Abstract
Lung cancer is the most common reason of cancer deaths and about 85% of these are non‐small‐cell lung cancer. Currently, lung cancer therapy is mainly based on the tumor node metastasis (TNM) disease staging and tumor histological classification. Despite therapeutic innovations, the prognosis for lung cancer patients has not significantly changed in the last years. Therefore, a proper understanding of cell signaling pathways involved in cancer pathogenesis seems to be essential for improvement in cancer therapy field. The knowledge of crosstalk between epidermal growth factor receptor (EGFR) and Notch pathway can lead to enhanced screening for the expression of these genes allowing patients to optimize treatment options and predict potential treatment resistance. This review focuses on recent advances related to the mechanisms of EGFR and Notch signaling in non‐small‐cell lung cancer and the effectiveness of current Notch‐ and EGFR‐targeted therapies.
Keywords: Epidermal growth factor receptor, non‐small‐cell lung cancer, Notch, tyrosine kinase inhibitors
Introduction
EGFR alterations in non‐small‐cell lung cancer in brief
Epidermal growth factor receptor (EGFR) has tyrosine kinase activity and is a transmembrane glycoprotein. The EGFR and members of its family play a significant role in carcinogenesis through contribution into cell proliferation, apoptosis, cell motility, and angiogenesis. EGFR alterations are involved in the pathogenesis and progression of many malignancies including lung cancer 1, 2, 3, 4, 5.
One of the most common alteration in non‐small‐cell lung cancer (NSCLC) patients and cells is overexpression of EGFR, which is demonstrated in more than half cases of NSCLC and is associated with a poor prognosis and chemoresistance 6, 7. Moreover, the expression of EGFR appears to be dependent on histological subtypes of NSCLC, and is most frequently expressed in squamous cell. In addition to EGFR overexpression, activating mutations of EGFR are observed in around 10% of all nonsquamous non‐small‐cell lung cancer patients 8.
Epidermal growth factor receptor mutations are significant predictors of treatment response to tyrosine kinase inhibitors (TKISs) in patients with non‐small‐cell lung cancer. However, according to researchers, diverse response to the treatment is common. Therefore, there are group of patients with mutations who do not show any response and some patients without mutations who can respond to the treatment. Moreover, other investigators 9 discovered additional alterations of EGFR in NSCLC patients (Table 1). Winter‐Larsen et al. identified genetic polymorphism of the EGFR gene and expected it may be important for prediction of clinical consequences in TKISs‐treated advanced NSCLC patients 10. Furthermore, increased EGFR copy numbers were described as a common modification in NSCLC. Altered EGFR copy numbers are present up to 59% of NSCLC 11, 12, 13, 14. According to Sholl et al. and other research groups 14, 16, gain of EGFR copy number is related to a positive effect after EGFR TKISs treatment; it has also been proposed to be a potential biomarker of TKISs responsiveness. Likewise, treatment with TKISs gives better results in positive EGFR samples 12, 15. In addition to described alteration, EGFR methylation and phosphorylation might have strong impact on the clinical outcome of NSCLC. Thus, Li et al. discovered the importance of EGFR gene methylation, which was associated with malignancy of this type of cancer 17. In other study, patients with phospho‐EGFR‐positive tumors demonstrated a longer survival 18. On the other hand, Hijiya et al. investigated 21 cases of NSCLC to examine correlations between the existence of EGFR mutations and the EGFR phosphorylation grade by immunohistochemistry. Moreover, the mutation status of the EGFR gene was correlated with immunoreactivity for phosphor‐EGFR and its immunoreactivity was significantly correlated with clinical responsiveness to one of the available drug—gefitinib 19. Taking together, the alterations of EGFR are common condition in NSCLC patients and usually correlate with poor prognosis and resistance to chemotherapy.
Table 1.
Aberrations in EGFR and Notch signaling pathways in NSCLC
| Pathway | Alteration | Clinical implication | Source |
|---|---|---|---|
| EGFR | Overexpression | Poor prognosis, chemoresistance | 6, 7 |
| Mutations | Predictors to TKISs response | 8 | |
| Copy number variations | Potential biomarker of TKISs responsiveness | 11, 12, 13, 14 | |
| Methylation status | Positive correlation with malignancy | 17 | |
| Phosphorylation status | Survival predictor | 18 | |
| Notch | Dysregulated expression | Poor prognosis | 24, 25, 26, 27 |
| Mutations | Poor prognosis | 28 |
EGFR, epidermal growth factor receptor; NSCLC, non‐small‐cell lung cancer; TKISs, tyrosine kinase inhibitors.
Notch in non‐small‐cell lung cancer in brief
The Notch signaling pathway is conservative and plays an important role in the cellular proliferation, differentiation, and apoptosis. The human Notch family includes four receptors (Notch 1 through 4 in mammalians) and five ligands (Jagged1, Jagged2, Dll1, Dll3, and Dll4) 20, 21. Activation of Notch pathway depends on interaction between specific ligand and receptor; nevertheless different mechanisms are involved in this process. The canonical way occurs when NICD is released after enzymatic intervention of ADAM family metalloprotease, which creates a substrate for a second cleavage by the γ‐secretase complex, releasing the Notch intracellular domain (NICD). The intracellular domain is later moved into the nucleus where it cooperates with CBF‐1 (transcription factor recombining binding protein suppressor of hairless). The noncanonical way can take place without γ‐secretase cleavage and CBF‐1 20. Although, the mechanisms of Notch activation are known in physiological conditions, the processes regulating this pathway in cancer are not so evident. It has been postulated that hypoxic tumor microenvironment may be crucial in regulation of Notch pathway in cancer. Moreover, evaluation of Notch pathway expression in cancer may not be related only to up‐ or downregulation of this signaling, but may be determined by compound interactions with EGFR through activation of PI3K/AKT/mTOR cascade which in turn increases the translation of hypoxia inducible factors (HIF‐1α). Therefore, according to some authors, hypoxia stabilizes NICD which can interact with hypoxia‐inducible factor 1 alpha (HIF‐1α) (Fig. 1) 22, 23.
Figure 1.
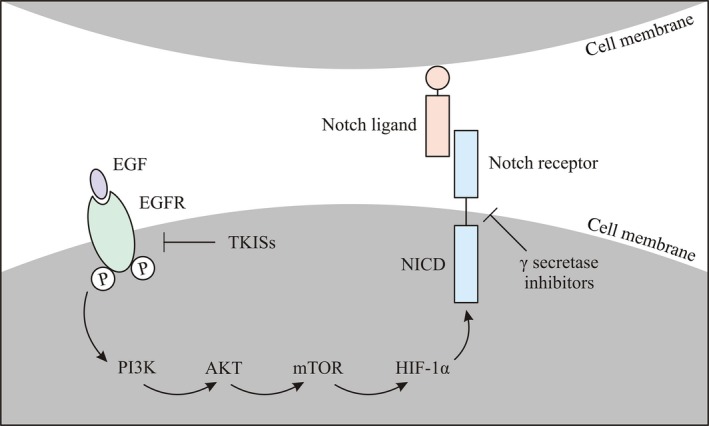
The scheme shows that activation of EGFR triggers PI3K/AKT/mTOR pathways cascade, which increases the translation of hypoxia‐inducible factors. Moreover, hypoxia can stabilize NICD by interaction with HIF‐1α. TKISs, tyrosine kinase inhibitors; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; PI3K/AKT, phosphatidylinositide 3‐kinases; mTOR, mechanistic target of rapamycin; NICD, intracellular domain of Notch; HIF‐1α, hypoxia‐inducible factors.
There are some studies indicating Notch is highly activated in NSCLC 24, 25. However, other studies demonstrated a reduced or undetectable Notch1 expression in NSCLC. This implies a supposed Notch1 tumor‐suppressive role in these tumors and again gives a notion that Notch function in NSCLC is more complicated than predicted 26, 27.
On the other hand, altered Notch genes may influence the stabilization of Notch in NSCLC. Hence, two types of alterations were detected in NSCLC: heterozygous mutations of the Notch‐1 locus in 10% of the cases, and loss of Numb expression in 30% of the cases of NSCLC 28.
Although, the role of Notch in non‐small‐cell lung cancer remains unclear, the observations reveal that Notch signaling in NSCLC depends on the specific tissue context, microenvironment, and crosstalk with other signaling pathways. Consequently, it might be important in development of tumor or can act as a tumor suppressor 24. Although the mechanism of Notch signaling in lung cancer pathogenesis is not fully understood, most likely other factors are involved.
Clinical relevance and therapeutic approaches aimed at targeting Notch and EGFR signaling in NSCLC
Many research groups try to explain crosstalk between Notch and EGFR in order to understand the mechanism of this cooperation and to know how cancer cells use the Notch pathway to compensate for EGFR‐targeted inhibition. Notch and epidermal growth factor receptor (EGFR) signaling are essential in cell proliferation, differentiation, and apoptosis, and thereby may contribute to the development of lung cancer.
It has been described that those pathways can cooperate in different mechanisms, either antagonistic or synergistic (Fig. 2), depending on tissue, developmental status, and microenvironment 29.
Figure 2.
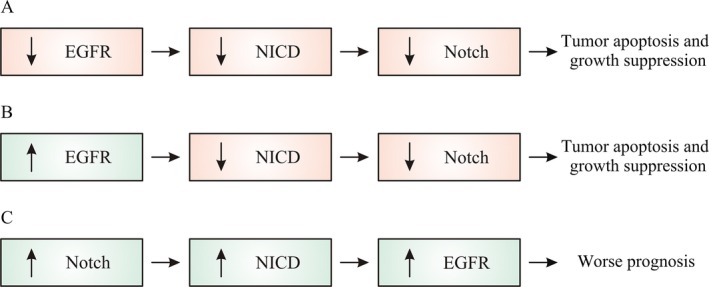
Possible mechanisms of crosstalk between EGFR and Notch and clinical consequences: (A) EGFR cooperates with intracellular domain of Notch by enhancing its effect on tumor apoptosis, (B) EGFR overexpression downregulates Notch, (C) Notch upregulates EGFR expression through p53 as a mediator of the Notch‐1. NICD, intracellular domain of Notch.
Although recent studies have shown that Notch and EGFR signaling are associated with drug resistance, antiangiogenic agent and EGFR tyrosine kinase inhibitors have been accepted for NSCLC treatment 30, 31.
Moreover, current clinical trials examine the efficacy and safety of antiangiogenic and anti‐EGFR agents combinations, as well as additional agents such as multitargeted antiangiogenic tyrosine kinase inhibitors 32, 33.
The researchers revealed that the expression of Notch‐1 was upregulated in EGFR‐TKISs developed resistant lung cancer cells. Additional, Notch‐1 contributed to the achievement of the epithelial–mesenchymal transition (EMT) phenotype, which was correlated with developed resistance to EGFR‐TKISs 34.
Another study showed that while inhibition of EGFR leads to reduction in tumor cell number, it also leads to a potent activation of the Notch pathway. Combined inhibition of EGFR and Notch3 receptors significantly reduced the growth of stem‐like cells. Taking together, investigators concluded that treatment of EGFR‐mutated lung cancer cell lines with erlotinib enriched then stem‐like cells with stem‐like cell potential through EGFR‐dependent activation of Notch3. Moreover, γ ‐secretase inhibitors could reverse this phenotype. Furthermore, the scientists noticed that phosphorylation of Notch3 can be linked to EGFR receptor, but no exact mechanism is known yet 35.
The crosstalk between Notch and EGFR pathway was also conducted by Konishi et al. and Kolev et al. The investigators demonstrated that the interaction between both pathways results in the inhibition of apoptosis 36, 37. Although independent results presented in gliomas indicated that Notch may upregulates EGFR through p53 38, another study showed that inhibition of Notch cleavage may not change cell number in the presence of EGFR mutations. Moreover, EGFR may affect Notch signaling suggesting that inhibition of both pathways could be promising in NSCLC. The researchers selected four NSCLC cell lines expressing different levels of NICD (intracellular domain of Notch) and EGFR protein levels and found that the cell lines exhibited different response to the γ‐secretase inhibitor DAPT (N‐[N‐(3,5‐difluorophenacetyl)‐l‐alanyl]‐S‐phenylglycine t‐butyl ester) and related this to EGFR status. DAPT was effective in proliferation of cells expressing wt EGFR (wild type), whereas it did not affect HCC827 cells expressing mutated EGFR. In addition, alterations were observed among the cells with wild‐type EGFR 39. Another groups of investigators focused on EGFR and Notch ligands. Correspondingly, Choi et al. examined Jag1 expression regulated by EGFR. Nevertheless, Jag2, which belongs to the same group of ligands, was not regulated by EGFR. To examine the role of EGFR using a different approach, wild‐type EGFR H1299 cells, which indicated low levels of Jag1 and Jag2 expression, were treated with EGF or transfected with wild‐type EGFR. As a result, two of the transfected agents increased only the expression of Jag1 and gefitinib treatment abolished EGFR‐induced Jag1 expression in H1299 cells 40. The discovery of EGFR mutations in non‐small‐cell lung cancer initiated the personalized medicine in advanced NSCLC. During the last decade, different EGFR‐TKISs have been developed. Three EGFR inhibitors, gefitinib, erlotinib, and afatinib, are already used in treatment for patients with NSCLC (Tables 2 and 3). Nevertheless, despite great advances have been made, novel treatment still should overcome the therapeutic challenges, such as resistance or metastases 41.
Table 2.
The most promising Notch and EGFR inhibitors list for targeted therapy of NSCLC
| Targets | |
|---|---|
| Notch inhibitors | |
| neutralizing monoclonal antibodies: OMP‐59R5, OMP‐21M18, NRR1, NRR2 | Notch receptors and ligands |
| γ‐secretase inhibitors: RO4929097, MRK‐0752, PF‐03084014, MRK‐003, BMS‐906024 | Blocking proteolytic activation of Notch receptors |
| EGFR inhibitors | |
| erlotinib, afatinib, gefitinib | EGFR gene mutations |
| osimertinib, rociletinib, dacomitinib | Cells with the T790M mutation |
| anti‐EGFR monoclonal antibodies: cetuximab, nimotuzumab, panitumumab | Three agents act on the same target (EGFR) |
EGFR, epidermal growth factor receptor; NSCLC, non‐small‐cell lung cancer.
Table 3.
Effectiveness of Notch‐ and EGFR‐targeted therapies in NSCLC
| Effectiveness of current Notch‐ and EGFR‐targeted therapy in NSCLC | References | |
|---|---|---|
| Notch‐targeted therapies | ||
| Inhibition of Notch signaling with available γ‐secretase inhibitors, mAbs, arsenic trioxide (animal model) | Affect tumor cells survival, differentiation, angiogenesis; drawbacks—toxicity | 42, 43, 44 |
| EGFR‐targeted therapies | ||
| Inhibition of mutated EGFR with TKISs inhibitors | Efficient in NSCLC patients with mutated EGFR, effectiveness in the treatment of brain metastases from NSCLC; drawbacks—cancer cells develop new mutations in the EGFR gene | 45, 46, 47 |
| Inhibition of mutated EGFR with mAbs | Used with chemotherapy as the first treatment in people with advanced squamous cell NSCLC inhibit tumor growth | 48, 49 |
| Combined Notch‐/EGFR‐targeted therapies | ||
| A phase I/II trial combining erlotinib (E) with γ‐secretase inhibitor RO4929097(R) in advanced NSCLC | Combination of R and E is safe in patients with NSCLC; clinical trial information: NCT01193881 | 50 |
| Combined Notch/EGFR therapy with γ‐secretase inhibitor (DAPT) N‐[N‐(3,5‐difluorophenacetyl)‐l‐alanyl]‐(S)‐phenylglycine t‐butyl ester and gefitinib (animal model) | Effective tumor growth inhibition, with decreased proliferative activity and increased apoptotic activity | 34 |
mAbs, monoclonal antibodies; E, erlotinib; R, γ‐secretase inhibitor RO4929097; TKISs, tyrosine kinase inhibitors; DAPT, N‐[N‐(3,5‐difluorophenacetyl)‐L‐alanyl]‐(S)‐phenylglycine t‐butyl ester.
Conclusions
As researchers have developed knowledge about the alterations in lung cancer cells that help them grow, they have developed newer drugs that specifically target these changes. Despite of new drugs and therapeutic regiments, the prognosis for lung cancer patients has not significantly transformed in the last years. There is now overwhelming data on the prognostic and predictive value of each EGFR signaling in NSCLC. Although the role of EGFR signaling in the pathogenesis and progression of NSCLC is well recognized, the importance of Notch pathway and its correlation with EGFR in lung cancer is still under investigation. Notch may act as an oncogene or a tumor suppressor gene in lung cancer cells depending on tissue, developmental context, and microenvironment. However, recently agents targeting the fundamental molecular signaling pathways in lung cancer are already under clinical trials with more promising results. Thus, the mechanism(s) of crosstalk between EGFR and Notch in non‐small‐cell lung cancer need to be identified.
Conflict of Interest
The author declares that there is no conflict of interests regarding the publication of this study.
Acknowledgments
This work is support by the budget for science in the years 2013–2015 Project No. IP2012 033872 (Iuventus Plus).
Cancer Medicine 2016; 5(12):3572–3578
References
- 1. Ciardiello, F. , and Tortora G.. 2008. EGFR antagonists in cancer treatment. N. Engl. J. Med. 358:1160–1174. [DOI] [PubMed] [Google Scholar]
- 2. Ciardiello, F. , and Tortora G.. 1998. Interactions between the epidermal growth factor receptor and type I protein kinase A: biological significance and therapeutic implications. Clin. Cancer Res. 4:821–828. [PubMed] [Google Scholar]
- 3. Lynch, T. J., Bell D. W., Sordella R., Gurubhagavatula S., Okimoto R. A., Brannigan B. W., et al. 2004. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N. Engl. J. Med. 350:2129–2139. [DOI] [PubMed] [Google Scholar]
- 4. Pao, W. , and Miller V. A.. 2005. Epidermal growth factor receptor mutations, small‐molecule kinase inhibitors, and non‐small‐cell lung cancer: current knowledge and future directions. J. Clin. Oncol. 23:556–2568. [DOI] [PubMed] [Google Scholar]
- 5. Cheng, L. , and Zhang D.. 2008. Molecular genetic pathology. Humana Press/Springer, New York, NY. [Google Scholar]
- 6. Reade, C. A. , and Ganti A. K.. 2009. EGFR targeted therapy in non‐small cell lung cancer: potential role of cetuximab. Biogeosciences 3:215–224. [PMC free article] [PubMed] [Google Scholar]
- 7. Cheng, L. , Zhang S., Alexander R., Yao Y., MacLennan G. T., Pan C. X., et al. 2011. The landscape of EGFR pathways and personalized management of non‐small‐cell lung cancer. Future Oncol. 7:519–541. [DOI] [PubMed] [Google Scholar]
- 8. Lopes, G. L. , Vattimo E. F., and de Castro Junior G.. 2015. Identifying activating mutations in the EGFR gene: prognostic and therapeutic implications in non‐small cell lung cancer. J Bras. Pneumol. 41:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tsiambas, E. , Ragos V., Lefas A. Y., Georgiannos S. N., Grapsa D., Patsouris E., et al. 2015. Chromosome 7 deregulation in non‐small cell lung carcinoma molecular landscape. J BUON 20:1635–1639. [PubMed] [Google Scholar]
- 10. Winther‐Larsen, A. , Nissen P. H., Jakobsen K. R., Demuth C., Sorensen B. S., and Meldgaard P.. 2015. Genetic polymorphism in the epidermal growth factor receptor gene predicts outcome in advanced non‐small cell lung cancer patients treated with erlotinib. Lung Cancer 90:314–320. [DOI] [PubMed] [Google Scholar]
- 11. Dahabreh, I. J. , Linardou H., Siannis F., Kosmidis P., Bafaloukos D., and Murray S.. 2010. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non‐small cell lung cancer. Clin. Cancer Res. 16:291–303. [DOI] [PubMed] [Google Scholar]
- 12. Hirsch, F. R. , Herbst R. S., Olsen C., Chansky K., Crowley J., Kelly K., et al. 2008. Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non‐small‐cell lung cancer patients treated with cetuximab and chemotherapy. J. Clin. Oncol. 26:3351–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cappuzzo F., Hirsch F. R., Rossi E., Bartolini S., Ceresoli G. L., Bemis L., et al. 2005. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non‐small‐cell lung cancer. J. Natl Cancer Inst. 97:643–655. [DOI] [PubMed] [Google Scholar]
- 14. Sholl, L. M. , John Iafrate A., Chou Y. P., Wu M. T., Goan Y. G., Su L., et al. 2007. Validation of chromogenic in situ hybridization for detection of EGFR copy number amplification in nonsmall cell lung carcinoma. Mod. Pathol. 20:1028–1035. [DOI] [PubMed] [Google Scholar]
- 15. Tsao, M. S. , Sakurada A., Cutz J. C., Zhu C. Q., Kamel‐Reid S., Squire J., et al. 2005. Erlotinib in lung cancer – molecular and clinical predictors of outcome. N. Engl. J. Med. 353:133–144. [DOI] [PubMed] [Google Scholar]
- 16. Cappuzzo, F. , Ligorio C., Jänne P. A., Toschi L., Rossi E., Trisolini R., et al. 2007. Prospective study of gefitinib in epidermal growth factor receptor fluorescence in situ hybridization‐positive/phospho‐AKT‐positive or never smoker patients with advanced non‐small‐cell lung cancer: the ONCOBELL trial. J. Clin. Oncol. 25:2248–2255. [DOI] [PubMed] [Google Scholar]
- 17. Li, J. , Jia X. F., Liu J., Liu J. J., and Zhao H. B.. 2015. Relationship of EGFR DNA methylation with the severity of non‐small cell lung cancer. Genet. Mol. Res. 14:11915–11923. [DOI] [PubMed] [Google Scholar]
- 18. Endoh, H. , Ishibashi Y., Yamaki E., Yoshida T., Yajima T., Kimura H., et al. 2009. Immunohistochemical analysis of phosphorylated epidermal growth factor receptor might provide a surrogate marker of EGFR mutation. Lung Cancer 63:241–246. [DOI] [PubMed] [Google Scholar]
- 19. Hijiya, N. , Miyawaki M., Kawahara K., Akamine S., Tsuji K., Kadota J., et al. 2008. Phosphorylation status of epidermal growth factor receptor is closely associated with responsiveness to gefitinib in pulmonary adenocarcinoma. Hum. Pathol. 39:316–323. [DOI] [PubMed] [Google Scholar]
- 20. Pancewicz, J. , and Nicot C.. 2011. Current views on the role of Notch signaling and the pathogenesis of human leukemia. BMC Cancer 11:502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu, N. , Zhang J., and Ji C.. 2013. Ankyrin/CDC10 repeats (ANK), necessary for protein‐protein interactions. Biomark. Res. 1:23.24252593 [Google Scholar]
- 22. Andersson, E. R. , Sandberg R., and Lendahl U.. 2011. Notch signaling: simplicity in design, versatility in function. Development 138:3593–3612. [DOI] [PubMed] [Google Scholar]
- 23. Yamaguchi, H. , Chang S. S., Hsu J. L., and Hung M. C.. 2014. Signaling cross‐talk in the resistance to HER family receptor targeted therapy. Oncogene 33:1073–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brizzi, M. F. , and Defilippi P.. 2013. Dll4/Notch1 signaling from tip/stalk endothelial cell specification to stroma‐dependent lung tumor inhibition: a flavor of Dll4/Notch1 pleiotropy in tumor cell biology. Transl. Lung Cancer Res. 2:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yuan, X. , Wu H., Xu H., Han N., Chu Q., Yu S., et al. 2015. Meta‐analysis reveals the correlation of Notch signaling with non‐small cell lung cancer progression and prognosis. Sci. Rep. 5:10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen, Y. , De Marco M. A., Graziani I., Gazdar A. F., Strack P. R., Miele L., et al. 2007. Oxygen concentration determines the biological effects of NOTCH‐1 signaling in adenocarcinoma of the lung. Cancer Res. 67:7954–7959. [DOI] [PubMed] [Google Scholar]
- 27. Konishi, J. , Kawaguchi K. S., Vo H., Haruki N., Gonzalez A., Carbone D. P., et al. 2007. Gamma‐secretase inhibitor prevents Notch3 activation and reduces proliferation in human lung cancers. Cancer Res. 67:8051–8057. [DOI] [PubMed] [Google Scholar]
- 28. Westhoff, B. , Colaluca I. N., D'Ario G., Donzelli M., Tosoni D., Volorio S., et al. 2009. Alterations of the Notch pathway in lung cancer. Proc. Natl Acad. Sci. USA 106:22293–22298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Doroquez, D. B. , and Rebay I.. 2006. Signal integration during development: mechanisms of EGFR and Notch pathway function and cross‐talk. Crit. Rev. Biochem. Mol. Biol. 41:339–385. [DOI] [PubMed] [Google Scholar]
- 30. Aita, M. , Fasola G., Defferrari C., Brianti A., Bello M. G., Follador A., et al. 2008. Targeting the VEGF pathway: antiangiogenic strategies in the treatment of non‐small cell lung cancer. Crit. Rev. Oncol. Hematol. 68:183–196. [DOI] [PubMed] [Google Scholar]
- 31. Adamo, V. , Franchina T., Adamo B., Denaro N., Gambadauro P., Chiofalo G., et al. 2009. Gefitinib in lung cancer therapy: clinical results, predictive markers of response and future perspectives. Cancer Biol. Ther. 8:206–212. [DOI] [PubMed] [Google Scholar]
- 32. Scagliotti, G. , and Govindan R.. 2010. Targeting angiogenesis with multitargeted tyrosine kinase inhibitors in the treatment of non‐small cell lung cancer. Oncologist 15:436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pennell, N. A. , and Lynch T. J. Jr. 2009. Combined inhibition of the VEGFR and EGFR signaling pathways in the treatment of NSCLC. Oncologist 14:399–411. [DOI] [PubMed] [Google Scholar]
- 34. Xie, M. , He C. S., Wei S. H., and Zhang L.. 2013. Notch‐1 contributes to epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance in non‐small cell lung cancer in vitro and in vivo. Eur. J. Cancer 49:3559–3572. [DOI] [PubMed] [Google Scholar]
- 35. Arasada, R. R. , Amann J. M., Rahman M. A., Huppert S. S., and Carbone D. P.. 2014. EGFR blockade enriches for lung cancer stem‐like cells through Notch3‐dependent signaling. Cancer Res. 74:5572–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Konishi, J. , Yi F., Chen X., Vo H., Carbone D. P., and Dang T. P.. 2010. Notch3 cooperates with the EGFR pathway to modulate apoptosis through the induction of bim. Oncogene 29:589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kolev, V. , Mandinova A., Guinea‐Viniegra J., Hu B., Lefort K., Lambertini C., et al. 2008. EGFR signaling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat. Cell Biol. 10:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Purow, B. W. , Sundaresan T. K., Burdick M. J., Kefas B. A., Comeau L. D., Hawkinson M. P., et al. 2008. Notch‐1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis 29:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Giannopoulou, E. , Nikolakopoulos A., Kotsirilou D., Lampropoulou A., Raftopoulou S., Papadimitriou E., et al. 2015. Epidermal growth factor receptor status and Notch inhibition in non‐small cell lung cancer cells. J. Biomed. Sci. 22:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Choi, K. , Ahn Y. H., Gibbons D. L., Tran H. T., Creighton C. J., Girard L., et al. 2009. Distinct biological roles for the Notch ligands Jagged‐1 and Jagged‐2. J. Biol. Chem. 284:17766–17774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Russo, A. , Franchina T., Ricciardi G. R., Picone A., Ferraro G., Zanghì M., et al. 2015. A decade of EGFR inhibition in EGFR‐mutated non‐ small cell lung cancer (NSCLC): old successes and future perspectives. Oncotarget 6:26814–26825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Purow, B. 2012. Notch inhibition as a promising new approach to cancer therapy. Adv. Exp. Med. Biol. 727:305–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luistro, L. , He W., Smith M., Packman K., Vilenchik M., Carvajal D., et al. 2009. Preclinical profile of a potent gamma‐secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res. 69:7672–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang, M. H. , Zang Y. S., Huang H., Chen K., Li B., Sun G. Y., et al. 2014. Arsenic trioxide exerts anti‐lung cancer activity by inhibiting angiogenesis. Curr. Cancer Drug Targets 14:557–566. [DOI] [PubMed] [Google Scholar]
- 45. Chonan, M. , Narita N., and Tominaga T.. 2016. Total regression of brain metastases in non‐small cell lung cancer patients harboring EGFR mutations treated with gefitinib without radiotherapy: two case reports. BMC Res. Notes 9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Protsenko, S. A. , and Rudakova A. V.. 2015. Gefitinib therapy in advanced non‐small cell lung cancer in patients with EGFR mutations: cost‐effectiveness analysis. Vopr. Onkol. 61:676–680. [PubMed] [Google Scholar]
- 47. Liu, L. L. , Li F., Pao W., and Michor F.. 2015. Dose‐dependent mutation rates determine optimum erlotinib dosing strategies for EGFR mutant non‐small cell lung cancer patients. PLoS ONE 10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bhardwaj, B. , Revannasiddaiah S., Bhardwaj H., Balusu S., and Shwaiki A.. 2016. Molecular targeted therapy to improve radiotherapeutic outcomes for non‐small cell lung carcinoma. Ann. Transl. Med. 4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hu, Y. , Dong X. Z., Liu X., Liu P., and Chen Y. B.. 2016. Enhanced antitumor activity of cetuximab in combination with the jak inhibitor CYT387 against non‐small‐cell lung cancer with various genotypes. Mol. Pharm. 13:689–697. [DOI] [PubMed] [Google Scholar]
- 50. Gold, K. A. , Byers L. A., Fan Y. H., Fujimoto J., Tse W. H., Jack Lee J., et al. 2013. A phase I/II trial combining erlotinib with gamma secretase inhibitor RO4929097 in advanced non‐small cell lung cancer (NSCLC). J. Clin. Oncol. 31(Suppl): abstr 8104. [Google Scholar]


