Synopsis
This article addresses the spectrum of atypia and dysplasia within the bladder epithelium and the diagnostic categories developed to further classify challenging lesions. In addition, the authors discuss the effects of inflammation, specific therapies, and instrumentation on the microscopic appearance of the bladder mucosa and the associated difficulty in achieving the appropriate diagnosis due to common findings seen in each of these clinical settings.
Keywords: Bladder, urothelial carcinoma, atypia, dysplasia, pathology
Introduction
The urothelial lining of the bladder presents diagnostic challenges, even under normal circumstances. A multitude of confounding factors such as inflammation, reparative changes, distension state, and treatment effects can hinder a diagnosis of early neoplasia. Thus, evaluation of biopsy and/or transurethral resection (TUR) specimens requires the pathologist to command a detailed understanding of the spectrum of histologic changes that can arise in the urinary bladder. Adding to the diagnostic challenge is the fact that available ancillary studies, such as immunohistochemical (IHC) stains, are suboptimal in the diagnosis of bladder neoplasia. In light of these difficulties, pathologists have developed a number of diagnostic categories to address the histologic “gray zones” in bladder pathology. Despite what progress has been made, these categories are relatively subjective and often do not correlate with outcomes.
II. What are atypia and dysplasia?
The definition of ‘atypia’ has been a contentious topic within the field of pathology, not restricted solely to the genitourinary subspecialty. The lack of consensus on one of the most commonly used terms in pathology can be attributed to a variety of causes, such as the subjective nature of histologic evaluation, institutional bias, prior diagnosis, and preference of the evaluating pathologist1. Furthermore, some pathologists have used the terms ‘atypia’ and ‘dysplasia’ interchangeably, which can introduce problems in clinical management. It is implied that atypia represents a benign process in many instances, while dysplasia describes a pre-neoplastic/neoplastic process1, although variation in defining these categories at the microscopic level often leads to confusion.
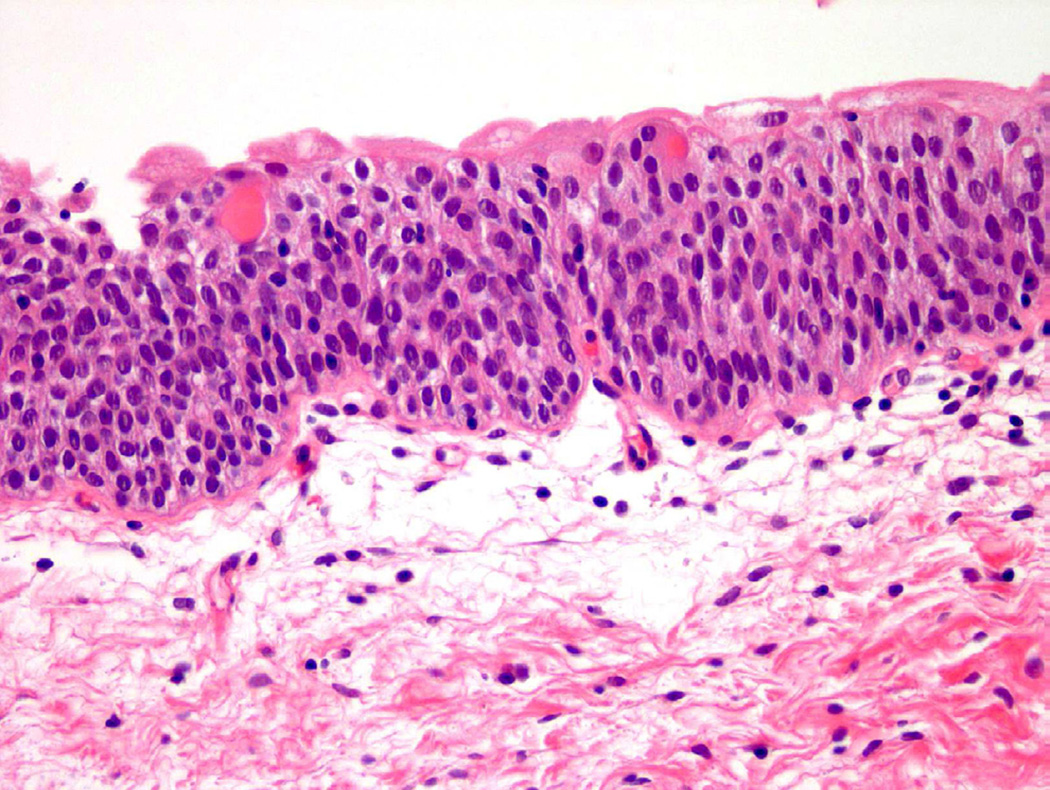
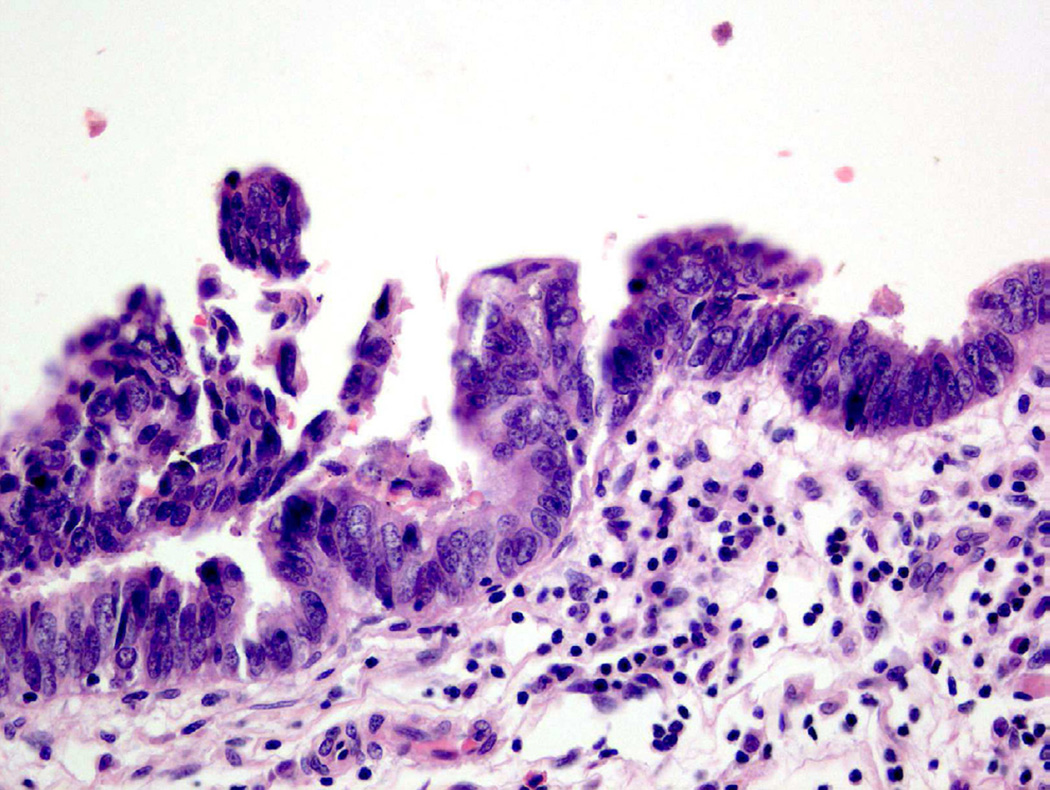
From a histologic perspective, “atypia” refers to a presence of one or more cellular or architectural features that deviate from that of an otherwise normal appearing cell or group of cells. Under normal conditions, urothelial cells contain oval nuclei, finely stippled chromatin, and minute to absent nucleoli, as well as ample cytoplasm and distinct cell membranes (Fig. 1)2. Even within the spectrum of “normal”, urothelial cells are permitted to have certain variations in cell size and cytoplasm, particularly in the most superficial layer of the urothelium (umbrella cell layer), which is in constant contact with the contents of the urinary space. These umbrella cells tend to be larger than cells in the intermediate and basal layers, with occasional binucleation and abundant eosinophilic cytoplasm. To further complicate matters for the pathologist, bladder distension can lead to flattening of superficial (or umbrella) cells to a point where the layer can be difficult to identify microscopically3.
Figure 1.
Normal urothelium.
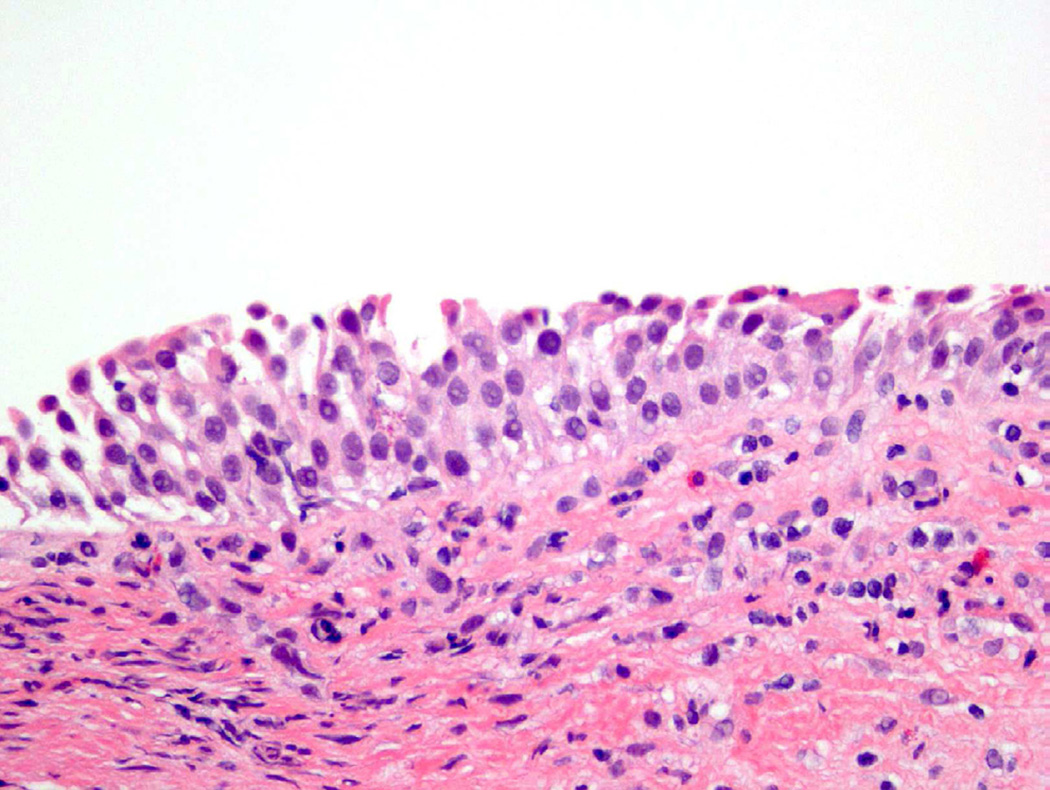
Despite the variety of appearances of a normal urothelial cell, certain specific cellular features are evaluated when determining the presence of atypia. Abnormal nuclear features, including increased size (nucleomegaly), deviation from typical ovoid shape, coarse chromatin, and irregular nuclear membrane contour, are all contributory factors in classifying a cell as atypical (Fig. 2)4. In addition, the identification of an enlarged, prominent nucleolus or multiple nucleoli should raise concern1. While eosinophilic (pink appearing) cytoplasmic features are acceptable in the superficial urothelium, these cytoplasmic changes in other layers, as well as loss of cytoplasmic clearing, are also considered atypical features4.
Figure 2.
Atypia demonstrates subtle changes from normal, which may include occasional hyperchromasia, disorganization, or increased nuclear/cellular variability.
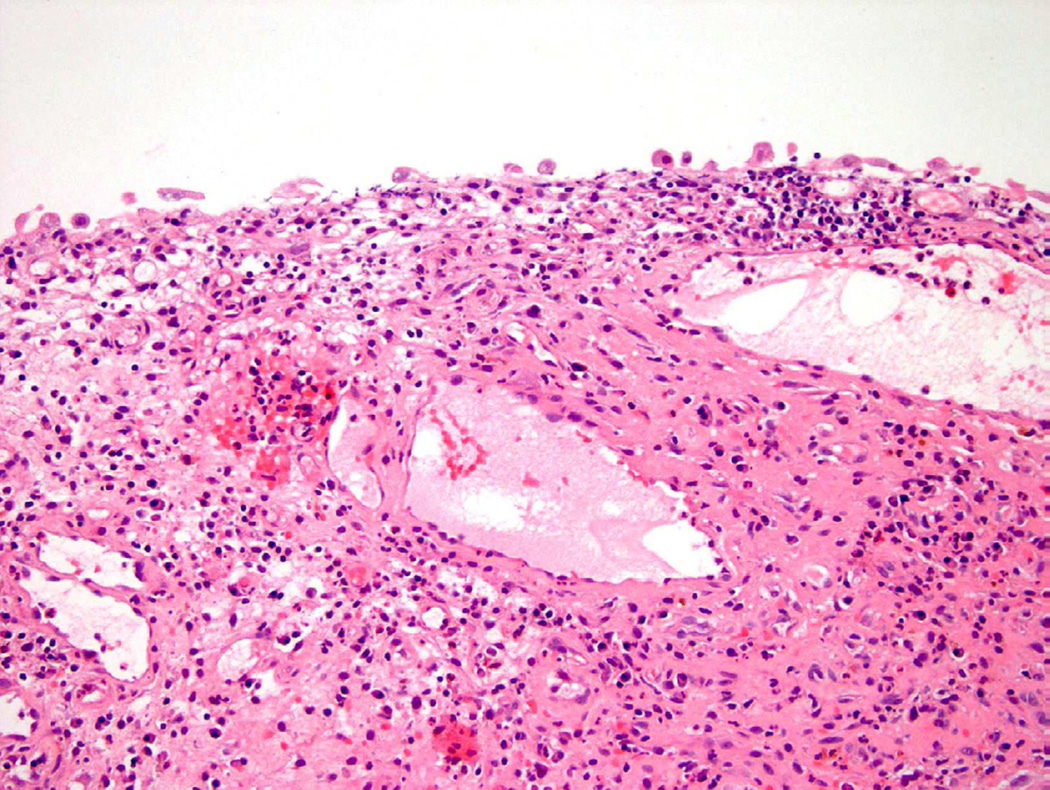
While atypia often refers specifically to cytologic (cellular) abnormalities, architectural changes can also guide the pathologist to the appropriate diagnosis. Denudation of the urothelium can be associated with previous instrumentation, inflammation, or a neoplastic process (Fig. 3)5. Conversely, thickening (hyperplasia) of the epithelium can result from a variety of causes ranging from a benign proliferation to a reactive process secondary to inflammation, as well as being simply an artifact of tangential sectioning6. The presence of atypical cells in the background of varied epithelial thickness can pose a difficult diagnostic challenge. Assessment of histologic changes throughout the entire specimen, detailed clinical history, and cystoscopic findings are all necessary to ensure the correct diagnosis is made. As such, close communication with the treating urologist is critical for the appropriate diagnosis and management of the patient7.
Figure 3.
Denudation may lead to a diagnosis of atypia.
III. Diagnostic categories of flat urothelial lesions
Reactive Urothelial Atypia
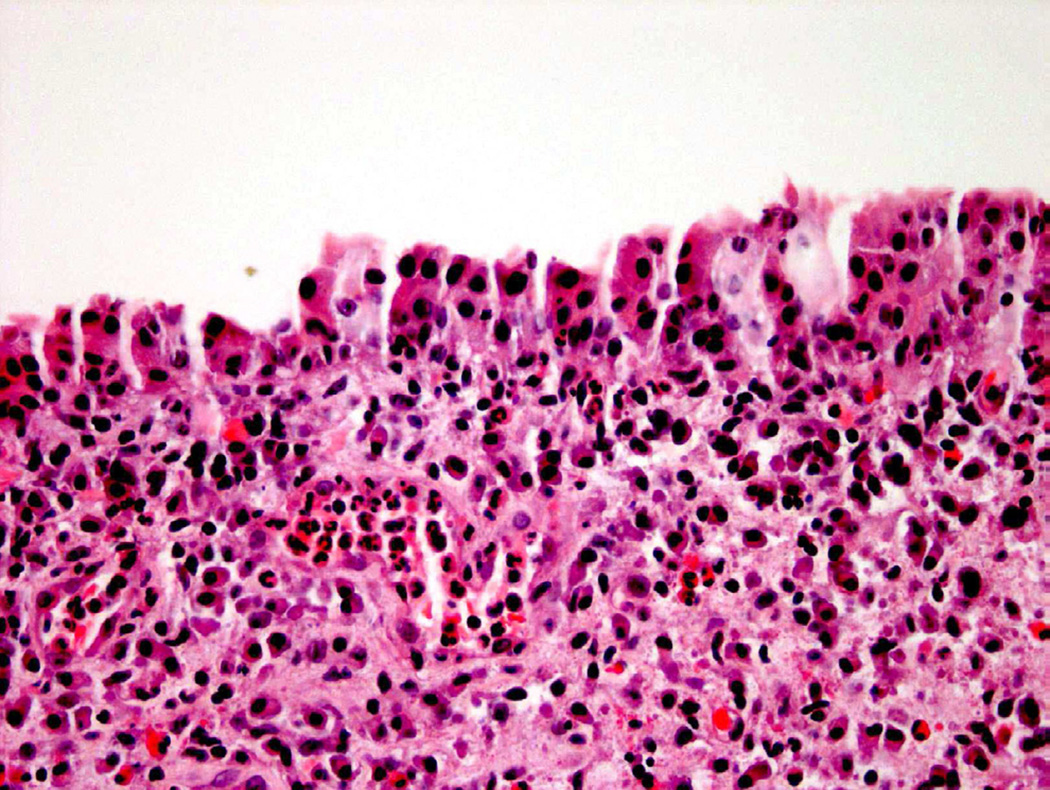
Reactive urothelial atypia remains one of the broadest categories used to describe abnormal-appearing urothelium, but is generally considered at diagnosis to represent a benign process5. Reactive atypia is most often diagnosed in the setting of an acute and/or chronic inflammatory process that may arise in the setting of past instrumentation, infection, prior treatment, and other clinical scenarios that incite inflammation. The cytologic and architectural abnormalities that arise in these contexts are generally mild and uniform4. Increased nuclear size, vesicular appearing chromatin, and pinpoint nucleoli are the most common features identified microscopically with reactive changes, which often occur in the presence of an inflammatory infiltrate within the urothelium (Fig. 4)1. A key distinction for reactive atypia is uniformity of findings throughout the specimen. Pleomorphism (variation in nuclear size), hyperchromasia, and nuclear crowding are features that should raise concern for a probable preneoplastic or overt neoplastic process8.
Figure 4.
Inflammation may complicate diagnostic accuracy, often leading to a diagnosis of atypia.
Atypia of Unknown Significance
‘Atypia of Unknown Significance’ is a term introduced by the International Society of Urologic Pathology consensus group in 1998 to classify histologic findings that meet various criteria for both reactive changes and dysplasia1. Typically, this diagnosis is used in the setting of reactive nuclear and cytoplasmic changes with mild degrees of pleomorphism and/or hyperchromasia that are concerning for dysplasia. Additionally, this term has been used to describe histologic findings where the level of atypia is disproportionally greater than expected for the amount of inflammation. There is ongoing debate on whether this term is even an appropriate diagnosis4. The classification of histologic findings as “atypia of unknown significance” warrants clinical follow-up, ideally in the setting of resolved inflammation. Despite the inclination to monitor these patients prospectively, there is no evidence in the literature that has shown that this category of patients is predisposed to neoplastic progression, although further studies are needed to better clarify this entity8.
Urothelial Dysplasia
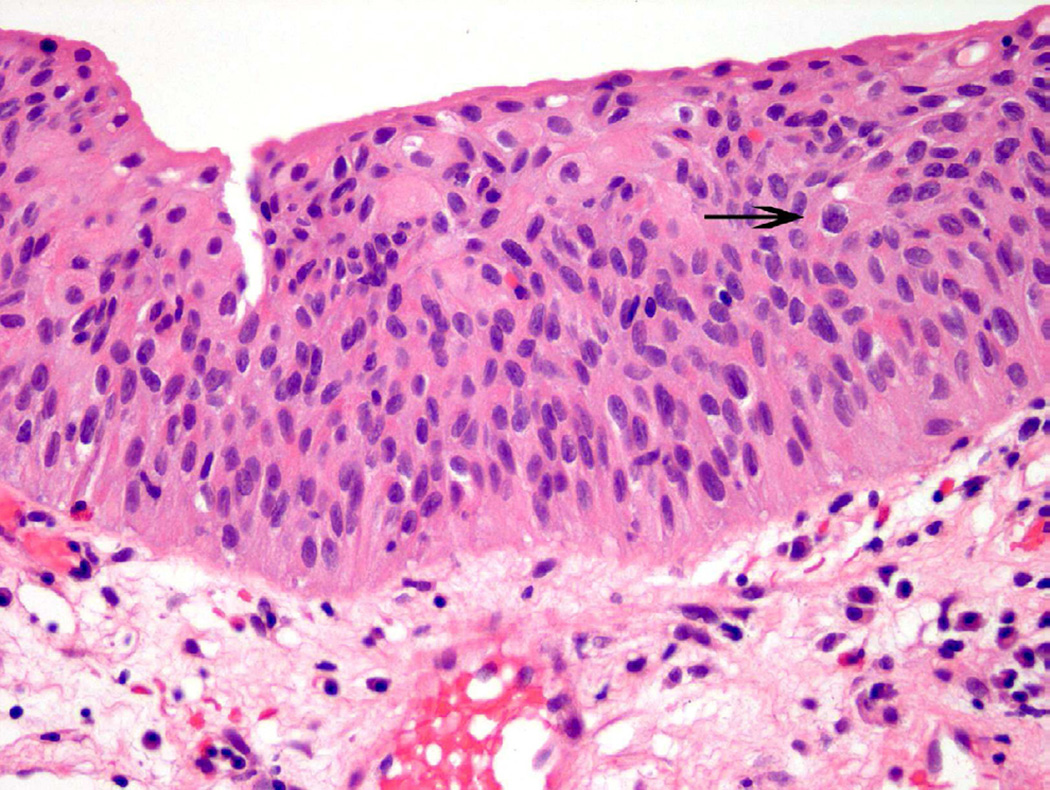
Urothelial dysplasia is considered to represent early pre-neoplastic/neoplastic change, with histologic features beyond that considered in the spectrum of benign or reactive urothelium9. In dysplasia, the thickness of the urothelium can be variable, but umbrella cells are usually present. Features of cellular crowding, multiple nucleoli, and mild hyperchromasia are common in this diagnostic category (Fig. 5)10. The diagnosis of ‘dysplasia’ is used when some, but not all, of the criteria necessary for urothelial carcinoma in situ (CIS) are met. Currently, further sub-classification of dysplasia into ‘mild’ or ‘moderate’ grade is no longer commonplace, whereas ‘severe dysplasia’ is generally considered CIS.
Figure 5.
Dysplasia is a subjective diagnosis. In this case, variable nuclear size, mild cellular disorganization and occasional abnormal nuclear contours (arrow) support the diagnosis.
Urothelial dysplasia can be further categorized into primary (de novo) and secondary dysplasia. Primary dysplasia is defined as dysplasia occurring in the absence of other histologic features of CIS and with no precedent history of urothelial neoplasia8. Since patients are usually asymptomatic and/or no gross lesion is visible on cystoscopy, true primary dysplasia is rarely seen by the pathologist. Secondary dysplasia is classified as dysplasia identified in the setting of known neoplasia of the bladder, either current or prior. In either presentation, the patient is currently undergoing screening to assess current symptoms or monitoring of a precedent high-grade lesion. While both primary and secondary dysplasia are classified as true premalignant lesions, secondary dysplasia is more likely to progress to carcinoma8.
Carcinoma in Situ
Urothelial carcinoma in situ (CIS) is a neoplastic diagnosis, with malignant cells restricted within the urothelial lining of the bladder (non-invasive disease). In addition to the features of dysplasia, these cells demonstrate marked nuclear and cellular pleomorphism and hyperchromasia (Fig. 6)9. CIS is commonly underdiagnosed when full-thickness cytologic atypia is absent or when umbrella cells are identified. Underdiagnosis of CIS may also occur in the setting of “clinging” CIS (single carcinoma cells are present in a background of otherwise denuded urothelium) and subtle involvement of von Brunn nests by CIS cells9.
Figure 6.
CIS typically shows overt neoplastic features.
IV. Inflammation as a contributor to a diagnosis of atypia/dysplasia
There are numerous inflammatory and infectious processes that incite reactive changes within the urinary bladder. Specifically, acute and chronic inflammation secondary to infectious, mechanical, or idiopathic causes can induce cytologic and architectural changes that can be interpreted by the pathologist within the spectrum of atypia. The following categories represent a sampling of some of the more common inflammatory processes that often lead to a diagnosis of atypia and/or dysplasia on bladder biopsy.
Acute and Chronic Cystitis
The most common cause of acute cystitis is infection, primarily but not restricted to, gram-negative bacteria11. Additional causes of acute cystitis include prior instrumentation, trauma, and catheterization. In the acute setting, inflammatory-mediated cell responses lead to an influx of neutrophils, lymphocytes, and macrophages within the urothelium and lamina propria of the bladder. Edema and vascular congestion are also common12. While reactive changes of the urothelium may not occur until the subacute or chronic inflammatory phase, pronounced inflammation can obscure underlying pathologic processes and make assessment extremely difficult for the pathologist, thus leading to a diagnosis of atypia. In the setting of chronic cystitis, a variety of changes can occur in the urothelium including denudation, ulceration, or hyperplasia. In addition to difficulties in adequately visualizing the specimen microscopically secondary to copious amounts of granulation tissue and/or fibrosis, reactive atypia due to a persistent inflammatory process can raise suspicion for dysplasia in the bladder8.
Polypoid Cystitis
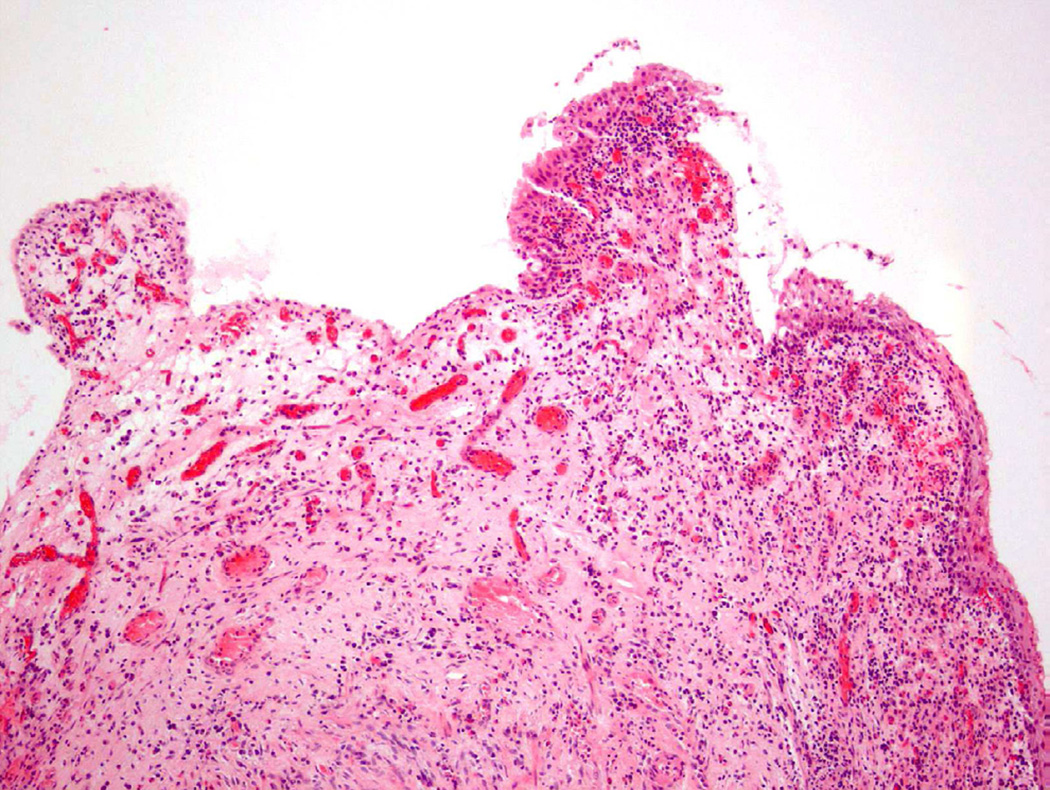
Polypoid cystitis, a common variant of cystitis in the bladder, remains a challenging inflammatory lesion for pathologists and urologists alike13 (fig 7). On cystoscopy, the often exophytic growth pattern of polypoid cystitis may clinically resemble a papillary neoplasm. These lesions tend to appear in patients with a history of indwelling catheters, fistulas, chronic obstruction, or calculi. Microscopically, the lesion consists of large, bulbous outpouchings of the urothelial lining, with the lamina propria often quite edematous (Fig. 8)13. Over time, ongoing inflammation may produce scarring of the lamina propria leading to a more papillary-like appearance, as well as induce squamous metaplasia and reactive atypia14. In a 2008 study, 41 cases of polypoid cystitis that had been previously misdiagnosed as papillary urothelial neoplasia were re-reviewed13. Despite some mild atypia and mitotic figures in many of the cases, the cytologic features included uniform cell enlargement, similar vesicular chromatin patterns, and a single prominent nucleolus. Each of the 41 cases lacked definitive hyperchromasia and nuclear pleomorphism which are key criteria in differentiating benign, reactive lesions from neoplasia.
Figure 7.
Polypoid cystitis.
Figure 8.
Flat urothelial hyperplasia recognized as thickened urothelium with no atypia.
Polyomavirus
BK and JC are double-stranded DNA viruses of the papovavirus family that can induce cytologic changes concerning for dysplasia15. These viruses live latently in the epithelium of the urinary tract and are asymptomatic. Use of systemic chemotherapeutic agents, primarily cyclophosphamide, as well as other clinical scenarios of immunodeficiency can lead to reactivation of these latent human polyoma viruses16. Atypical cellular features are usually first identified on urine cytology sampling where nuclear inclusions and/or chromatin irregularities are noted. Polyoma-virus cellular changes often do not appear on histologic examination of biopsy specimens due to the virus’ preference to infect the superficial cell layer, which can be lost in processing15. Efforts to use analysis of DNA content to differentiate virally-infected cells from true neoplastic cells have historically been unsuccessful due to aneuploidy17. Correlation with clinical history, knowledge of the patient’s immune status, and familiarity with common nuclear changes associated with re-activated viral infections are all crucial to avoid overdiagnosis of malignancy in this patient population16.
V. Hyperplasia as a contributor to a diagnosis of atypia/dysplasia
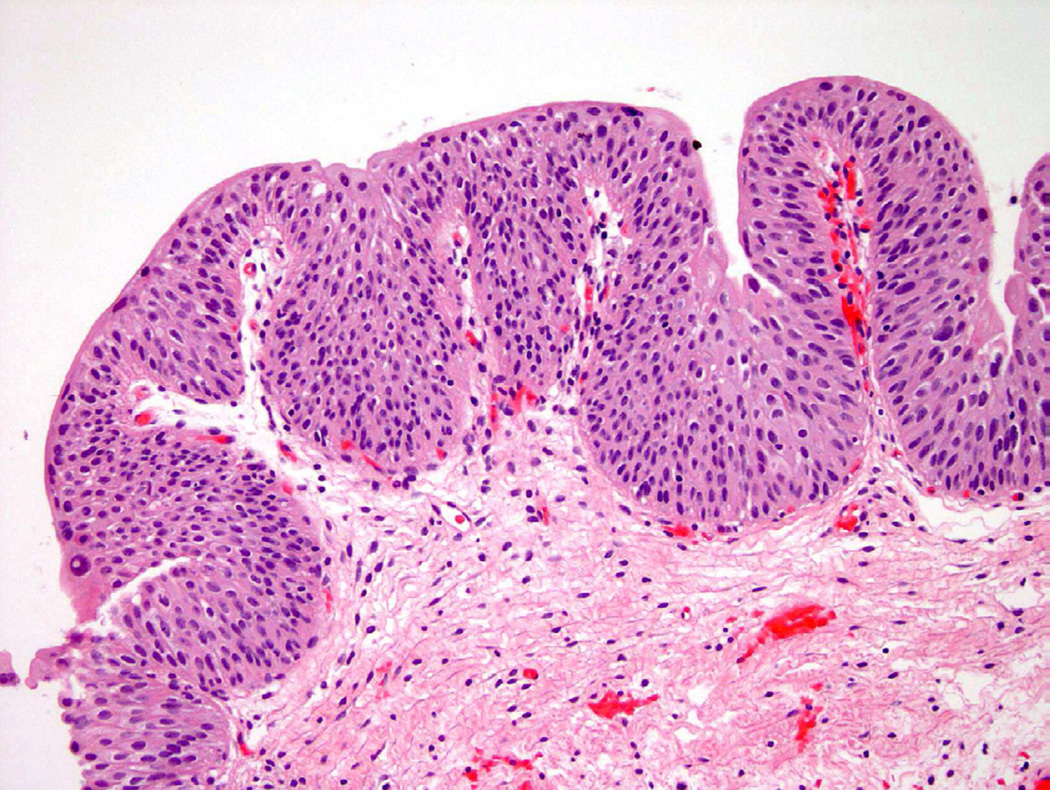
The two most common types of hyperplasia identified in the urinary bladder are simple (flat) and papillary. Flat hyperplasia is defined as thickened, but cytologically normal urothelium, usually greater than 7 cell layers in thickness (Fig. 8)1. Flat hyperplasia has occasionally been associated with the presence of mild atypia, but the evaluating pathologist should have a low threshold for addressing this in his or her diagnosis. Difficulty in definitively determining the presence of flat hyperplasia may be secondary to tangential sectioning of the tissue during processing6. Flat urothelial hyperplasia usually occurs in the clinical setting of inflammation or lithiasis (bladder stones), but has also been identified adjacent to papillary-type neoplasms9.
Papillary urothelial hyperplasia appears histologically as undulating epithelial thickening devoid of significant cellular atypia (Fig. 9). These papillary fronds may contain small, dilated vessels that appear as rudimentary vascular cores, but lack the branching or more complex architecture that would place this into a papillary neoplastic category18. Similar to flat hyperplasia, mild cytologic changes in the background of inflammation and instrumentation are permitted. Any indication that the atypical features are disproportional given the clinical setting, should guide the pathologist to consider a pre-neoplastic process and thus a diagnosis of “papillary hyperplasia with dysplasia”19. Taylor et al. examined 16 cases of papillary hyperplasia and were unable to definitively determine if papillary hyperplasia, with no evidence of atypia, would progress to neoplasia without intervention. Furthermore, the authors postulated that diagnosing papillary hyperplasia in a patient with a history of urothelial neoplasia likely represented a recurrence18. Conversely, papillary hyperplasia with atypical features, dysplasia, or focal CIS may predispose the patient to increased risk for disease progression19.
Figure 9.
Papillary hyperplasia shows upward tenting of vessels, suggesting a possible early papillary lesion in some instances.
VI. Metaplastic processes and their relationship to bladder atypia and neoplasia
Metaplasia is defined as a change (often reversible) from one differentiated cell type to another20. In the case of the urothelium, metaplasia often occurs in the setting of an inflammatory process or following instrumentation. While metaplasia, by definition, is not a neoplastic process, atypia in these metaplastic cells can raise concern for less common malignancies of the bladder, such as adenocarcinoma or squamous cell carcinoma.
Squamous metaplasia
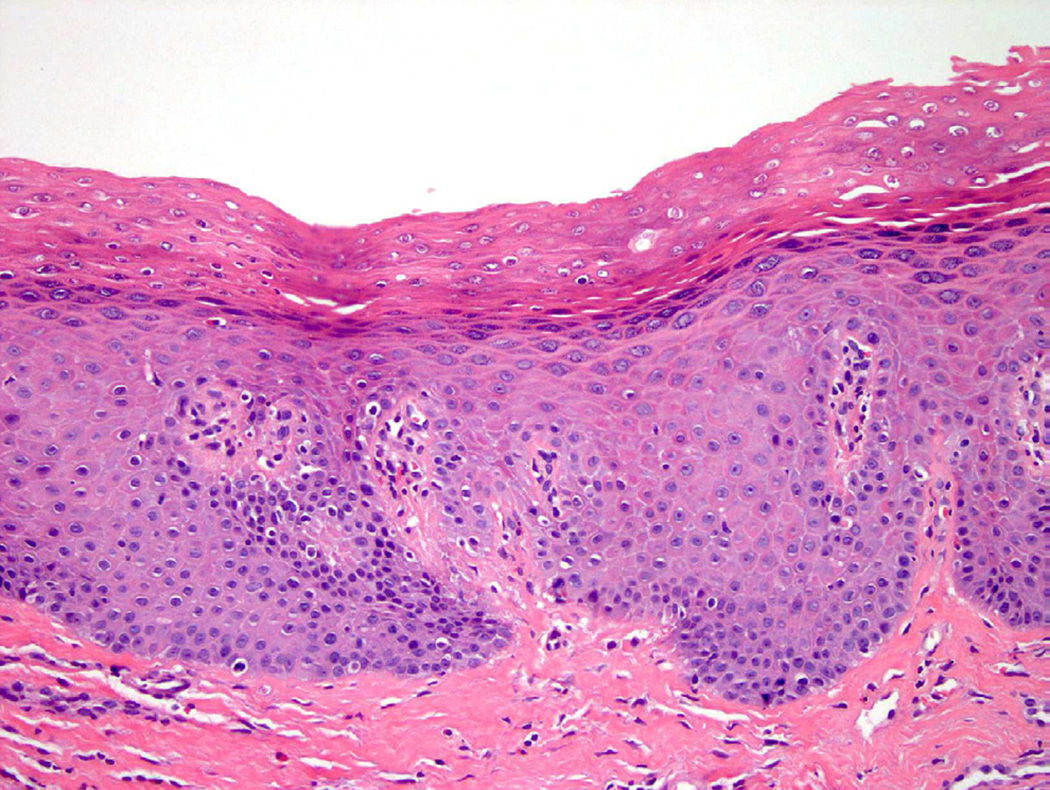
Squamous metaplasia of the bladder involves replacement of urothelium by squamous epithelium and may be either non-keratinizing or keratinizing. In some instances, non-keratinizing squamous metaplasia is normal, such as in the trigone of the bladder in women21. Chronic inflammatory processes, often seen in patients with spinal cord injury or persistent in-dwelling catheters, may increase the likelihood of developing squamous metaplasia, which can present as leukoplakia on cystoscopy. Although keratinizing squamous metaplasia is traditionally associated with cystitis, bladder stones, and schistosomiasis, it may also be associated with concurrent or subsequent malignancy and thus should be regarded with higher suspicion and additional clinical followup (Fig. 10)22.
Figure 10.
Keratinizing squamous metaplasia.
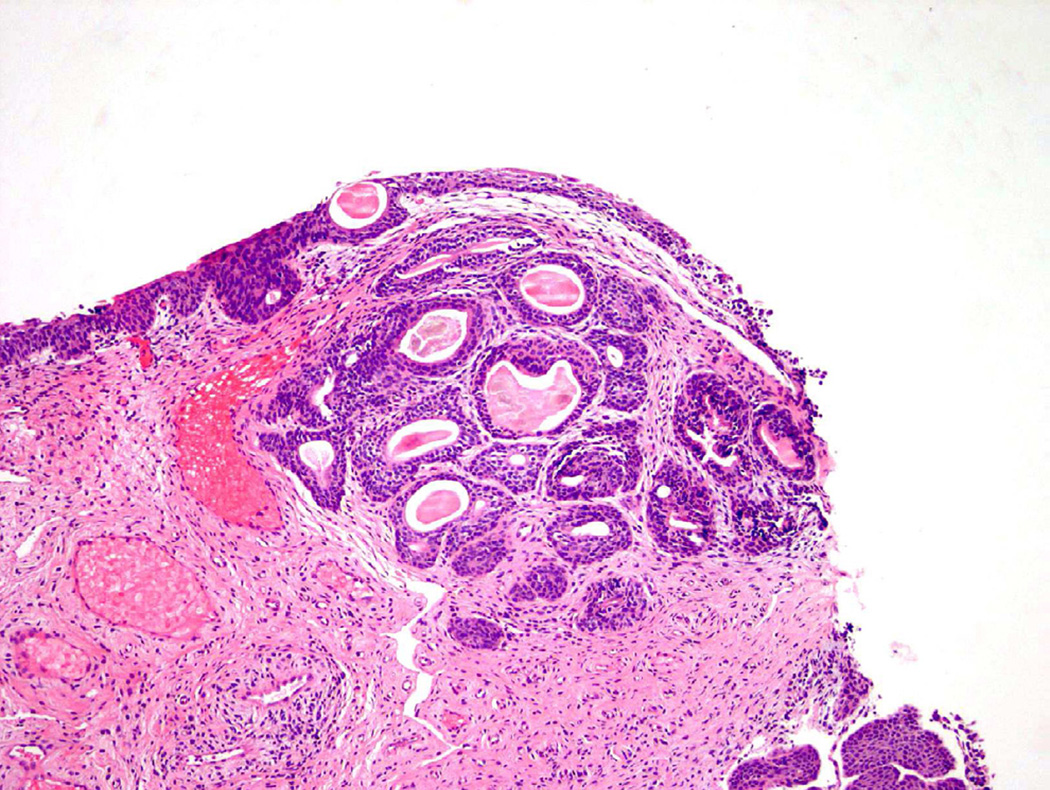
Cystitis cystica et glandularis
Cystitis cystica et glandularis (CCEG) is a benign process defined as cystic changes within von Brunn nests (cystitis cystica) that develop metaplastic changes of the cellular lining to columnar or cuboidal epithelium (Fig. 11)23. Similar to other metaplastic processes, CCEG is attributed to chronic inflammation/irritation and favors, but is not restricted to, the trigone of the bladder. CCEG is further subdivided into usual and intestinal types, although in practice this sub-categorization is often not frequently used.
Figure 11.
Cystitis cystica et glandularis.
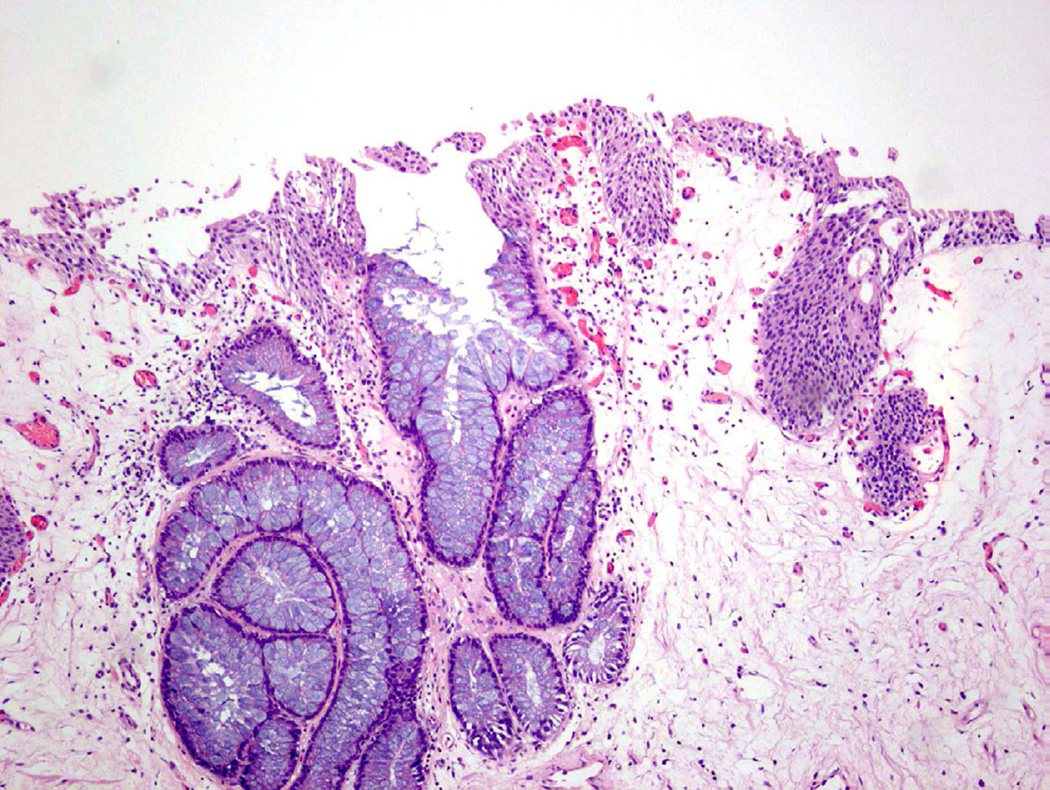
Intestinal metaplasia
Intestinal metaplasia (IM) is a proliferation of epithelium in the urinary bladder characterized by gland-like structures lined with mucin-secreting cells (Fig. 12)24. These mucin-secreting cells resemble goblet cells, which are found in the colonic mucosa of the lower gastrointestinal tract23. In a study by Corica et al., 53 cases of IM in the bladder were evaluated with long-term follow up (mean >12 years among the two case groups24). The authors determined that the presence of IM was not a strong risk factor for the development of overt malignancy24. Smith et al. evaluated 19 cases of IM and found no evidence carcinoma at follow-up (mean 4.4 years23). Additionally, while 37% of the cases with IM had co-existing carcinoma, the authors felt that the association between the two processes did not imply that IM was the precursor lesion23. One study has suggested that pure adenocarcinomas of the urinary bladder (as opposed to urothelial carcinoma with glandular differentiation) are derived from IM, but attempts to definitively associate IM as pre-malignant has yet to be proven25. Extensive IM has been suggested to predispose patients to a greater risk for developing adenocarcinoma, but this frequently cited association is based on a single study26 and no additional evidence has been identified in the current literature.
Figure 12.
Intestinal metaplasia showing mucin-containing goblet cells.
Glandular metaplasia with dysplasia
Glandular metaplasia (sometimes used interchangeably with intestinal metaplasia24) has been associated with cytologic atypia in rare cases and can be further divided into glandular metaplasia with low/high grade dysplasia27. Low-grade dysplasia shares morphologic features in common with tubular adenomas of the colon, including columnar nuclei that maintain polarity and uniformity. In contrast, adenocarcinoma in situ (glandular metaplasia with high-grade dysplasia) demonstrates overt atypia, including nuclear stratification, atypical mitoses and nuclear pleomorphism (Fig. 13)28.
Figure 13.
Adenocarcinoma in situ (glandular metaplasia with high-grade dysplasia) shows overt neoplastic alterations.
Villous adenomas of the urinary tract (also referred to as glandular metaplasia with an exophytic growth pattern8) histologically appear identical to colonic villous adenomas and consist of pseudostratified columnar mucosa with mucin-secreting cells and epithelial projections of variable size. Villous adenomas in the bladder have been associated with invasive adenocarcinoma, squamous cell carcinoma, and urothelial carcinoma in situ29. These lesions are exceedingly rare, but when identified without concurrent carcinoma appear to have an excellent prognosis for the patient30. As with villous adenomas of the colon, extensive sampling is imperative to definitively rule out stromal invasion or focal adenocarcinoma in situ8.
VII. Treatment effect as a cause of atypia and dysplasia
Non-surgical intervention for bladder and/or prostate neoplasia involves a variety of treatments including systemic chemotherapy, intravesicular therapy, and radiotherapy. Each treatment modality poses its own challenge to the pathologist secondary to the inflammation and atypia associated with each intervention. Accurate determination of the cytologic and morphologic changes secondary to treatment effect requires a detailed understanding of what effects should be expected with individual therapies. Although many therapies may induce numerous reactive changes that may be interpreted as atypia, some – like radiation cystitis – may actually be a risk factor for the development of bladder neoplasia as well.
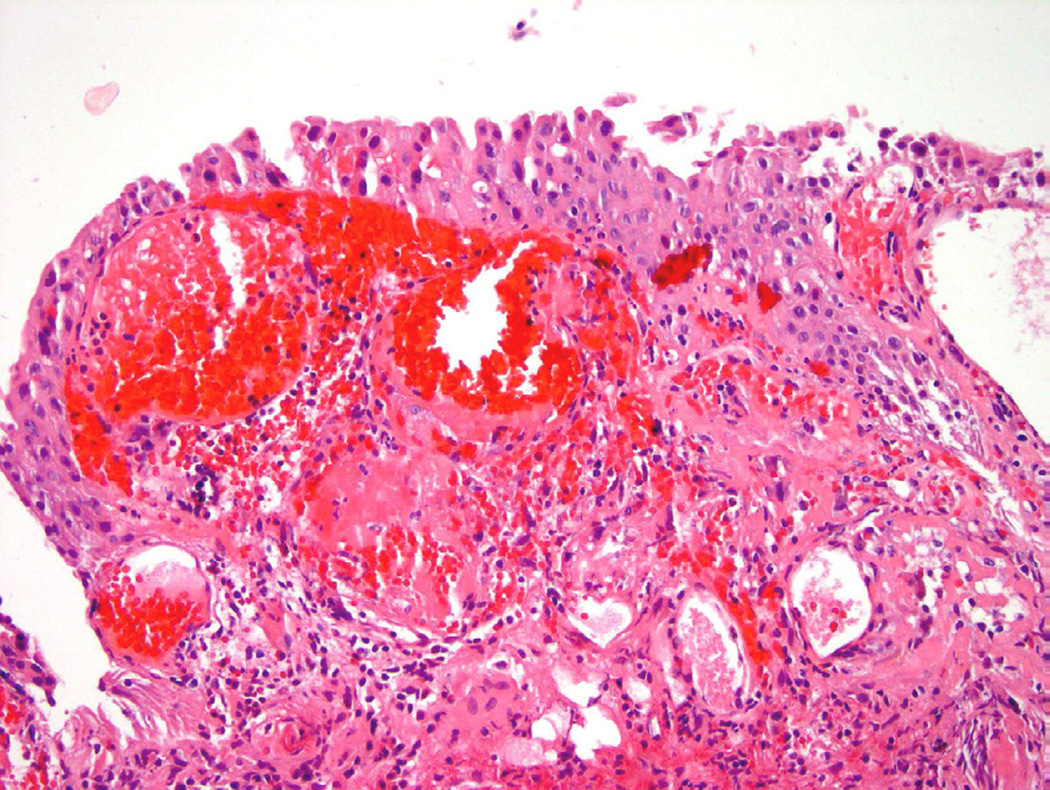
Radiation therapy
Approximately one-fourth of all patients with muscle invasive bladder carcinoma will undergo some form of radiotherapy31. In addition, many patients diagnosed with prostate cancer will also undergo various forms of radiation therapy that can ultimately affect the bladder lining based on its proximity. On cystoscopy, edema, ulceration, and erythema are common findings in addition to areas of mucosal hemorrhage. Histologically, the urothelium shows “radiation atypia”, which includes cellular enlargement, multinucleation, vacuolization, and chromatin clearing (Fig. 14)31. In addition to inflammation and cytologic atypia identified in the epithelium, vascular changes secondary to the radiation include edema, vascular proliferation, vessel wall hyalinization, and thrombosis. Ischemic changes caused by exposure to radiation can lead to long-term sequelae such as mucosal denudation, contractures, and fistula formation32.
Figure 14.
Radiation atypia with a background of inflammation, nuclear atypia and edema.
Chemotherapy
Chemotherapeutic regimens in the treatment of bladder cancer consist of intravesical and/or systemic agents, many of which can incite atypia of the urothelium. Two of the most common intravesical agents are triethylenethiophosphoramide (Thiotepa) and mitomycin C. Thiotepa (an alkalating agent) and mitomycin C (an antibiotic with antitumor properties) both produce similar cytologic and architectural changes to the urothelium, including denudation and atypia of the umbrella cells with features of increased size, multinucleation and vacuolization31. Additional forms of cellular atypia that occur following treatment with these two agents include degenerative changes and membrane alterations that may incite a diagnosis of atypia. Other intravesicular topical agents, such as doxorubicin and epirubicin, have been implicated in causing cystitis and similar cytologic changes, but details of these changes have not been studied thoroughly31.
In addition to intravesicular chemotherapy with the oxazophoshporine class of alkylating agents, systemic chemotherapy with cyclophosphamide has been identified as a cause of bladder urothelial atypia33,34. Often used in the therapy of autoimmune conditions, such as systemic lupus erythematous and rheumatoid arthritis, and lymphoproliferative disorders, this alkylating agent undergoes metabolism to produce acrolein and phosphoramide mustard, which are excreted in the urine and are exposed to the superficial bladder mucosa35. Clinically, patients can present with urinary symptoms and hematuria, which may prompt the clinician for cystoscopic evaluation and biopsy. Grossly, the mucosal surface can appear erythematous, edematous, and possibly hemorrhagic. Microscopically, large, multinucleated cells are seen, caused by the arrest of cell division secondary to exposure to the active metabolites of cyclophosphamide. Nuclei are variably enlarged with irregular membranes, coarse chromatin, and irregularly sized and shaped nucleoli31. These effects are similar to changes induced by radiation therapy. Cyclophosphamide has also been associated with hemorrhagic cystitis36.
From the pathologist’s perspective, one of the greatest difficulties in the setting of chemotherapeutic treatment is determining whether atypia is the result of therapy or whether the atypia represents early neoplastic changes secondary to new or recurrent disease. Several studies have determined that cyclophosphamide therapy is associated with up to a nine-fold increase in the development of bladder carcinoma37. Furthermore, chemotherapeutic agents have been associated with benign epithelial proliferations which can mimic invasive carcinoma due to an infiltrative growth pattern into the lamina propria of the bladder mucosa38. These “pseudocarcinomatous” proliferations cause several challenges for the pathologist due to the elevated risk for developing cancer in patients treated with chemotherapy and the limited sampling on biopsy38.
Bacillus Calmette-Guérin (BCG) therapy
Bacillus Calmette-Guérin (BCG) therapy is the most common intravesicular immunotherapy agent used worldwide for the treatment of superficial and non-invasive high-grade bladder neoplasia39. BCG is a pleiotropic immune stimulator that can limit tumor progression by recruitment of an anti-tumoral immune response28. Microscopically, BCG therapy most notably causes acute and chronic inflammation associated with non-caseating granulomas, but may also induce mucosal ulceration, denudation and reactive urothelial atypia40.
Photodynamic therapy
Photodynamic therapy is a newer, less common modality being used in treatment of bladder neoplasia41. Photodynamic treatment is administered either systemically or intravesicularly, subsequently inducing necrosis of tumor cells when exposed to a light source42. In addition to inducing coagulative necrosis and hemorrhage, adjacent benign mucosa may be affected by the treatment. Cytologic atypia is not traditionally associated with this therapy, and should raise suspicion if identified on microscopy examination28.
Ketamine cystitis
An exceedingly rare, but notable, mimicker of CIS is ketamine cystitis, which can occur in the setting of chronic exposure to this drug. These patients will typically report clinical symptoms of dysuria, urgency, hematuria, and incontinence. On cystoscopy, a small capacity, erythematous bladder is seen. Microscopically, ulceration and acute inflammation are the most common findings. A 2009 study from Oxley et al. looked at 17 patients with reported ketamine cystitis, 10 of which were described as having significant urothelial atypia on histologic examination concerning for CIS. The authors reported that despite nuclear enlargement and disorganization of cells seen on biopsy, the changes identified were likely reactive and did not represent a preneoplastic process43.
VIII. Immunohistochemistry as an ancillary tool
Though extensively studied, the use of immunohistochemistry (IHC) in the diagnosis of urothelial neoplasia has remained somewhat challenging. As previously discussed, discerning reactive atypia from dysplasia and CIS remains one of the greatest topics of contention in urologic pathology. Several studies have examined the utility of IHC in non-invasive lesions of urothelium, particularly atypia and CIS, to determine what role, if any, they may serve in reaching the appropriate diagnosis.
Cytokeratin 20, CD44, and p53
Several studies have been done to determine which immunohistochemical markers could be useful to the pathologist to differentiate reactive atypia from dysplasia. In 2001, one study evaluated 25 cases of non-neoplastic urothelium (15 cases of reactive atypia and 10 cases of normal urothelium) using three common IHC stains: cytokeratin 20 (CK20), CD44, and p5344. CK20 stains cytoskeletal intermediate filaments found in gastrointestinal mucosa, urothelium and Merkel cells and is generally restricted to the umbrella cell layer in normal urothelium. p53 is a tumor suppressor gene implicated in many malignancies, including bladder carcinoma45; under normal conditions, p53 demonstrates a weak cytoplasmic signal in the urothelium. Finally, CD44 is an immunomarker previously studied as a possible prognostic factor in papillary urothelial neoplasms and has been found under normal conditions to reside in the basal layers of the urothelium45. A combinatorial approach has been used with these three IHC markers to aid in the distinction between normal urothelium with atypia and CIS. Specifically, intense nuclear p53 expression (that correlates with p53 mutation), coupled with full thickness CK20 expression and loss of CD44 favors CIS44, 46. Although this overall finding in larger studies appears promising, use of these markers routinely on individual cases can often be problematic, with variability in intensity and distribution of markers common.
IX. Conclusion
Evaluation of the bladder urothelium is a diagnostically challenging process that involves not only subtleties in morphologic evaluation, but an awareness of the patient history to elucidate sources of atypia unrelated to neoplastic transformation. Common challenges in distinguishing the categories of reactive atypia from atypia of uncertain significance and dysplasia include the presence of inflammation, therapy, and prior instrumentation. Despite improved investigation into the utility of IHC markers, microscopic assessment remains the definitive approach to achieving the most accurate urothelial diagnosis possible.
Key Points.
Atypia and dysplasia are terms used to describe cellular abnormalities in the spectrum of reactive changes to neoplasia.
Acute and chronic inflammation secondary to infectious, mechanical, or idiopathic causes can induce cytologic and architectural changes that can be interpreted by the pathologist within the spectrum of atypia.
Treatments including systemic chemotherapy, intravesicular therapy, and radiotherapy incite specific, often common, cellular changes that can microscopically be concerning for a neoplastic process.
Despite improved investigation, the utility of immunohistochemistry as an ancillary tool in distinguishing benign processes from urothelial neoplasia remains unclear
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998 Dec;22(12):1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Epstein JI, Amin MB, Reuter VE. Biopsy Interpretation of the Bladder. Philadelphia: Lippincott Williams & Wilkins; 2010. Normal bladder anatomy and variants of normal histology; pp. 1–13. [Google Scholar]
- 3.Reuter VE. Histology for Pathologists 3ed. Philadelphia: Lippincott Williams & Wilkins; 2010. Urinary bladder, ureter, and renal pelvis; pp. 910–922. [Google Scholar]
- 4.Banerjee Suman K, MD, PhD, Lopez-Beltran Antonio., MD Urothelial Dysplasia and Its Mimics. Pathology Case Reviews. 2008 Jul-Aug;13(4):149–153. [Google Scholar]
- 5.Amin MB, McKenney JK, Paner GP, Hansel DE, Grignon DJ, Montironi R, Lin O, Jorda M, Jenkins LC, Soloway M, Epstein JI, Reuter VE. ICUD-EAU International Consultation on Bladder Cancer 2012: Pathology. Eur Urol. 2012 Oct 5; doi: 10.1016/j.eururo.2012.09.063. [DOI] [PubMed] [Google Scholar]
- 6.Koss LG. Tumors of the Urinary Bladder, Fasicle 11. Washington, DC: Armed Forces Institute of Pathology; 1975. [Google Scholar]
- 7.Gorin MA, Ayyathurai R, Soloway MS. Diagnosis and treatment of bladder cancer: how can we improve? Postgrad Med. 2012 May;124(3):28–36. doi: 10.3810/pgm.2012.05.2545. [DOI] [PubMed] [Google Scholar]
- 8.Sesterhenn I, van det Kwast KT, Mazerolles C. Preneoplastic non-papillary lesions and conditions of the urinary bladder: an update based on the Ancona International Consultation. Virchows Arch. 2002 Jan;440(1):3–11. doi: 10.1007/s00428-001-0577-6. [DOI] [PubMed] [Google Scholar]
- 9.Sauter G, Algaba F, Amin MB, Busch C. World Health Organization Classification of Tumours Pathology and Genetics: Tumours of the Urinary System and Male Genital Organs. In: Epstein JI, Eble JN, Sesterhenn I, Sauter G, editors. Tumours of the urinary system. Lyon: IARC Press; 2004. pp. 89–157. [Google Scholar]
- 10.Cheng L, Cheville JC, Neumann RM, Bostwick DG. Natural history of urothelial dysplasia of the bladder. Am J Surg Pathol. 1999 Apr;23(4):443–447. doi: 10.1097/00000478-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Filiatrault L, McKay RM, Patrick DM, Roscoe DL, Quan G, Brubacher J, Collins KM. Antibiotic resistance in isolates recovered from women with community-acquired urinary tract infections presenting to a tertiary care emergency department. CJEM. 2012 Sep;14(5):295–305. doi: 10.2310/8000.2012.120666. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Amin MB, Reuter VE. Biopsy Interpretation of the Bladder. Philadelphia: Lippincott Williams & Wilkins; 2010. Cystitis; pp. 226–259. [Google Scholar]
- 13.Lane Z, Epstein JI. Polypoid/papillary cystitis: a series of 41 cases misdiagnosed as papillary urothelial neoplasia. Am J Surg Pathol. 2008 May;32(5):758–764. doi: 10.1097/PAS.0b013e31816092b5. [DOI] [PubMed] [Google Scholar]
- 14.Young RH. Papillary and polypoid cystitis. A report of eight cases. Am J Surg Pathol. 1988 Jul;12(7):542–546. doi: 10.1097/00000478-198807000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Herawi M, Parwani AV, Chan T, Ali SZ, Epstein JI. Polyoma virus-associated cellular changes in the urine and bladder biopsy samples: a cytohistologic correlation. Am J Surg Pathol. 2006 Mar;30(3):345–350. doi: 10.1097/01.pas.0000179117.38787.57. [DOI] [PubMed] [Google Scholar]
- 16.Wojcik EM, Miller MC, Wright BC, Veltri RW, O'Dowd GJ. Comparative analysis of DNA content in polyomavirus-infected urothelial cells, urothelial dysplasia and high grade transitional cell carcinoma. Anal Quant Cytol Histol. 1997 Oct;19(5):430–436. [PubMed] [Google Scholar]
- 17.Koss LG, Sherman AB, Eppich E. Image analysis and DNA content of urothelial cells infected with human polyomavirus. Anal Quant Cytol. 1984 Jun;6(2):89–94. [PubMed] [Google Scholar]
- 18.Taylor DC, Bhagavan BS, Larsen MP, Cox JA, Epstein JI. Papillary urothelial hyperplasia. A precursor to papillary neoplasms. Am J Surg Pathol. 1996 Dec;20(12):1481–1488. doi: 10.1097/00000478-199612000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Swierczynski SL, Epstein JI. Prognostic significance of atypical papillary urothelial hyperplasia. Hum Pathol. 2002 May;33(5):512–517. doi: 10.1053/hupa.2002.124031. [DOI] [PubMed] [Google Scholar]
- 20.Abbas AK, Aster JC, Fausto N, Kumar V. Kumar: Robbins and Cotran Pathologic Basis of Disease, Professional Edition. 8th ed. Philadelphia: Saunders; 2009. Cellular Responses to Stress and Toxic Insults: Adaptation, Injury, and Death. [Google Scholar]
- 21.Epstein JI, Amin MB, Reuter VE. Biopsy Interpretation of the Bladder. Philadelphia: Lippincott Williams & Wilkins; 2010. Flat urothelial lesions; pp. 14–42. [Google Scholar]
- 22.Lagwinski N, Thomas A, Stephenson AJ, Campbell S, Hoschar AP, El-Gabry E, Dreicer R, Hansel DE. Squamous cell carcinoma of the bladder: a clinicopathologic analysis of 45 cases. Am J Surg Pathol. 2007 Dec;31(12):1777–1787. doi: 10.1097/PAS.0b013e31805c9cd9. [DOI] [PubMed] [Google Scholar]
- 23.Smith AK, Hansel DE, Jones JS. Role of cystitis cystica et glandularis and intestinal metaplasia in development of bladder carcinoma. Urology. 2008 May;71(5):915–918. doi: 10.1016/j.urology.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 24.Corica FA, Husmann DA, Churchill BM, Young RH, Pacelli A, Lopez-Beltran A, Bostwick DG. Intestinal metaplasia is not a strong risk factor for bladder cancer: study of 53 cases with long-term follow-up. Urology. 1997 Sep;50(3):427–431. doi: 10.1016/S0090-4295(97)00294-X. [DOI] [PubMed] [Google Scholar]
- 25.Behzatoğlu K. Malignant glandular lesions and glandular differentiation in invasive/noninvasive urothelial carcinoma of the urinary bladder. Ann Diagn Pathol. 2011 Dec;15(6):422–426. doi: 10.1016/j.anndiagpath.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Elem B, Alam SZ. Total intestinal metaplasia with focal adenocarcinoma in a Schistosoma-infested defunctioned urinary bladder. Br J Urol. 1984 Jun;56(3):331–333. doi: 10.1111/j.1464-410x.1984.tb05401.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan TY, Epstein JI. In situ adenocarcinoma of the bladder. Am J Surg Pathol. 2001 Jul;25(7):892–899. doi: 10.1097/00000478-200107000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Montironi R, Lopez-Beltran A, Scarpelli M, Mazzucchelli R, Cheng L. Morphological classification and definition of benign, preneoplastic and non-invasive neoplastic lesions of the urinary bladder. Histopathology. 2008 Dec;53(6):621–633. doi: 10.1111/j.1365-2559.2008.03025.x. [DOI] [PubMed] [Google Scholar]
- 29.Seibel JL, Prasad S, Weiss RE, Bancila E, Epstein JI. Villous adenoma of the urinary tract: a lesion frequently associated with malignancy. Hum Pathol. 2002 Feb;33(2):236–241. doi: 10.1053/hupa.2002.31293. [DOI] [PubMed] [Google Scholar]
- 30.Cheng L, Montironi R, Bostwick DG. Villous adenoma of the urinary tract: a report of 23 cases, including 8 with coexistent adenocarcinoma. Am J Surg Pathol. 1999 Jul;23(7):764–771. doi: 10.1097/00000478-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Beltran A, Luque RJ, Mazzucchelli R, Scarpelli M, Montironi R. Changes produced in the urothelium by traditional and newer therapeutic procedures for bladder cancer. J Clin Pathol. 2002 Sep;55(9):641–647. doi: 10.1136/jcp.55.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pavlidakey PG, MacLennan GT. Radiation cystitis. J Urol. 2009 Sep;182(3):1172–1173. doi: 10.1016/j.juro.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 33.Siddiqui A, Melamed MR, Abbi R, et al. Mucinous (colloid) carcinoma of urinary bladder following long-term cyclophosphamide for Waldenstrom macroglobulinemia. Am J Surg Pathol. 1996;20:500–504. doi: 10.1097/00000478-199604000-00015. [DOI] [PubMed] [Google Scholar]
- 34.Wall RL, Clausen KP. Carcinoma of the urinary bladder in patients receiving cyclophosphamide. N Engl J Med. 1975;293:271–273. doi: 10.1056/NEJM197508072930604. [DOI] [PubMed] [Google Scholar]
- 35.Plotz PH, Klippel JH, Decker JL, Grauman D, Wolff B, Brown BC, Rutt G. Bladder complications in patients receiving cyclophosphamide for systemic lupus erythematosus or rheumatoid arthritis. Ann Intern Med. 1979 Aug;91(2):221–223. doi: 10.7326/0003-4819-91-2-221. [DOI] [PubMed] [Google Scholar]
- 36.Lawrence HJ, Simone J, Aur RJA. Cyclophosphamide-induced hemorrhagic cystitis in children with leukemia. Cancer. 1975;36:1572–1576. doi: 10.1002/1097-0142(197511)36:5<1572::aid-cncr2820360506>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Fairchild WV, Spence CR, Solomon HD, Gangai MP. The incidence of bladder cancer after cyclophosphamide therapy. J Urol. 1979 Aug;122(2):163–164. doi: 10.1016/s0022-5347(17)56306-5. [DOI] [PubMed] [Google Scholar]
- 38.Lane Z, Epstein JI. Pseudocarcinomatous epithelial hyperplasia in the bladder unassociated with prior irradiation or chemotherapy. Am J Surg Pathol. 2008 Jan;32(1):92–97. doi: 10.1097/PAS.0b013e3180eaa1dc. [DOI] [PubMed] [Google Scholar]
- 39.Pinke LA, Lamm DL. Intravesical immunotherapy: BCG. In: Lerner, Schoenberg, Sternberg, editors. Textbook of Bladder Cancer. 1st. Oxon: Taylor & Francis; 2006. pp. 353–358. [Google Scholar]
- 40.Bassi P, Milani C, Meneghini A, et al. Clincial value of pathologic changes after intravesical BCG therapy of superificial bladder cancer. Urology. 1992;40:175–179. doi: 10.1016/0090-4295(92)90523-y. [DOI] [PubMed] [Google Scholar]
- 41.Pinthus JH, Bogaards A, Weersink R, Wilson BC, Trachtenberg J. Photodynamic therapy for urological malignancies: past to current approaches. J Urol. 2006 Apr;175(4):1201–1207. doi: 10.1016/S0022-5347(05)00701-9. [DOI] [PubMed] [Google Scholar]
- 42.Jichlinski P, Leisinger HJ. Photodynamic therapy in superficial bladder cancer: past, present, and future. Urol Res. 2001;29:396–405. doi: 10.1007/s002400100215. [DOI] [PubMed] [Google Scholar]
- 43.Oxley JD, Cottrell AM, Adams S, Gillatt D. Ketamine cystitis as a mimic of carcinoma in situ. Histopathology. 2009 Dec;55(6):705–708. doi: 10.1111/j.1365-2559.2009.03437.x. [DOI] [PubMed] [Google Scholar]
- 44.McKenney JK, Desai S, Cohen C, Amin MB. Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: an analysis of cytokeratin 20, p53, and CD44 antigens. Am J Surg Pathol. 2001 Aug;25(8):1074–1078. doi: 10.1097/00000478-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Netto GJ, Epstein JI. Dabbs: Diagnostic Immunohistochemistry. 3rd ed. Philadelphia: Saunders; 2010. Immunohistology of the Prostate, Bladder, Kidney, and Testis in; pp. 593–661. [Google Scholar]
- 46.Hodges KB, Lopez-Beltran A, Emerson RE, Montironi R, Cheng L. Clinical utility of immunohistochemistry in the diagnoses of urinary bladder neoplasia. Appl Immunohistochem Mol Morphol. 2010 Oct;18(5):401–410. doi: 10.1097/PAI.0b013e3181e04816. [DOI] [PubMed] [Google Scholar]