Abstract
Invasive mucinous lung adenocarcinoma (IMA) is a rare subtype of lung adenocarcinoma with no effective treatment option in advanced disease. KRAS mutations occur in 28–87% of the cases. NRG1 fusions were recently discovered in KRAS‐negative IMA cases and otherwise negative for known driver oncogenes and could represent an attractive therapeutic target. Published data suggest that NRG1 fusions occur essentially in nonsmoking Asian women. From an IMA cohort of 25 French patients of known ethnicity, driver oncogenes EGFR, KRAS, BRAF, ERBB2 mutations, and ALK and ROS1 rearrangements presence were analyzed. In the IMA samples remaining negative for these driver oncogenes, an NRG1 rearrangement detection was performed by FISH. A driver oncogene was identified in 14/25 IMA, namely 12 KRAS mutations (48%), one ROS1 rearrangement (4%), and one ALK rearrangement (4%). The detection of NRG1 rearrangement by FISH was conducted in the 11 pan‐negative IMA. One sample was NRG1 FISH‐positive and 100% of the tumor nuclei analyzed were positive. This NRG1‐positive patient was a 61‐year‐old nonsmoking woman of Vietnamese ethnicity and was the sole patient of Asian ethnicity of the cohort. She died 6 months after the diagnosis with a pulmonary multifocal disease. NRG1 FISH detection should be considered in patients with IMA pan‐negative for known driver oncogenes. These results might suggest that NRG1 fusion is more frequent in IMA from Asian patient. Larger studies are needed.
Keywords: FISH, invasive mucinous adenocarcinoma, lung adenocarcinoma, molecular oncology, NRG1
Introduction
Invasive mucinous adenocarcinoma (IMA) of the lung represents 2–10% of all lung adenocarcinomas (LUAD) 1, 2, 3. This histological subtype is considered as being one of the most malignant subtypes of LUAD, and is associated with a poor prognosis, probably due to frequent late‐stage diagnosis 1, 2, 3. Standard chemotherapy is the unique treatment option at advanced stages, as to date no effective targeted therapy has shown its effectiveness.
The most commonly found genetic alterations in IMA are KRAS mutations, with a prevalence of 28–87% of cases 4, 5, 6, 7, 8, 9, 10, 11, 12. Recently, recurrent CD74‐NRG1 somatic gene fusions were discovered in IMA cases otherwise negative for known driver oncogenes (EGFR, KRAS, BRAF, ERBB2, ALK, ROS1) 13. NRG1 (neuregulin 1) is usually not expressed in normal lung and in LUAD, but NRG1 fusions lead to NRG1 III‐b3 isoform expression in IMA. By means of an extracellular EGF‐like domain, NRG1 III‐b3 binds the extracellular domain of ERBB3, leading to heterodimerization of ERBB3 with ERBB2. The resulting activation of the downstream PI3K‐AKT and MAPK pathways promotes anchorage‐independent growth of LUAD cell lines. As ERBB2‐ERBB3 dimers and PI3K‐AKT and MAPK pathways could be targetable, NRG1 fusions represent promising therapeutic targets 14. Indeed, NRG1 fusion‐mediated signaling could be effectively suppressed in vitro by tyrosine kinase inhibitors such as lapatinib and afatinib approved for clinical use.
CD74 is the most frequently found NRG1 fusion partner, but novel NRG1 partners have been described, such as SLC3A2‐NRG1 and VAMP2‐NRG1 in two independent cohorts of IMA and RBPMS‐NRG1, WRN‐NRG1, and SDC4‐NRG1 in a cohort of LUAD and squamous lung carcinomas 11, 12, 15.
NRG1 fusions could drive 7–27% of IMA and published data suggest that these oncogenic fusions essentially occur in nonsmoking women of Asian origin 11, 13, 16. In this study, we sought to examine the prevalence and the clinical profile associated with NRG1 fusions in a French cohort of IMA patients.
Materials and Methods
Population studied
Twenty‐five consecutive IMA patients surgically treated at Tenon Hospital (AP‐HP), France, from 1991 to 2013, were retrieved from the Chest department database. The diagnosis was confirmed by a lung cancer pathologist (MA) and was based on the 2015 WHO classification of tumors of the lung 1. Clinical findings at diagnosis and follow‐up data were recorded. All patients signed a research informed consent form, permitting analysis of their biological samples. This study was approved by our hospital's ethics human research committee.
EGFR, KRAS, BRAF, and ERBB2 mutation analyses
For each formalin‐fixed paraffin‐embedded (FFPE) specimen, a 3‐μm tissue section was stained with H&S and examined by light microscopy to determine the percentage of tumor cells. After DNA isolation (QIAamp DNA mini kit®, Qiagen, Courtaboeuf, France) from three 20 μm tissue sections, EGFR mutations G719S, T790M, and L858R (exons 18, 20, and 21, respectively), KRAS mutations G12S, G12R, G12C, G12A, G12V, and G13D (exon 2), and BRAF mutations V600E and V600K (exon 15) were detected with TaqMan®Assays (Custom TaqMan® SNP Genotyping Assays, Life Technologies SAS, Saint Aubin, France). EGFR exon 19 deletions, EGFR exon 20 insertions and ERBB2 exon 20 insertion were detected by sizing analysis. Sequencing data were then analyzed using SeqScape software.
ALK and ROS1 immunohistochemistry
Immunostainings of the ALK and ROS1 proteins were performed on 3‐μm tissue sec tions on a Benchmark Ventana staining module (Ventana®, Roche Diagnostics, Meylan, France) using a primary monoclonal ALK antibody (Clone 5A4, Ab 17127; Abcam, Paris, France) diluted at 1:50 for 2 h at 37°C, or a primary monoclonal ROS1 antibody (Clone D4D6, #3287, Cell Signaling Technology®, Danvers, MA) at a dilution of 1:50 2 h at 20°C, as previously described. Positive external controls were performed, using a LUAD specimen that had been previously validated for ALK rearrangement by fluorescent in situ hybridization and the ROS1‐rearranged cell line HCC78. The staining scores were assessed as follows: 0, no staining; 1+, faint cytoplasmic staining; 2+, moderate cytoplasmic staining; and 3+, intense granular cytoplasmic staining. The presence of 10% of cells stained with an intensity of ≥2 was considered as positive staining. Specimens with a positive staining score were tested for ALK or ROS1 rearrangement by FISH.
ALK, ROS1, and NRG1 break‐apart FISH
FISH was performed on unstained 4‐μm FFPE tumor‐tissue sections using an ALK break‐apart probe set (Vysis LSI ALK Dual Color®, Break Apart Rearrangement Probe; Abbott Molecular, Rungis, France) or a ZytoLight® SPEC ROS1 Dual Color Break Apart Probe (ZytoVision, Bremerhaven, Germany) and a paraffin‐pretreated reagent kit (Vysis®, Abbott Molecular) according to the manufacturer's instructions. Tumor tissues were considered ALK‐positive if >15% of the cells showed split orange and green signals and/or single orange signals or ROS1‐positive if >15% of the cells showed split orange and green signals and/or single green signals.
As NRG1 fusions have been described in tumors without EGFR/KRAS/BRAF/HER2 mutations and ALK/ROS1 rearrangements, NRG1 break‐apart FISH was performed only in pan wild‐type samples.
An NRG1‐specific fluorescent DNA probe was used kindly provided by ZytoVision (Zytolight SPEC NRG1 Dual Color Break Apart, ZytoVision, Bremerhaven, Germany). This probe contains green and orange‐labeled polynucleotides, which target sequences mapping in 8p12 proximal to the NRG1 break point region. The 3' NRG1 probe is labeled with an orange spectrum fluorophore and the 5' NRG1 probe with a green spectrum fluorophore. The quality of each FISH experiment was categorized as good, moderate, or poor, according to the quality of the hybridization signals, and the presence of no to a very high fluorescent background noise, respectively. Tumor tissues were considered NRG1 FISH‐positive when >15% of the nuclei harbored either a split pattern with 3′ and 5′ signals separated by a distance superior to the diameter of the largest signal, or isolated 3′ (orange) signals. This threshold was chosen by analogy with the threshold commonly used for other FISH assays for gene rearrangement detection in FFPE lung tumor samples, such as ALK, ROS1, or RET gene rearrangements.
Nuclei were counterstained with DAPI/Vectashield® (Vektor Laboratories, Burlingame, CA) and were analyzed with a Leica CytoVision GSL10 FISH fluorescence capture system® (Leica, Nanterre, France) under a 63x oil immersion objective. Signals were enumerated with the CytoVision imaging system® (Leica). At least 100 nuclei were analyzed (mean = 126) for each tumor sample.
Results
Clinical and molecular findings for the 25 IMA patients are shown in Table 1. All the driver oncogenes detected were mutually exclusive.
Table 1.
Individual clinical and molecular characteristics of patients with invasive mucinous adenocarcinoma
| Samples | Sex | Age | Ethny | Smoking (pack year) | Driver oncogene |
|---|---|---|---|---|---|
| 1 | F | 78 | Caucasian | Never | None |
| 2 | F | 60 | Caucasian | Never | None |
| 3 | M | 62 | Caucasian | Never | None |
| 4 | F | 60 | Caucasian | Never | None |
| 5 | M | 47 | North African | Ever | None |
| 6 | M | 56 | Caucasian | Ever | None |
| 7 | F | 55 | Caucasian | Never | None |
| 8 | F | 68 | Caucasian | Never | None |
| 9 | F | 61 | Asian | Never | NRG1 |
| 10 | M | 46 | North African | Never | None |
| 11 | M | 57 | Caucasian | Ever | None |
| 12 | M | 63 | Caucasian | Ever | KRAS |
| 13 | M | 87 | Caucasian | Ever | KRAS |
| 14 | M | 54 | Caucasian | Ever | KRAS |
| 15 | M | 58 | Caucasian | Ever | KRAS |
| 16 | M | 71 | Caucasian | Ever | KRAS |
| 17 | F | 77 | Caucasian | Ever | KRAS |
| 18 | M | 70 | Caucasian | Ever | KRAS |
| 19 | M | 69 | Caucasian | Ever | KRAS |
| 20 | M | 73 | Caucasian | Ever | KRAS |
| 21 | F | 58 | Caucasian | Ever | KRAS |
| 22 | M | 78 | Caucasian | Ever | KRAS |
| 23 | M | 78 | Caucasian | Never | KRAS |
| 24 | F | 55 | Caucasian | Ever | ALK |
| 25 | F | 82 | Caucasian | Never | ROS1 |
After analysis for EGFR, KRAS, BRAF, and ERBB2 mutations and ALK and ROS1 rearrangements, 11 samples remained wild‐type for all driver oncogenes and were analyzed for NRG1 rearrangement by break‐apart FISH. The FISH patterns found in our cohort are depicted in Figure 1. The clinical findings and FISH quality and characteristics of each sample analyzed are shown in Table 2.
Figure 1.
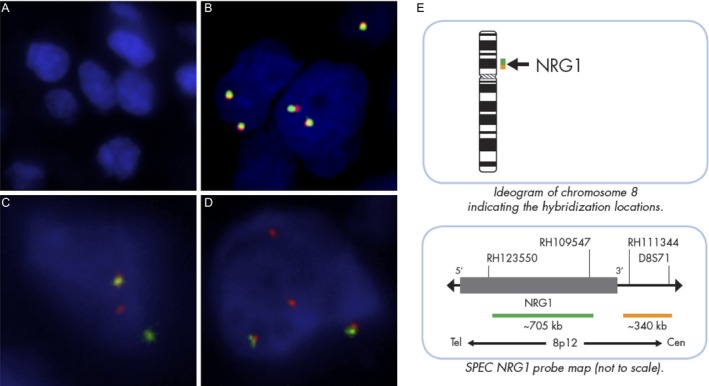
Patterns of NRG1 FISH hybridization in our study (A) noninterpretable (absence of FISH signal), (B) negative with two fusion signals per nucleus, (C) negative with the presence of a split signal (one orange and one green signal) in <15% of the nuclei, (D) positive with at least one isolated orange signal in more than 15% of the nuclei. Original magnification ×630. (E) Ideogram of chromosome 8 and NRG1 probe map for the ZytoLight® SPEC NRG1 Dual Color Break‐apart Probe (ZytoVision), kindly provided by ZytoVision.
Table 2.
Patient characteristics and NRG1 FISH results in invasive mucinous adenocarcinoma tested for NRG1 fusion
| Samples | Date of samples conditioning | Sex | Age | Ethny | Smoking (pack year) | FISH results | Positives tumor cells (%) | Hybridation quality |
|---|---|---|---|---|---|---|---|---|
| 1 | 1991 | F | 78 | Caucasian | Never | NI | _ | No FISH signal |
| 2 | 2005 | F | 60 | Caucasian | Never | Negative | 1.0 | Poor |
| 3 | 1999 | M | 62 | Caucasian | Never | NI | No FISH signal | |
| 4 | 2009 | F | 60 | Caucasian | Never | Negative | 1.0 | Poor |
| 5 | 2010 | M | 47 | North African | Ever (40) | Negative | 6.8 | Moderate |
| 6 | 1994 | M | 56 | Caucasian | Ever (58) | NI | No FISH signal | |
| 7 | 2001 | F | 55 | Caucasian | Never | NI | No FISH signal | |
| 8 | 2013 | F | 68 | Caucasian | Never | Negative | 7.4 | Good |
| 9 | 2006 | F | 61 | Asian | Never | Positive | 100 | Good |
| 10 | 2000 | M | 46 | North African | Never | NI | No FISH signal | |
| 11 | 1995 | M | 57 | Caucasian | Ever (65) | NI | No FISH signal |
F, female; M, Male; NI, Not interpretable.
One sample was NRG1 FISH‐positive and 100% of the tumor nuclei analyzed were positive, harboring at least one isolated orange signal, together with at least 1 fusion signal (Fig. 2). The frequency of each driver oncogene is shown in Figure 3.
Figure 2.
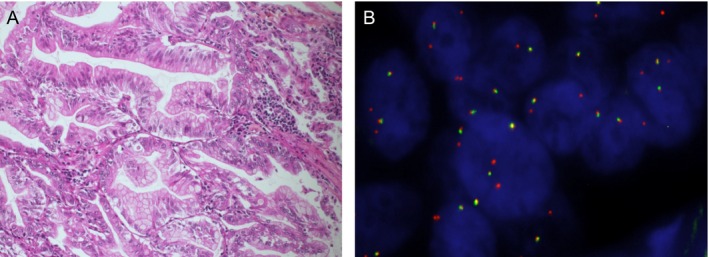
Representative histopathological features (A) and break‐apart NRG1 FISH result (B) of the NRG1‐positive IMA case. (A) Goblet or columnar well differentiated tumoral cells with abundant intracytoplasmic mucin and small basally located nuclei (Hematoxylin‐Eosin‐Saffron, original magnification ×20) (B) Tumor nuclei hybridized with the ZytoLight® SPEC NRG1 dual color beak‐apart probe (ZytoVision). All tumor cell nuclei analyzed were positive, showing at least one isolated 3' (orange) signal. Original magnification ×630.
Figure 3.
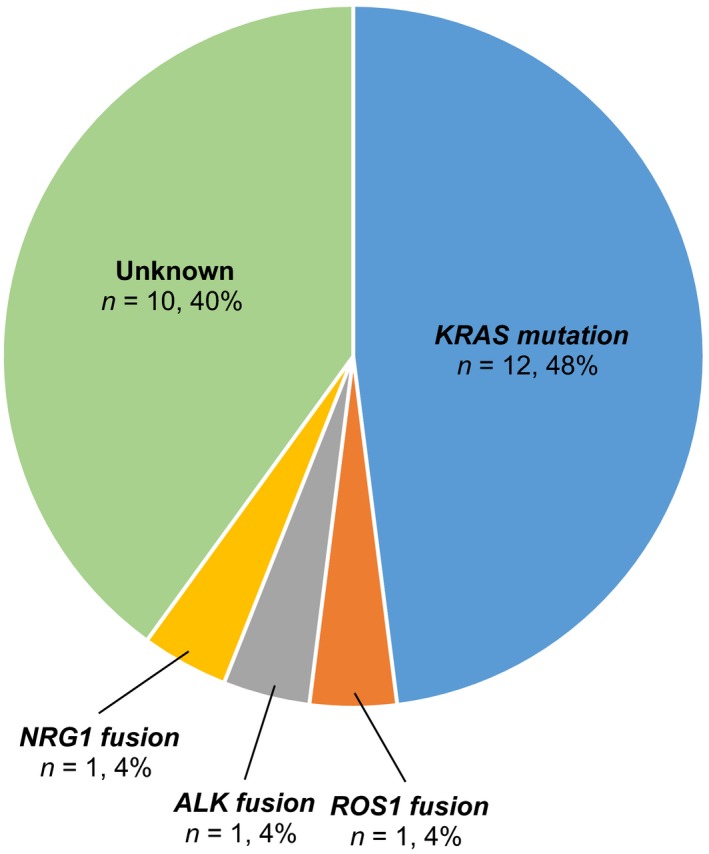
Pie chart of the frequencies of driver oncogenes detected. All driver oncogenes detected were mutually exclusive. Note that NRG1 FISH was performed only in the 11 samples wild‐type for EGFR,KRAS,ERBB2, and BRAF mutations and ALK and ROS1 rearrangements.
This NRG1‐positive patient was a 61‐year‐old nonsmoking woman. She was born in Vietman to Vietnamese parents and migrated in France in 1976. She had a history of cured left breast cancer in 1988 treated with sequential neoadjuvant chemotherapy, radical mastectomy and chest wall irradiation, an ileal and pulmonary tuberculosis in 2003 successfully treated with antibiotics, a minimal change nephrotic syndrome requiring a daily corticosteroid treatment until 2004 and an insulin‐dependent diabetes. She presented with cough and dyspnea in April 2006. Chest computed tomography (CT) showed diffuse pulmonary parenchymal involvement with alveolar consolidation and pseudo nodules with peripheral ground‐glass opacities in the lower left lobe. The upper left lobe was destroyed by sequelae of tuberculosis. Diagnosis was obtained by bronchoscopic cytology. Abdominal CT, brain magnetic resonance imaging (MRI), and positron emission tomography using 18F‐fluorodeoxyglucose revealed no evidence of mediastinal node involvement or extra thoracic metastasis. Because of upper left lobe destruction, a left pneumonectomy was performed. Pathological analysis revealed an IMA which was TTF1 negative and CK7 and CK20 positive. The chest wall was invaded in an extent inferior to 1 centimeter and tumor cells were observed in one intralobar node. The tumor was classified as pT3N1M0. In view of the medical history of the patient, adjuvant chemotherapy was not administered and radiotherapy of the chest wall was performed. The disease relapsed 5 months after the surgery with appearance of numerous nodules in the remaining right lung on chest CT. The patient was enrolled in the IFCT‐0504 clinical trial evaluating erlotinib or carboplatin/paclitaxel in advanced lepidic adenocarcinoma and was randomized in the erlotinib arm. After 4 weeks of erlotinib, the patient presented a respiratory failure secondary to a nondocumented right interstitial lung disease (ILD) which could be related to a disease progression or an erlotinib‐induced ILD. She died after two weeks in intensive critical care unit.
Discussion
NRG1 rearrangements may be found by FISH in IMA wild‐type for EGFR, KRAS, BRAF, ERBB2, ALK, and ROS1. Our series of 25 IMA showed one NRG1 FISH‐positive case, corresponding to a prevalence of 4%. Previous works using high throughput transcriptome sequencing in frozen samples or anchored multiplex PCR and next‐generation sequencing in FFPE samples estimated prevalence for NRG1 fusions in IMA of 7–27% 11, 12, 13. The lower prevalence in our study could be due to the lesser sensitivity of FISH assay in FFPE. The FISH signals were of poor quality in 6/11 cases and corresponded to samples fixed prior to 2003 when preanalytical tissue handling steps were less standardized.
The NRG1 FISH‐positive case was a Vietnamese nonsmoking woman, corresponding to the expected clinical profile reported in previous study (Table 3) 11, 13, 16. It is remarkable that the only NRG1‐positive case occurred in the sole patient of Asian ethnicity in our cohort. We speculate that NRG1 fusions might occur at a lower prevalence in IMA from Caucasian patients. Shim et al. reported the molecular analysis of two cohorts of IMA, one from Caucasian patients (n = 31) and one form Asian patients (n = 41). A trend for a lower prevalence of fusion in Caucasian was found but type of fusion according to ethnicity was not given.
Table 3.
Characteristics of published patients with NRG1‐positive invasive mucinous adenocarcinoma
| Sex | Age | Ethny | Smoking (pack year) | Gene fusion | Reference | |
|---|---|---|---|---|---|---|
| 1 | Female | 64 | Caucasian | Never | CD74‐NRG1 | Fernandez‐Cuesta et al. 13 |
| 2 | Female | 73 | Asian | Never | CD74‐NRG1 | Fernandez‐Cuesta et al. 13 |
| 3 | Female | 72 | Asian | Never | CD74‐NRG1 | Fernandez‐Cuesta et al. 13 |
| 4 | Female | 66 | Asian | Never | CD74‐NRG1 | Fernandez‐Cuesta et al. 13 |
| 5 | Female | 31 | Asian | Never | CD74‐NRG1 | Fernandez‐Cuesta et al. 13 |
| 6 | Male | 55 | Asian | Ever (47) | CD74‐NRG1 | Nakaoku et al. 11 |
| 7 | Female | 68 | Asian | Never | CD74‐NRG1 | Nakaoku et al. 11 |
| 8 | Female | 78 | Asian | Never | CD74‐NRG1 | Nakaoku et al. 11 |
| 9 | Female | 47 | Asian | Never | CD74‐NRG1 | Nakaoku et al. 11 |
| 10 | Female | 53 | Asian | Never | CD74‐NRG1 | Nakaoku et al. 11 |
| 11 | Female | 66 | Asian | Never | SLC3A2‐NRG1 | Nakaoku et al. 11 |
| 12 | Female | 89 | Asian | Never | CD74‐NRG1 | Gow et al. 16 |
| 13 | Female | 65 | NA | Never | CD74‐NRG1 | Shim et al. 12 |
| 14 | Male | 84 | NA | Never | CD74‐NRG1 | Shim et al. 12 |
| 15 | Male | 56 | NA | Ever | CD74‐NRG1 | Shim et al. 12 |
| 16 | Female | 73 | NA | Never | CD74‐NRG1 | Shim et al. 12 |
| 17 | Female | 58 | NA | Never | VAMP2‐NRG1 | Shim et al. 12 |
| 18 | Female | 62 | Asian | Never | NRG1 a | Duruisseaux et al. (this issue) |
Partner gene unknown.
The results of our study might indirectly suggest the scarcity of NRG1 fusions in IMA in Caucasian patients. However, there is a need of a dedicated study to answer the question of whether or not the prevalence of NRG1 fusion differs according to ethnicity. As NRG1 fusions could be targetable, NRG1 FISH detection should be considered in patients with IMA pan‐negative for EGFR, KRAS, BRAF, ERBB2, ALK, and ROS1.
Conflict of Interest
None declared.
Acknowledgments
M. Duruisseaux is a doctoral fellow funded by “Fonds de dotation Recherche en Santé Respiratoire 2010”, “AgiràDom”, and “Comité des maladies respiratoires (COMARES) de l'Isère”. This study also received support from “Subvention 2010 et 2011 Leg Poix ‐ La Chancellerie des Universités de Paris” and “ITMO Cancer 2012 Institut National du Cancer Plan Cancer 2009‐2013 Modèles de tumeurs spontanées chez l'animal pour la recherche translationnelle en cancérologie”.
We would like to thank the Tumorothèque des Hôpitaux Universitaires de l'Est Parisien (HUEP), (the East Paris University Hospitals Tumor Bio‐bank), AP‐HP, Hôpital Tenon, Service d'Anatomie Pathologique, F‐75970, Paris, for providing us with the samples.
Cancer Medicine 2016; 5(12):3579–3585
Michael Duruisseaux and Anne McLeer‐Florin contributed equally to this work.
References
- 1. Travis, W. D. , Brambilla E., and Muller‐Hermelink H. K.. 2004. Pathology and genetics of tumors of the lung, pleura, thymus and heart World Health Organisation classification of tumours. IARC Press, Lyon, France. [Google Scholar]
- 2. Yoshizawa, A. , Motoi N., Riely G. J., Sima C. S., Gerald W. L., Kris M. G., et al. 2011. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod. Pathol. 24:653–664. [DOI] [PubMed] [Google Scholar]
- 3. Warth, A. , Muley T., Meister M., Stenzinger A., Thomas M., Schirmacher P., et al. 2012. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage‐independent predictor of survival. J. Clin. Oncol. 30:1438–1446. [DOI] [PubMed] [Google Scholar]
- 4. Marchetti, A. , Pellegrini S., Bertacca G., Buttitta F., Gaeta P., Carnicelli V., et al. 1998. FHIT and p53 gene abnormalities in bronchioloalveolar carcinomas. Correlations with clinicopathological data and K‐ras mutations. J. Pathol. 184:240–246. [DOI] [PubMed] [Google Scholar]
- 5. Kadota, K. , Yeh Y.‐C., D'Angelo S. P., Moreira A. L., Kuk D., Sima C. S., et al. 2014. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am. J. Surg. Pathol. 38:1118–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hata, A. , Katakami N., Fujita S., Kaji R., Imai Y., Takahashi Y., et al. 2010. Frequency of EGFR and KRAS mutations in Japanese patients with lung adenocarcinoma with features of the mucinous subtype of bronchioloalveolar carcinoma. J. Thorac. Oncol. 5:1197–1200. [DOI] [PubMed] [Google Scholar]
- 7. Finberg, K. E. , Sequist L. V., Joshi V. A., Muzikansky A., Miller J. M., Han M., et al. 2007. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J. Mol. Diagn. 9:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sakuma, Y. , Matsukuma S., Yoshihara M., Nakamura Y., Noda K., Nakayama H., et al. 2007. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K‐ras gene‐mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am. J. Clin. Pathol. 128:100–108. [DOI] [PubMed] [Google Scholar]
- 9. Wislez, M. , Antoine M., Baudrin L., Poulot V., Neuville A., Pradere M., et al. 2010. Non‐mucinous and mucinous subtypes of adenocarcinoma with bronchioloalveolar carcinoma features differ by biomarker expression and in the response to gefitinib. Lung Cancer 68:185–191. [DOI] [PubMed] [Google Scholar]
- 10. Hu, H. , Pan Y., Li Y., Wang L., Wang R., Zhang Y., et al. 2014. Oncogenic mutations are associated with histological subtypes but do not have an independent prognostic value in lung adenocarcinoma. Onco. Targets Ther. 7:1423–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakaoku, T. , Tsuta K., Ichikawa H., Shiraishi K., Sakamoto H., Enari M., et al. 2014. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin. Cancer Res. 20:3087–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shim, H. S. , Kenudson M., Zheng Z., Liebers M., Cha Y. J., Hoang Ho Q., et al. 2015. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J. Thorac. Oncol. 10:1156–1162. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez‐Cuesta, L. , Plenker D., Osada H., Sun R., Menon R., Leenders F., et al. 2014. CD74‐NRG1 fusions in lung adenocarcinoma. Cancer Discov. 4:415–422. [DOI] [PubMed] [Google Scholar]
- 14. Fernandez‐Cuesta, L. , and Thomas R. K.. 2015. Molecular pathways: targeting NRG1 fusions in lung cancer. Clin. Cancer Res. 21:1989–1994. [DOI] [PubMed] [Google Scholar]
- 15. Dhanasekaran, S. M. , Balbin O. A., Chen G., Nadal E., Kalyana‐Sundaram S., Pan J., et al. 2014. Transcriptome meta‐analysis of lung cancer reveals recurrent aberrations in NRG1 and Hippo pathway genes. Nat. Commun. 5:5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gow, C.‐H. , Wu S.‐G., Chang Y.‐L., and Shih J.‐Y.. 2014. Multidriver mutation analysis in pulmonary mucinous adenocarcinoma in Taiwan: identification of a rare CD74‐NRG1 translocation case. Med. Oncol. 31:34. [DOI] [PubMed] [Google Scholar]


