initial infection[Abdel-Hakeem et al., 2014]. Thus, the immune responses among “protected” individuals are seemingly the perfect lesson-learn examples that can be highly exploitable for the vaccine development.
Series of GAWS studies revealed that the SNPs near IL28B (IFN-λ3) gene serves as a determinant that distinguish the protected and the susceptible population to HCV infection[Prokunina-Olsson et al., 2013; Thomas et al., 2009]. These discoveries implied the clinical significance of endogenous IFN system in regulation of HCV. Historically, abundant intrahepatic ISGs expression is believed to be prerequisite for the clearance of HCV. Upon IFN injection, a rapid decline of serum HCV RNA is observed during the 1st phase in sustained virological responders (SVR)[Neumann et al., 1998]. The 1st phase takes place within 48 hours thereafter there is a slow decline of serum HCV RNA the 2nd phase. The induction of intrahepatic ISGs is the major attribution for the suppression of HCV during the 1st phase while removal of infected cells plays a role in the 2nd phase[Lau et al., 2013; Watanabe et al., 2013]. Moreover, a study with chimpanzee demonstrated that the potent induction of hepatic ISGs were observed in the animal resolving HCV infection during acute phase[Bigger et al., 2001]. These results suggest that the abundance of ISGs expression represent the degree of viral suppression. In agreement with the notion, it has been puzzling that non-responders (NR) of IFN-therapy express higher level of baseline ISGs than that of SVR population[Honda et al., 2010; Lau et al., 2013]. The key to solve this paradoxical phenomenon requires an understanding of the concept of “IFN refractoriness”. IFN treatment provokes the greatest induction of ISGs upon initial treatment, thereafter the cells become nearly irresponsive despite constitutive exposure to high dose of IFN. The mechanism of the refractoriness is likely due to the induction of SOCS1, SOCS3 and USP18, which are ISGs themselves but are also potent inhibitors of Jak-STAT signaling (Figure 2)[Ivashkiv and Donlin, 2014]. Thus, the constant exposure to IFN prohibits the cells from inducing ISGs at the magnitude that exceeds the threshold required for the resolution of infection.
It has been known that African ancestry tend to be refractory to IFN therapy. The mechanism has been unclear for many years; however, the racial difference in IFN-λ3 SNPs provided clues to solve this puzzle[Ge et al., 2009; Thomas et al., 2009]. The “favorable IFN-λ3 genotype”, which is represented by rs12979860-CC (strong linkage disequilibrium (LD) with rs8099917-TT), associates with lower basal hepatic ISGs expression[Honda et al., 2010]. In contrast, the unfavorable IFN-λ3 genotype (rs12979860-TT or rs8099917-GG), which is the prevalent genotype in African ancestry, correlate with higher baseline ISGs expression. Follow up studies discovered two additional SNPs, which are both in strong LD with rs12979860, provided compelling explanations for the longstanding paradox on why NR express higher baseline ISGs. A SNP at rs480321 located in the 3’UTR determines the stability of IFN-λ3 mRNA by regulating the affinity to microRNA[McFarland et al., 2014]. The other SNP at rs368234815 determines the production of a newly identified gene, IFN-λ4[Prokunina-Olsson et al., 2013]. The rs368234815-ΔG allows IFN-λ4 gene to be in frame while rs368234815-TT introduce premature termination. Thus, the population who carries the unfavorable IFN-λ3 genotype is equipped with functional IFN-λ4 production. These mechanisms are likely the explanation for the higher baseline ISGs seen in African ancestry infected with HCV. Lastly, the baseline HCV RNA titer among the unfavorable IFN-λ3 genotype population is lower than that of favorable IFN-λ3 genotype[Ge et al., 2009]. This phenomenon is likely mediated by the baseline ISGs induced by IFN-λ4. Taken together, these observations perfectly fit to the concept that the degree of ISGs expression correlates the antiviral pressure.
Although it became much clearer how IFN system regulates HCV infection, there are still a number of important questions that need to be answered. For example, which cell type is the most responsible for the production of IFN-λ in a patient liver? Both infected hepatocytes and mDC2 are involved in IFN-λ production[Thomas et al., 2012; Yoshio et al., 2013; Zhang et al., 2013]. A study investigating the IFN-λ3 GT of liver transplant recipients and donors showed that the favorable IFN-λ3 genotype of the donor associates with either spontaneous clearance or better treatment outcome upon post-transplant recurrent infection[Fukuhara et al., 2010]. Based on this notion, it is speculated that the hepatocytes may play a predominant role because hematopoietic cells in the liver such as DCs in donor livers will likely be replaced by that of the recipient.
The clinical perspective of the effectors of IFN, “ISGs”, has been yet far primitive. Aforementioned ISG-wide screening study illustrated the anti-HCV potency of individual ISGs[Schoggins et al., 2011]. It is important to recognize the potential pitfall when applying this result to the understanding of innate immunity occurring in patient liver. The ISGs expression pattern is highly specific to cell types. Indeed, type I IFN-treated primary human hepatocytes showed that only one-third of total ISGs were significantly up-regulated[He et al., 2010], indicating that some of the potent suppressor of HCV proposed by the ISGs-wide screening study may not be relevant. It is also important to consider that the results from in vitro studies with Huh7 cells, which is the platform for the in vitro studies of HCV, may not well represent the event occurring in bona fide primary hepatocytes.
B. Clinical significance of the hepatic innate immune cells
There are only handfuls of studies that address this clinical importance of hepatic innate immune cells despite it has been presumed to play a substantial role in the organ system level immunity against HCV infection.
KCs are expected to be immune tolerant due to the constant exposure to microorganism or toxin derived from portal vein. Upon HCV infection, the activation of KCs occurs. The level of soluble CD163 (sCD163), which is a macrophage activation marker, increases in the serum of the HCV infected individuals[Kazankov et al., 2014]. In addition, the degree of sCD163 elevation correlates with the degree of liver disease progression, implying that the activation of KCs by HCV contribute to the hepatic inflammation. However, it has not been specifically addressed the critical question: whether KCs are the source of sCD163. Thus, the next step should be to first define the activation status of KCs in HCV infected liver.
The involvement of DCs in anti-HCV immunity again has also been clinically implicated. Chronic HCV infection significantly reduces the frequency of both mDCs and pDCs in peripheral blood[Kanto et al., 2004]. In return, the number of intrahepatic DCs is increased. The enrichment of DCs in the liver appears predominantly in mDC population. DC subsets analysis demonstrated that both mDC1 and mDC2 are significantly increased in the HCV infected liver[Yoshio et al., 2013]. The DCs extracted from HCV infected liver possess intact functionality at least for the production of type I/III IFN and IL-12. These results indicate that mDCs are recruited to the infected liver and play a role in antiviral innate immune.
NKs dynamically respond to HCV infection. During acute HCV infection, both CD56bright and CD56dim NKs undergo the activation, which polarize towards cytotoxicity or increase the capacity of type II IFN production[Amadei et al., 2010]. Importantly, an enhanced expression of one of the NKs activation markers, CD107a was observed among the individuals who spontaneously cleared HCV[Amadei et al., 2010; Pelletier et al., 2010]. Thus, strong activation of NKs might be required for the resolution of infection. Nevertheless, NKs remain activated during or after transition to chronic infection[Serti et al., 2015]. Moreover, the cytotoxic status of NKs well correlates with the degree of hepatic necroinflammation[Ahlenstiel et al., 2010]. One of the proposed mechanism of continuous activation of NKs in HCV infected liver is due to IL-26 secreted from infiltrated T cells. IL-26 support the polarization to cytotoxicity, resulting in the production of TRAIL in CD56bright NKs[Miot et al., 2015]. TRAIL is the major inducer of apoptosis, therefore this mechanism likely contributes to the necroinflammation of hepatocytes. Taken together, NKs are double sided swords that play an important role in clearance of HCV infection while the long standing cytotoxicity result in the development of cirrhosis through constitutive promotion of hepatocyte death.
The change of NKs phenotype during IFN therapy may be less relevant at present time due to the emergence of DAAs. However, the study results obtained during IFN-era have provided valuable information on NKs biology in human body. For example, the degree of NKs polarization to cytotoxicity during IFN therapy well correlates with treatment outcomes[Ahlenstiel et al., 2010]. This observation also proves the critical relationship between the IFN system and the antiviral activity of NKs.
Concluding Remarks: Importance of the better understanding of innate immunity in the era of DAA
The value of our knowledge on innate immunology obtained during the IFN era substantially progressed our understanding of the virus-host interaction followed by the innate and adaptive immune response. This knowledge was well translated in the investigation of other pathogens, liver diseases, and vaccine strategies. To date, at least 11 anti-HCV DAAs have been approved by FDA in the US. Since the introduction of the DAAs, the clinical management of HCV infection underwent the paradigm shift. The DAA combination therapy relieved the patient from the side effects of IFNs and more importantly offered much more promising treatment outcomes. Thus, the weekly injection of exogenous IFN is now retiring from clinical practice and is no longer recommended in the guidelines. However, the criticalness of the effectors of IFNs, over 300 ISGs, and the downstream immune event regulated by these effectors remain solid even in the era of DAAs.
The currently approved DAAs are targeting NS3/4A, NS5A, and NS5B proteins. These DAAs are believed to target and inhibit the function of specific viral proteins necessary for the continuation of the viral replication. However, the detailed mechanism by which these DAAs exhibit their potent antiviral properties is not fully understood. DAA treatments are presumably capable of abrogating the innate immune evasion strategy mediated by individual viral proteins. A good example for this case is that NS3 protease inhibitor restores the RIG-I signalling by inhibiting the cleavage of MAVS[Johnson et al., 2007]. Upon the restoration of RIG-I pathway, the HCV RNA in turn stimulate the ISGs induction, thereby supporting the antiviral action of NS3 protease inhibitors. This effect can be achieved with other classes of DAAs that targets NS5B such Sofosbuvir (SOF) as the inhibition of HCV genome replication ultimately reduces the abundance of NS3/4A. This notion is well supported in clinical observation that the SVR upon IFN-free DAA treatment (SOF) is coupled with significantly higher intrahepatic ISGs than that of treatment failure group[Meissner et al., 2014]. This evidence provides a proof of the concept that DAAs suppress HCV replication through at least two distinct mechanisms: 1) direct inhibition of viral replication via antagonizing the function of viral protein, and 2) the restoration of endogenous IFNs system to the robust induction of ISGs. In agreement with this notion, therapeutic IFNs may redeem its position upon emergence of “difficult to treat” virus that harbours multi-DAAs resistant in cis[Chen et al., 2016].
Lastly, the improved therapeutic outcome by DAAs is expected to have minimal impact on the global burdens of HCV infection. This is attributed to the fact that the majority of the affected population is unaware of their infection or receive fragmented medical care. Moreover, history shows that the only medical intervention to mitigate infectious disease burden is to establish a definitive vaccine. Studies discussed in this review emphasized the critical role of hepatic IFN system and encourages us to the adjuvant effect of fine-tuned innate immunity that likely serves as a key to a successful vaccine development. Therefore, furthering our knowledge on innate immunity and its cross-talk with adaptive immunity will eventually lead us to conquer this significant threat to public health.
Acknowledgments
Financial support: This work was supported by funds from NIH NIAAA (R21AA022751:TS) and NIDDK (RO1DK101773:TS)
Abbreviations footnote
- HCV
hepatitis C virus
- IFN
interferon
- PAMPs
Pathogen-associated molecular patterns
- DAMPs
Danger-associated molecular patterns
- TLR
toll like receptor
- ISG
interferon stimulated gene
- DC
dendritic cell
- KC
Kupffer cell
- NK
natural killer cell
- HSC
hepatic stellate cell
- LSEC
liver sinusoidal endothelial cells
References
- Abdel-Hakeem MS, Bedard N, Murphy D, Bruneau J, Shoukry NH. Signatures of protective memory immune responses during hepatitis C virus reinfection. Gastroenterology. 2014;147(4):870–881. e878. doi: 10.1053/j.gastro.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Kaname Y, Hamamoto I, Tsuda Y, Wen X, Taguwa S, Moriishi K, Takeuchi O, Kawai T, Kanto T, Hayashi N, Akira S, Matsuura Y. Hepatitis C virus nonstructural protein 5A modulates the toll-like receptor-MyD88-dependent signaling pathway in macrophage cell lines. J Virol. 2007;81(17):8953–8966. doi: 10.1128/JVI.00649-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, Ghany MG, Hoofnagle JH, Liang TJ, Heller T, Rehermann B. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138(1):325–335. e321–e322. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, Missale G, Ferrari C, Khakoo SI. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138(4):1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75(15):7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6(4):718–722. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- Broering R, Wu J, Meng Z, Hilgard P, Lu M, Trippler M, Szczeponek A, Gerken G, Schlaak JF. Toll-like receptor-stimulated non-parenchymal liver cells can regulate hepatitis C virus replication. J Hepatol. 2008;48(6):914–922. doi: 10.1016/j.jhep.2008.01.028. [DOI] [PubMed] [Google Scholar]
- Cao X, Ding Q, Lu J, Tao W, Huang B, Zhao Y, Niu J, Liu YJ, Zhong J. MDA5 plays a critical role in interferon response during hepatitis C virus infection. J Hepatol. 2015;62(4):771–778. doi: 10.1016/j.jhep.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Chen ZW, Li H, Ren H, Hu P. Global prevalence of pre-existing HCV variants resistant to direct-acting antiviral agents (DAAs): mining the GenBank HCV genome data. Sci Rep. 2016;6:20310. doi: 10.1038/srep20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney IW, Lai VC, Zhong W, Brodhag T, Dempsey S, Lim C, Hong Z, Lau JY, Tam RC. Comparative analysis of anti-hepatitis C virus activity and gene expression mediated by alpha, beta, and gamma interferons. J Virol. 2002;76(21):11148–11154. doi: 10.1128/JVI.76.21.11148-11154.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho NE, Bang BR, Gurung P, Li M, Clemens DL, Underhill TM, James LP, Chase JR, Saito T. Retinoid Regulation of Antiviral Innate Immunity in Hepatocytes. Hepatology. 2015 doi: 10.1002/hep.28380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, Carson WE, Caligiuri MA. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97(10):3146–3151. doi: 10.1182/blood.v97.10.3146. [DOI] [PubMed] [Google Scholar]
- Cormier EG, Durso RJ, Tsamis F, Boussemart L, Manix C, Olson WC, Gardner JP, Dragic T. L-SIGN (CD209L) and DC-SIGN (CD209) mediate transinfection of liver cells by hepatitis C virus. Proc Natl Acad Sci U S A. 2004;101(39):14067–14072. doi: 10.1073/pnas.0405695101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispe IN. Liver antigen-presenting cells. J Hepatol. 2011;54(2):357–365. doi: 10.1016/j.jhep.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denniston MM, Klevens RM, McQuillan GM, Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology. 2012;55(6):1652–1661. doi: 10.1002/hep.25556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickensheets H, Sheikh F, Park O, Gao B, Donnelly RP. Interferon-lambda (IFN-lambda) induces signal transduction and gene expression in human hepatocytes, but not in lymphocytes or monocytes. J Leukoc Biol. 2013;93(3):377–385. doi: 10.1189/jlb.0812395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Q, Cao X, Lu J, Huang B, Liu YJ, Kato N, Shu HB, Zhong J. Hepatitis C virus NS4B blocks the interaction of STING and TBK1 to evade host innate immunity. J Hepatol. 2013;59(1):52–58. doi: 10.1016/j.jhep.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Dreux M, Garaigorta U, Boyd B, Decembre E, Chung J, Whitten-Bauer C, Wieland S, Chisari FV. Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity. Cell Host Microbe. 2012;12(4):558–570. doi: 10.1016/j.chom.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlin BR, Carden MR. Injection drug users: the overlooked core of the hepatitis C epidemic. Clin Infect Dis. 2006;42(5):673–676. doi: 10.1086/499960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher NF, Sutaria R, Jo J, Barnes A, Blahova M, Meredith LW, Cosset FL, Curbishley SM, Adams DH, Bertoletti A, McKeating JA. Activated macrophages promote hepatitis C virus entry in a tumor necrosis factor-dependent manner. Hepatology. 2014;59(4):1320–1330. doi: 10.1002/hep.26911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florentin J, Aouar B, Dental C, Thumann C, Firaguay G, Gondois-Rey F, Soumelis V, Baumert TF, Nunes JA, Olive D, Hirsch I, Stranska R. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood. 2012;120(23):4544–4551. doi: 10.1182/blood-2012-02-413286. [DOI] [PubMed] [Google Scholar]
- Florimond A, Chouteau P, Bruscella P, Le Seyec J, Merour E, Ahnou N, Mallat A, Lotersztajn S, Pawlotsky JM. Human hepatic stellate cells are not permissive for hepatitis C virus entry and replication. Gut. 2015;64(6):957–965. doi: 10.1136/gutjnl-2013-305634. [DOI] [PubMed] [Google Scholar]
- Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134(6):1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, Uchiyama H, Soejima Y, Shirabe K, Matsuura Y, Maehara Y. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139(5):1577–1585. 1585, e1571–e1573. doi: 10.1053/j.gastro.2010.07.058. [DOI] [PubMed] [Google Scholar]
- Gao B, Radaeva S, Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J Leukoc Biol. 2009;86(3):513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaminza P, Dryden KA, Boyd B, Wood MR, Law M, Yeager M, Chisari FV. Ultrastructural and biophysical characterization of hepatitis C virus particles produced in cell culture. J Virol. 2010;84(21):10999–11009. doi: 10.1128/JVI.00526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge DL, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ, Sulkowski M, McHutchison JG, Goldstein DB. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461(7262):399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- Giugliano S, Kriss M, Golden-Mason L, Dobrinskikh E, Stone AE, Soto-Gutierrez A, Mitchell A, Khetani SR, Yamane D, Stoddard M, Li H, Shaw GM, Edwards MG, Lemon SM, Gale M, Jr, Shah VH, Rosen HR. Hepatitis C virus infection induces autocrine interferon signaling by human liver endothelial cells and release of exosomes, which inhibits viral replication. Gastroenterology. 2015;148(2):392–402. e313. doi: 10.1053/j.gastro.2014.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Rosen HR. Natural killer cells: multifaceted players with key roles in hepatitis C immunity. Immunol Rev. 2013;255(1):68–81. doi: 10.1111/imr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller Hasskamp J, Zapas JL, Elias EG. Dendritic cell counts in the peripheral blood of healthy adults. Am J Hematol. 2005;78(4):314–315. doi: 10.1002/ajh.20296. [DOI] [PubMed] [Google Scholar]
- He XS, Nanda S, Ji X, Calderon-Rodriguez GM, Greenberg HB, Liang TJ. Differential transcriptional responses to interferon-alpha and interferon-gamma in primary human hepatocytes. J Interferon Cytokine Res. 2010;30(5):311–320. doi: 10.1089/jir.2009.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermant P, Demarez C, Mahlakoiv T, Staeheli P, Meuleman P, Michiels T. Human but not mouse hepatocytes respond to interferon-lambda in vivo. PLoS One. 2014;9(1):e87906. doi: 10.1371/journal.pone.0087906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiet MS, Bauhofer O, Zayas M, Roth H, Tanaka Y, Schirmacher P, Willemsen J, Grunvogel O, Bender S, Binder M, Lohmann V, Lotteau V, Ruggieri A, Bartenschlager R. Control of temporal activation of hepatitis C virus-induced interferon response by domain 2 of nonstructural protein 5A. J Hepatol. 2015 doi: 10.1016/j.jhep.2015.04.015. [DOI] [PubMed] [Google Scholar]
- Honda M, Sakai A, Yamashita T, Nakamoto Y, Mizukoshi E, Sakai Y, Nakamura M, Shirasaki T, Horimoto K, Tanaka Y, Tokunaga K, Mizokami M, Kaneko S. Hepatic ISG expression is associated with genetic variation in interleukin 28B and the outcome of IFN therapy for chronic hepatitis C. Gastroenterology. 2010;139(2):499–509. doi: 10.1053/j.gastro.2010.04.049. [DOI] [PubMed] [Google Scholar]
- Israelow B, Narbus CM, Sourisseau M, Evans MJ. HepG2 cells mount an effective antiviral interferon-lambda based innate immune response to hepatitis C virus infection. Hepatology. 2014;60(4):1170–1179. doi: 10.1002/hep.27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CL, Owen DM, Gale M., Jr Functional and therapeutic analysis of hepatitis C virus NS3.4A protease control of antiviral immune defense. J Biol Chem. 2007;282(14):10792–10803. doi: 10.1074/jbc.M610361200. [DOI] [PubMed] [Google Scholar]
- Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, Oki C, Itose I, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190(11):1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205(7):1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kazankov K, Barrera F, Moller HJ, Bibby BM, Vilstrup H, George J, Gronbaek H. Soluble CD163, a macrophage activation marker, is independently associated with fibrosis in patients with chronic viral hepatitis B and C. Hepatology. 2014;60(2):521–530. doi: 10.1002/hep.27129. [DOI] [PubMed] [Google Scholar]
- Kelly A, Fahey R, Fletcher JM, Keogh C, Carroll AG, Siddachari R, Geoghegan J, Hegarty JE, Ryan EJ, O’Farrelly C. CD141(+) myeloid dendritic cells are enriched in healthy human liver. J Hepatol. 2014;60(1):135–142. doi: 10.1016/j.jhep.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Kern M, Popov A, Scholz K, Schumak B, Djandji D, Limmer A, Eggle D, Sacher T, Zawatzky R, Holtappels R, Reddehase MJ, Hartmann G, Debey-Pascher S, Diehl L, Kalinke U, Koszinowski U, Schultze J, Knolle PA. Virally infected mouse liver endothelial cells trigger CD8+ T-cell immunity. Gastroenterology. 2010;138(1):336–346. doi: 10.1053/j.gastro.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Khakoo SI, Thio CL, Martin MP, Brooks CR, Gao X, Astemborski J, Cheng J, Goedert JJ, Vlahov D, Hilgartner M, Cox S, Little AM, Alexander GJ, Cramp ME, O’Brien SJ, Rosenberg WM, Thomas DL, Carrington M. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305(5685):872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- Kumthip K, Chusri P, Jilg N, Zhao L, Fusco DN, Zhao H, Goto K, Cheng D, Schaefer EA, Zhang L, Pantip C, Thongsawat S, O’Brien A, Peng LF, Maneekarn N, Chung RT, Lin W. Hepatitis C virus NS5A disrupts STAT1 phosphorylation and suppresses type I interferon signaling. J Virol. 2012;86(16):8581–8591. doi: 10.1128/JVI.00533-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau DT, Negash A, Chen J, Crochet N, Sinha M, Zhang Y, Guedj J, Holder S, Saito T, Lemon SM, Luxon BA, Perelson AS, Gale M., Jr Innate immune tolerance and the role of kupffer cells in differential responses to interferon therapy among patients with HCV genotype 1 infection. Gastroenterology. 2013;144(2):402–413. e412. doi: 10.1053/j.gastro.2012.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159(6):1312–1326. doi: 10.1016/j.cell.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CH, Chang CJ, Lin YJ, Yeh CN, Chen MF, Hsieh SY. Viral hepatitis-associated intrahepatic cholangiocarcinoma shares common disease processes with hepatocellular carcinoma. Br J Cancer. 2009;100(11):1765–1770. doi: 10.1038/sj.bjc.6605063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerat H, Rumin S, Habersetzer F, Berby F, Trabaud MA, Trepo C, Inchauspe G. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood. 1998;91(10):3841–3849. [PubMed] [Google Scholar]
- Li K, Li NL, Wei D, Pfeffer SR, Fan M, Pfeffer LM. Activation of chemokine and inflammatory cytokine response in hepatitis C virus-infected hepatocytes depends on Toll-like receptor 3 sensing of hepatitis C virus double-stranded RNA intermediates. Hepatology. 2012;55(3):666–675. doi: 10.1002/hep.24763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Choe WH, Hiasa Y, Kamegaya Y, Blackard JT, Schmidt EV, Chung RT. Hepatitis C virus expression suppresses interferon signaling by degrading STAT1. Gastroenterology. 2005;128(4):1034–1041. doi: 10.1053/j.gastro.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Liu S, Cai X, Wu J, Cong Q, Chen X, Li T, Du F, Ren J, Wu YT, Grishin NV, Chen ZJ. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347(6227):aaa2630. doi: 10.1126/science.aaa2630. [DOI] [PubMed] [Google Scholar]
- Lodoen MB, Lanier LL. Viral modulation of NK cell immunity. Nat Rev Microbiol. 2005;3(1):59–69. doi: 10.1038/nrmicro1066. [DOI] [PubMed] [Google Scholar]
- Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Owen DM, Li K, Erickson AK, Johnson CL, Fish PM, Carney DS, Wang T, Ishida H, Yoneyama M, Fujita T, Saito T, Lee WM, Hagedorn CH, Lau DTY, Weinman SA, Lemon SM, Gale M. Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(15):6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–783. e764. doi: 10.1053/j.gastro.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi K, Saito T, Crochet N, Barton DJ, Gale M, Jr, Silverman RH. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA. 2010;16(11):2108–2119. doi: 10.1261/rna.2244210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, Rice CM. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131(6):1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- McFarland AP, Horner SM, Jarret A, Joslyn RC, Bindewald E, Shapiro BA, Delker DA, Hagedorn CH, Carrington M, Gale M, Jr, Savan R. The favorable IFNL3 genotype escapes mRNA decay mediated by AU-rich elements and hepatitis C virus-induced microRNAs. Nat Immunol. 2014;15(1):72–79. doi: 10.1038/ni.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medvedev AE. Toll-like receptor polymorphisms, inflammatory and infectious diseases, allergies, and cancer. J Interferon Cytokine Res. 2013;33(9):467–484. doi: 10.1089/jir.2012.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner EG, Wu D, Osinusi A, Bon D, Virtaneva K, Sturdevant D, Porcella S, Wang H, Herrmann E, McHutchison J, Suffredini AF, Polis M, Hewitt S, Prokunina-Olsson L, Masur H, Fauci AS, Kottilil S. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J Clin Invest. 2014;124(8):3352–3363. doi: 10.1172/JCI75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miot C, Beaumont E, Duluc D, Le Guillou-Guillemette H, Preisser L, Garo E, Blanchard S, Hubert Fouchard I, Creminon C, Lamourette P, Fremaux I, Cales P, Lunel-Fabiani F, Boursier J, Braum O, Fickenscher H, Roingeard P, Delneste Y, Jeannin P. IL-26 is overexpressed in chronically HCV-infected patients and enhances TRAIL-mediated cytotoxicity and interferon production by human NK cells. Gut. 2015;64(9):1466–1475. doi: 10.1136/gutjnl-2013-306604. [DOI] [PubMed] [Google Scholar]
- Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nature Reviews Microbiology. 2007;5(6):453–463. doi: 10.1038/nrmicro1645. [DOI] [PubMed] [Google Scholar]
- Myong S, Cui S, Cornish PV, Kirchhofer A, Gack MU, Jung JU, Hopfner KP, Ha T. Cytosolic Viral Sensor RIG-I Is a 5 ‘-Triphosphate-Dependent Translocase on Double-Stranded RNA. Science. 2009;323(5917):1070–1074. doi: 10.1126/science.1168352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, Delker DA, Jo J, Bertoletti A, Hagedorn CH, Gale M., Jr IL-1beta Production through the NLRP3 Inflammasome by Hepatic Macrophages Links Hepatitis C Virus Infection with Liver Inflammation and Disease. Plos Pathog. 2013;9(4):e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science. 1998;282(5386):103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- Nitta S, Sakamoto N, Nakagawa M, Kakinuma S, Mishima K, Kusano-Kitazume A, Kiyohashi K, Murakawa M, Nishimura-Sakurai Y, Azuma S, Tasaka-Fujita M, Asahina Y, Yoneyama M, Fujita T, Watanabe M. Hepatitis C virus NS4B protein targets STING and abrogates RIG-I-mediated type I interferon-dependent innate immunity. Hepatology. 2013;57(1):46–58. doi: 10.1002/hep.26017. [DOI] [PubMed] [Google Scholar]
- Okuse C, Rinaudo JA, Farrar K, Wells F, Korba BE. Enhancement of antiviral activity against hepatitis C virus in vitro by interferon combination therapy. Antiviral Res. 2005;65(1):23–34. doi: 10.1016/j.antiviral.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Ooi EL, Chan ST, Cho NE, Wilkins C, Woodward J, Li M, Kikkawa U, Tellinghuisen T, Gale M, Jr, Saito T. Novel antiviral host factor, TNK1, regulates IFN signaling through serine phosphorylation of STAT1. Proc Natl Acad Sci U S A. 2014;111(5):1909–1914. doi: 10.1073/pnas.1314268111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange JS, Fassett MS, Koopman LA, Boyson JE, Strominger JL. Viral evasion of natural killer cells. Nat Immunol. 2002;3(11):1006–1012. doi: 10.1038/ni1102-1006. [DOI] [PubMed] [Google Scholar]
- Osburn WO, Fisher BE, Dowd KA, Urban G, Liu L, Ray SC, Thomas DL, Cox AL. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138(1):315–324. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier S, Drouin C, Bedard N, Khakoo SI, Bruneau J, Shoukry NH. Increased degranulation of natural killer cells during acute HCV correlates with the magnitude of virus-specific T cell responses. J Hepatol. 2010;53(5):805–816. doi: 10.1016/j.jhep.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokunina-Olsson L, Muchmore B, Tang W, Pfeiffer RM, Park H, Dickensheets H, Hergott D, Porter-Gill P, Mumy A, Kohaar I, Chen S, Brand N, Tarway M, Liu L, Sheikh F, Astemborski J, Bonkovsky HL, Edlin BR, Howell CD, Morgan TR, Thomas DL, Rehermann B, Donnelly RP, O’Brien TR. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nat Genet. 2013;45(2):164–171. doi: 10.1038/ng.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe IA, Galsinh SK, Wilson GK, Parker R, Durant S, Lazar C, Branza-Nichita N, Bicknell R, Adams DH, Balfe P, McKeating JA. Paracrine signals from liver sinusoidal endothelium regulate hepatitis C virus replication. Hepatology. 2014;59(2):375–384. doi: 10.1002/hep.26571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Gale M. Principles of intracellular viral recognition. Current Opinion in Immunology. 2007;19(1):17–23. doi: 10.1016/j.coi.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Saito T, Gale M. Regulation of innate immunity against hepatitis C virus infection. Hepatology Research. 2008;38(2):115–122. doi: 10.1111/j.1872-034X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Saito T, Hirai R, Loo Y-M, Owen D, Johnson CL, Sinha SC, Akira S, Fujita T, Gale M., Jr Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):582–587. doi: 10.1073/pnas.0606699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454(7203):523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarobe P, Lasarte JJ, Zabaleta A, Arribillaga L, Arina A, Melero I, Borras-Cuesta F, Prieto J. Hepatitis C virus structural proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J Virol. 2003;77(20):10862–10871. doi: 10.1128/JVI.77.20.10862-10871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011 doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schorey JS, Cheng Y, Singh PP, Smith VL. Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep. 2015;16(1):24–43. doi: 10.15252/embr.201439363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48(1):322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- Serti E, Chepa-Lotrea X, Kim YJ, Keane M, Fryzek N, Liang TJ, Ghany M, Rehermann B. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology. 2015;149(1):190–200. e192. doi: 10.1053/j.gastro.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serti E, Werner JM, Chattergoon M, Cox AL, Lohmann V, Rehermann B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology. 2014;147(1):209–220. e203. doi: 10.1053/j.gastro.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR. How cells respond to interferons revisited: from early history to current complexity. Cytokine Growth Factor Rev. 2007;18(5–6):419–423. doi: 10.1016/j.cytogfr.2007.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syal G, Fausther M, Dranoff JA. Advances in cholangiocyte immunobiology. Am J Physiol Gastrointest Liver Physiol. 2012;303(10):G1077–G1086. doi: 10.1152/ajpgi.00227.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210(2–4):237–247. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, Isogawa M, Chisari FV. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc Natl Acad Sci U S A. 2010;107(16):7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif KD, Siddiqui A. Cell surface expression of major histocompatibility complex class I molecules is reduced in hepatitis C virus subgenomic replicon-expressing cells. J Virol. 2003;77(21):11644–11650. doi: 10.1128/JVI.77.21.11644-11650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DR, Puig M, Darnell ME, Mihalik K, Feinstone SM. New antiviral pathway that mediates hepatitis C virus replicon interferon sensitivity through ADAR1. J Virol. 2005;79(10):6291–6298. doi: 10.1128/JVI.79.10.6291-6298.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Thio CL, Martin MP, Qi Y, Ge D, O’HUigin C, Kidd J, Kidd K, Khakoo SI, Alexander G, Goedert JJ, Kirk GD, Donfield SM, Rosen HR, Tobler LH, Busch MP, McHutchison JG, Goldstein DB, Carrington M. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461(7265) doi: 10.1038/nature08463. 798-U752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E, Gonzalez VD, Li Q, Modi AA, Chen W, Noureddin M, Rotman Y, Liang TJ. HCV infection induces a unique hepatic innate immune response associated with robust production of type III interferons. Gastroenterology. 2012;142(4):978–988. doi: 10.1053/j.gastro.2011.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195(1):43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Z, Pierce RH, Kurtis J, Kuroki Y, Crispe IN, Orloff MS. Hepatitis C virus core protein subverts the antiviral activities of human Kupffer cells. Gastroenterology. 2010;138(1):305–314. doi: 10.1053/j.gastro.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Wang B, Trippler M, Pei R, Lu M, Broering R, Gerken G, Schlaak JF. Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J Hepatol. 2009a;51(6):1037–1045. doi: 10.1016/j.jhep.2009.06.020. [DOI] [PubMed] [Google Scholar]
- Wang C, Pflugheber J, Sumpter R, Jr, Sodora DL, Hui D, Sen GC, Gale M., Jr Alpha interferon induces distinct translational control programs to suppress hepatitis C virus RNA replication. J Virol. 2003;77(7):3898–3912. doi: 10.1128/JVI.77.7.3898-3912.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Liang Y, Devaraj S, Wang J, Lemon SM, Li K. Toll-like receptor 3 mediates establishment of an antiviral state against hepatitis C virus in hepatoma cells. J Virol. 2009b;83(19):9824–9834. doi: 10.1128/JVI.01125-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46(5):1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Sugauchi F, Tanaka Y, Matsuura K, Yatsuhashi H, Murakami S, Iijima S, Iio E, Sugiyama M, Shimada T, Kakuni M, Kohara M, Mizokami M. Hepatitis C virus kinetics by administration of pegylated interferon-alpha in human and chimeric mice carrying human hepatocytes with variants of the IL28B gene. Gut. 2013;62(9):1340–1346. doi: 10.1136/gutjnl-2012-302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins C, Woodward J, Lau DT, Barnes A, Joyce M, McFarlane N, McKeating JA, Tyrrell DL, Gale M., Jr IFITM1 is a tight junction protein that inhibits hepatitis C virus entry. Hepatology. 2013;57(2):461–469. doi: 10.1002/hep.26066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Peisley A, Richards C, Yao H, Zeng X, Lin C, Chu F, Walz T, Hur S. Structural basis for dsRNA recognition, filament formation, and antiviral signal activation by MDA5. Cell. 2013;152(1–2):276–289. doi: 10.1016/j.cell.2012.11.048. [DOI] [PubMed] [Google Scholar]
- Yi G, Wen Y, Shu C, Han Q, Konan KV, Li P, Kao CC. Hepatitis C Virus NS4B Can Suppress STING Accumulation To Evade Innate Immune Responses. J Virol. 2015;90(1):254–265. doi: 10.1128/JVI.01720-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshio S, Kanto T, Kuroda S, Matsubara T, Higashitani K, Kakita N, Ishida H, Hiramatsu N, Nagano H, Sugiyama M, Murata K, Fukuhara T, Matsuura Y, Hayashi N, Mizokami M, Takehara T. Human blood dendritic cell antigen 3 (BDCA3)(+) dendritic cells are a potent producer of interferon-lambda in response to hepatitis C virus. Hepatology. 2013;57(5):1705–1715. doi: 10.1002/hep.26182. [DOI] [PubMed] [Google Scholar]
- Zhang S, Kodys K, Li K, Szabo G. Human type 2 myeloid dendritic cells produce interferon-lambda and amplify interferon-alpha in response to hepatitis C virus infection. Gastroenterology. 2013;144(2):414–425. e417. doi: 10.1053/j.gastro.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]