Abstract
Intramembrane proteases are an ancient and diverse group of multi-spanning membrane proteins that cleave transmembrane substrates inside the membrane to effect a wide range of biological processes. As proteases, a clear understanding of their function requires kinetic dissection of their catalytic mechanism, but this is difficult to achieve for membrane proteins. Kinetic measurements in detergent systems are complicated by micelle fusion/exchange, which introduces an additional kinetic step and imposes system-specific behaviors (e.g. cooperativity). Conversely, kinetic analysis in proteoliposomes is hindered by premature substrate cleavage during co-reconstitution, and lack of methods to quantify proteolysis in membranes in real-time. In this chapter, we describe a method for the real-time kinetic analysis of intramembrane proteolysis in model liposomes. Our assay is inducible, because the enzyme is held inactive by low pH during reconstitution, and fluorogenic, since fluorescence emission from the substrate is quenched near lipids but restored upon proteolytic release from the membrane. The precise measurement of initial reaction velocities continuously in real time facilitates accurate steady-state kinetic analysis of intramembrane proteolysis and its inhibition inside the membrane environment. Using real data we describe a step-by-step strategy to implement this assay for essentially any intramembrane protease.
1. INTRODUCTION
Although many important reactions are catalyzed by enzymes that are tethered to the cell membrane, proteolysis occurring directly inside the cell membrane has been discovered more recently (Sakai et al., 1996; Rawson et al., 1997; Wolfe et al., 1999; De Strooper et al., 1999; Urban et al., 2001; Weihofen et al., 2002). These enzymes evolved in four distinct families that are conserved in all forms of life from bacteria to man, and act as control points for a wide range of cellular processes.
The site-2 protease family of metalloproteases release membrane-anchored transcription factors to control cholesterol and fatty acid biosynthesis in humans, and virulence circuits in a broad range of bacterial species (Brown et al., 2000; Makinoshima & Glickman, 2006). Several members of the aspartyl intramembrane protease family play prominent roles in the immune system, while the γ-secretase complex is expressed ubiquitously and has been implicated in Alzheimer’s disease and certain malignancies (De Strooper & Annaert, 2010; Wolfe, 2009). Rhomboid proteases are intramembrane serine proteases that activate signaling in animals, and a mitochondrial rhomboid protease regulates mitophagy with direct implications in Parkinson’s disease (Jin et al., 2010). Parasite-encoded rhomboid proteases modulate adhesion between host and parasite in a range of pathogens including the malaria parasite (Urban, 2009). Finally, the most recently discovered Rce-1 family of glutamyl intramembrane proteases process prenylated proteins as a final step in their maturation (Manolaridis et al., 2013). A prominent target of Rce-1 in humans is the G-protein Ras, which is one of the most commonly mutated oncogenes in all cancers.
The remarkable ubiquity of intramembrane proteases (Koonin et al., 2003; Lemberg & Freeman, 2007; Kinch et al., 2006) and their numerous implications in disease processes (as reviewed in Urban, 2009; De Strooper & Annaert, 2010; Urban & Dickey, 2011) has sparked an interest in deciphering their unique catalytic mechanism as a means to understand such biomedically important enzymes. A major advance towards this goal was establishing the heterologous expression and purification of intramembrane proteases in catalytically active form (Urban & Wolfe, 2005; Lemberg et al., 2005; Akiyama et al., 2004). Appropriate detergents (usually gentle alkyl glycosides) were instrumental to the success of these studies; detergents solubilize membrane proteins from the cell membrane and thus allow separation of intramembrane proteases away from other membrane-resident proteins of a cell. In doing so, detergents convert the complex, 2-dimensional system of the membrane (for which few biochemical techniques are available) to a 3-dimensional environment that allows established purification, analysis, and crystallization techniques to be applied.
One model intramembrane protease in particular, the rhomboid serine protease GlpG from Escherichia coli, has been the focus of intense biochemical investigation over the past decade. Early experiments were first to establish that these proteins alone catalyze intramembrane proteolysis (Urban & Wolfe, 2005). Ultimately, applying standard crystallization techniques to active GlpG in detergent systems culminated in determination of the first intramembrane protease structure (Wang et al., 2006; Wu et al., 2006; Ben-Shem et al., 2007; Lemieux et al., 2007). Ongoing studies are continuing to yield a wealth of information through structure-based functional assays and thermodynamic analyses conducted in detergent (Baker & Urban, 2012; Paslawski et al., 2015).
Despite these important advances, achieving a complete understanding of this unique class of enzymes requires careful kinetic analysis to uncover their precise catalytic properties. Indeed, since intramembrane proteases did not evolve from their soluble counterparts, we should not expect them to share identical catalytic mechanisms. In this regard detergents should be used only as a stepping-stone to analyzing intramembrane protease catalysis in their natural membrane environment. Here we describe a method for temporarily and reversibly inactivating an intramembrane protease during reconstitution with its substrate into liposomes. This inducible system prevents the premature processing of substrate during reconstitution, allowing the precise measurement of initial reaction rates. An amino-terminal fluorescent label on the substrate is quenched by the lipid environment and becomes highly fluorescent when released by proteolytic cleavage of the substrate. This fluorogenic signal enables the real-time monitoring of proteolysis occurring in the membrane and facilitates steady-state kinetic analysis. Our inducible and fluorogenic assay (outlined schematically in Fig. 1) is also amenable to the study of protease inhibition kinetics as potential inhibitors can easily be introduced concomitant with enzyme reactivation to evaluate potency in the actual membrane environment, which is the only natural setting for these enzymes in the cell. Finally, this strategy could be applied to any intramembrane protease.
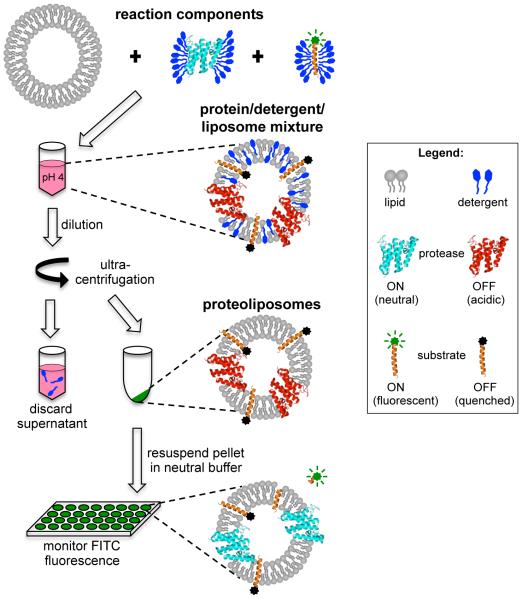
Figure 1.
Schematic diagram of the inducible reconstitution and fluorogenic intramembrane protease assay. Pure rhomboid protease and FITC-TatA substrate in detergent micelles are mixed with liposomes at low pH to inactivate the protease during reconstitution. Detergent is removed from the protein/lipid/detergent complexes by means of dilution followed by ultracentrifugation. Resuspension of the proteoliposome pellet in neutral reaction buffer re-activates the protease. The FITC fluorophore on the reconstituted substrate is quenched by its proximity to the membrane lipids, allowing detection of a fluorogenic signal as evidence of proteolytic release of the FITC-labeled amino-terminus.
2. ENZYMATIC CONSIDERATIONS FOR KINETIC ANALYSIS OF PROTEOLYSIS IN DETERGENT MICELLE SYSTEMS
Conducting kinetic analysis of intramembrane protease catalysis in detergent systems instead of in membranes affords the major advantage of applying standard techniques to these unusual enzymes. However, micellar systems like detergents introduce several unappreciated hydrodynamic features that are not true characteristics of the enzymes, and intramembrane proteases themselves have been found to display different properties in membranes. Given the importance of these considerations, we will detail each specifically in turn (and summarized in Table 1).
Table 1.
| DETERGENT MICELLE SYSTEM PROPERTIES | IMPLICATIONS FOR KINETIC ANALYSIS |
|---|---|
|
Sequestration: enzyme and substrate are in separate detergent micelles |
Imicellar fusion/fission rates introduce additional and unaccounted kinetic step |
|
Micelle Dilution Effect: concentration of micelles is the effective 'volume' of the reaction |
kinetic parameters scale with both type and concentration of detergent, not just concentration of proteins |
|
Cooperativity: detergents self-associate in a strongly cooperative manner |
danger of misappropriating cooperativity to enzyme:substrate interaction |
|
Solubility: limit of solubility imposed by maximal protein:detergent ratios |
substrate solubility limit and/or distorted micelle structure may plateau reaction rates before enzyme is truly saturated |
| Altered Dynamics: increased protein dynamics in detergent | cleavage at ectopic sites in substrates or even non-substrates |
|
Accessibility: altered accessibility of inhibitors to enzyme active- site |
loverestimation of inhibitor efficacies |
|
Altered Conformation: non-native environment changes enzyme conformation/function |
catalysis may be enhanced, diminished, or even disallowed |
|
Enzyme Bias: only some intramembrane proteases are active in detergent (many require a membrane environment) |
kinetic properties of the subset of enzymes active in detergent may not be generally applicable to entire class of proteases |
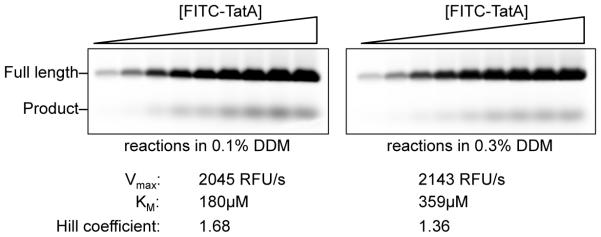
Kinetic rate measurements performed in detergent micelle systems are confounded by several inherent limitations. Reactions in detergents occur as a result of micelle-micelle fission/fusion events; since substrates and enzymes are contained within separate detergent micelles, catalysis relies on the rate of exchange between different micelles (Barzykin & Tachiya, 1994) and therefore may not accurately reflect the enzyme’s rate in natural membranes. Second, the true transmembrane substrate concentration in a detergent system is the concentration of protein relative to micelles, not the protein relative to buffer solution. As such, the measured KM for the same protein concentration of substrate/enzyme changes depending on the amount and/or type of detergent used in the reaction. For example, a Michaelis-Menten kinetic analysis performed in the presence of identical protein concentrations and buffer constituents, but 0.1% versus 0.3% n-dodecyl-β-D-maltoside (DDM) detergent (not an uncommon difference between labs), results in a 2-fold change in KM when measured exactly in parallel (Fig. 2). Changing the type of detergent is likely to have an even greater impact. Third, detergents by their nature are inherently cooperative systems, and thus observing cooperativity in a kinetic experiment conducted in detergent should be expected and not ascribed to the enzyme without further evidence. In fact, the degree of cooperativity changes depending on detergent concentration when all other parameters are held constant (Fig. 2). Fourth, steady-state enzyme kinetics relies on achieving a plateau of enzyme velocity with increasing substrate as evidence of enzyme saturation. However, substrate solubility limits are often reached before saturation of enzyme with substrate can be achieved. In fact, often the reaction rate plateaus that are observed (and erroneously interpreted as enzyme saturation) result from substrate aggregation or altered detergent/protein complexes as the ratio of protein to micelle is changed (because detergent concentration is usually held constant, increasing the amount of protein alters the number of substrates in each micelle). As such, perceived KM constants in detergent systems appear much lower (‘tighter’) than the true binding constant for a substrate (Dickey et al., 2013).
Figure 2.
Steady-state kinetic analysis in detergent. FITC-TatA substrate titrations performed under identical reaction conditions except detergent concentration was either 0.1% (left panel) or 0.3% (right panel). While the kinetic parameter, Vmax, was unaffected, KM was approximately double in the higher detergent condition. The increased concentration of detergent also resulted in decreased cooperativity, as evidenced by the lower Hill coefficient.
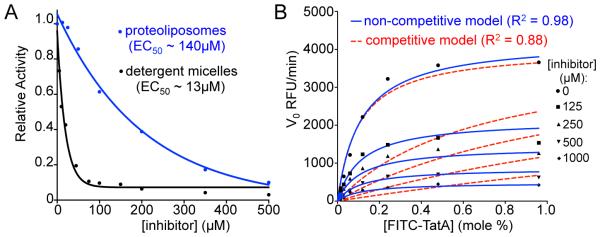
In addition to the inherent properties that surfactants impose, intramembrane proteases behave differently in detergent versus membrane systems: GlpG, for example, generates incorrect cleavage sites in substrates, has a 10-fold higher turnover constant (kcat), and even cleaves some non-substrates in detergent systems (Moin & Urban, 2012; Dickey et al., 2013). This is because the membrane environment plays an active role in ‘tempering’ enzyme and substrate dynamics, while both are radically changed in detergent systems (Moin & Urban, 2012). Inhibitor accessibility to the active site is also altered in detergent versus membrane systems. For example, the small-molecule inhibitor JLK6 (7-amino-4-chloro-3-methoxy-isocoumarin) has a >10-fold more potent EC50 in detergent versus membrane with exactly the same substrate/enzyme (Fig. 6A). Finally, most medically relevant rhomboid proteases are inactive in detergent systems; the only eukaryotic rhomboid protease that has been characterized biochemically, Drosophila melanogaster Rhomboid-4 (Baker & Urban, 2015), absolutely requires reconstitution for activity. Ascribing enzymatic properties to rhomboid proteases as a family based on analysis of a minority that remain active in detergents introduces a serious sampling bias.
Figure 6.
Analysis of inhibition kinetics of intramembrane proteolysis. (A) Inhibition of FITC-TatA cleavage by GlpG with increasing concentration of 7-amino-4-chloro-3-methoxy-isocoumarin (JLK6) in proteoliposomes (blue) compared to detergent micelles (black) reveals that the membrane environment decreases efficacy of inhibition approximately 10-fold. (B) Initial velocities plotted against substrate concentration in the absence or presence of increasing concentrations of peptide aldehyde inhibitor (Ac-VRMA-CHO). Non-linear regression analysis using a non-competitive inhibition model (blue solid line) gives a significantly better fit (R2 = 0.98) than a competitive inhibition model (red dashed line).
3. A NEW STRATEGY: QUANTITATIVE ANALYSIS IN PROTEOLIPOSOMES
An appealing, although challenging, alternative is to study intramembrane protease kinetics in model liposomes that better approximate the native membrane environment. Biochemical analysis of membrane protein cleavage in the context of a lipid bilayer requires co-reconstitution of the protease and substrate into model liposomes. The first step in this process involves mixing detergent micelles containing each of the proteins with liposomes, which leads to partial ‘penetration’ of the liposome membranes by the detergents/proteins, thereby generating lipid/protein/detergent mixed complexes (Fig. 1). Removal of the detergent allows reformation of the lipid bilayer around the incorporated membrane protein(s), and these protein-containing lipid vesicles are called proteoliposomes (Fig. 1). There are several methods for achieving detergent removal, including dialysis, dilution, gel filtration, and polystyrene bead adsorption (Rigaud & Lévy, 2003).
The limitation of any reconstitution protocol in its application to intramembrane proteases is its time-consuming nature. Co-reconstitution of an active intramembrane protease with its substrate into liposomes inevitably results in the premature processing of substrate, thereby preventing the accurate measurement of initial reaction rates. We circumvent this problem by inactivating the protease during co-reconstitution with substrate into liposomes, and subsequently re-activating the protease at the onset of kinetic analysis (Fig. 1). Inactivation is achieved by lowering the pH to 4, thereby protonating the active-site histidine of GlpG. We use sodium acetate buffer (pKa = 4.76) because it is an effective buffer in the low pH range and has been observed to enhance inhibition by occupying the oxyanion hole in the active site of serine proteases. Importantly, the temporary low pH treatment does not perturb the overall stability or structure of the enzyme nor does it have any residual effect on protease activity (Dickey et al., 2013). While low pH (or high pH with aspartyl proteases, for example) is a general strategy applicable to any intramembrane protease, alternative methods of protease inactivation, for example, by the inclusion of reversible small molecule inhibitors or the exclusion of any required cation cofactors (e.g. Zn2+ for metalloproteases) during reconstitution, should also prove effective.
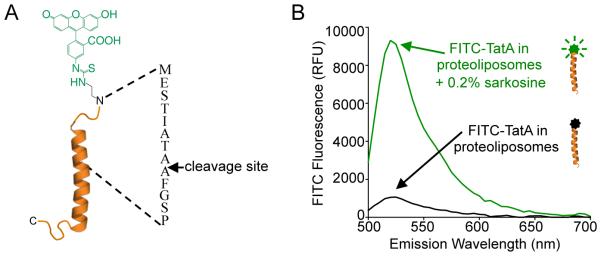
The ability to monitor product formation directly in ‘real-time’ streamlines the experimental process. Our strategy in designing the FITC-TatA substrate was to use fluorescence anisotropy as a readout of proteolysis since the small, FITC-labeled cleavage product is expected to tumble more quickly than the full-length substrate immobilized in liposomes. However, we noticed that upon reconstitution into liposomes, the fluorescence signal emitted from the FITC-labeled TatA substrate is effectively quenched by proximity to the lipids, compared to the robust fluorescence emission in detergent micelles (Fig. 3B). Cleavage of the substrate by rhomboid proteases releases the FITC-labeled amino-terminus, thereby relieving the lipid-imposed quenching to generate a signal that can be detected in real-time fluorometrically (Fig. 4B).
Figure 3.
Fluorogenic FITC-TatA substrate. (A) A peptide comprised of residues 1-33 of TatA is labeled amino-terminally through β-alanine linkage to fluorescein isothiocyanate. The rhomboid cleavage site between two adjacent alanine residues is indicated. (B) FITC fluorescence emission is quenched when the FITC-TatA substrate is reconstituted in proteoliposomes (black line) but robust fluorescence emission is restored when the liposomes are solubilized by 0.2% (w/v) sarcosine detergent (green line).
Figure 4.
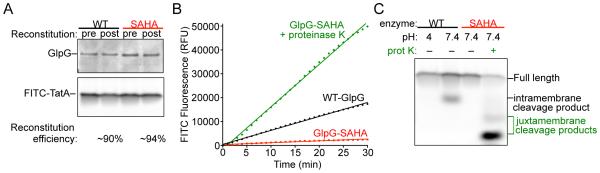
Key parameters for inducible intramembrane protease assay development. (A) Representative samples taken pre- and post-reconstitution for reactions with either wildtype GlpG or its catalytic mutant (SAHA is GlpG-S201A+H254A) were resolved electrophoretically and imaged for GlpG protease levels by Krypton infrared protein staining followed by Odyssey infrared scanning (upper panel) and for FITC-TatA using a Typhoon fluorescence scanner (lower panel). Reconstitution efficiency of 90-95% was observed for both the protease and substrate. (B) Real-time reaction time courses show a linear increase in FITC-fluorescence over time for wildtype GlpG (black line) compared to a negligible but common ‘drift’ in fluorescence with the catalytic mutant (red line). A robust fluorescence signal is generated when proteinase K is added to the SAHA reaction (green line). (C) SDS gel electrophoresis followed by fluorescent imaging of reaction products confirms that the FITC fluorescence signal detected for wildtype GlpG in real-time corresponds to an intramembrane proteolytic cleavage product (lane 2) that is not detected when the catalytic residues are mutated (lane 3) or when the wildtype enzyme is assayed at pH 4 (lane 1). Smaller reaction products corresponding to cleavage sites outside the membrane were detected in the presence of proteinase K (lane 4).
As a starting point, we will assume that the reader wishes to study the kinetics of a new intramembrane protease that has been purified to homogeneity in active form, and that a substrate (natural or surrogate) has been identified. We will outline our protocol with ‘real data’ using the model rhomboid protease GlpG from E. coli, which is facile to study in this context and could even be used as an instructive parallel control.
4. PREPARATION OF LIPOSOMES
Unilamellar liposomes, which are spherical vesicles comprised of a single lipid bilayer (Bangham & Horne, 1964), provide an excellent model of natural membranes and can be used to study membrane proteins. Liposomes may be custom tailored to the membrane protein under investigation to best reflect its native environment, including such parameters as lipid composition, cholesterol content, and membrane curvature (vesicle diameter). For the GlpG protease that has been expressed, detergent-solubilized, and purified from bacteria (Baker & Urban, 2012), we use liposomes prepared from a natural E. coli lipid extract to study membrane-immersed proteolysis.
1. Place 100mg of chloroform-solubilized extract of E. coli polar lipids at 25mg/mL (100600C, Avanti Polar Lipids, Inc.) in a round-bottom 14/20 borosilicate flask. If a defined mixture of lipids is desired, chloroform solutions of the component lipids should be mixed in the appropriate ratio during this step. This is the only way to ensure proper mixing of the lipid constituents. To produce a thin, uniform lipid film, evaporate the solvent at 37°C using a rotary evaporator (Buchi) with a digital vacuum regulator set to maintain pressure at 200 Torr. It is important that solvent is removed to completion by placing the flask under high vacuum overnight at room temperature.
2. Rehydrate lipids at a concentration of 10mg/mL in aqueous buffer consisting of 10mM HEPES, pH 7.3, 10mM NaCl, and 1mM DTT by mixing at 37°C. Complete rehydration requires extensive mixing by repeated pipetting.
3. Sonicate with 2-minute pulses (in a cup horn sonicator attached to a circulating waterbath set at 37°C) to produce small unilamellar vesicles, as evidenced by a change in appearance from a cloudy suspension to a uniform solution.
4. Prepare lipid vesicles of more uniform size distribution by passing the aqueous lipid solution through a Nucleopore Track-Etched Polycarbonate filter (Whatman) of defined pore size (typically 30, 100 or 200nm) using an Avanti Mini-Extruder (Avanti Polar Lipids, Inc.) 12-14 times on a digitally controlled hotplate set to 40°C.
5. PREPARATION OF FLUOROPHORE-LABELED SUBSTRATE
We use long, peptidic transmembrane substrates with amino-terminal, β-alanine linked fluorescein isothiocyanate (FITC) and amidated carboxy-termini that were synthesized with standard Fmoc solid-phase chemistry and purified to >90% via reverse-phase HPLC. We incorporate a carboxy-terminal cysteine residue for additional site-specific labeling (e.g. membrane-impermeable probe accessibility studies, biotinylation, etc). The twin arginine transporter A (TatA) component of Providencia stuartii is the only known natural bacterial rhomboid substrate (Stevenson et al., 2007) and is efficiently processed by many rhomboid proteases, including GlpG (Dickey et al., 2013). While the method we describe here uses a peptide corresponding to amino acids 1-33 of the 99 residues that comprise TatA naturally (Fig. 3A), other peptides based on eukaryotic rhomboid substrates (e.g. Spitz and Gurken) have been synthesized and used to study rhomboid proteolysis in our lab. We generally incorporate the entire transmembrane segment of the substrate, plus at least 5 amino-terminal and carboxy-terminal juxtamembrane residues, into the substrate peptide. This approach should be easily adapted to accommodate other intramembrane protease substrates of interest.
1. Resuspend the lyophilized peptide to a concentration of 200μM in 2,2,2-Trifluoroethanol (Sigma) and 1mM DTT by mixing end-over-end for ~1h at room temperature.
2. Transfer the peptide to a pear-shaped 14/20 borosilicate flask and evaporate the solvent at 37°C using a rotary evaporator (as described above). Place the peptide under high vacuum overnight at room temperature to remove any residual solvent.
3. Resuspend the thin peptide film to a concentration of 400μM with a buffer comprised of 20mM HEPES, pH 7.3, 150mM NaCl, 1mM DTT, and 0.2% (w/v) sodium dodecanoyl sarcosine (Anatrace). As with lipids, extensive pipetting is essential to rehydrate the peptide film. Subject the resusupended peptide to indirect sonication in a temperature-controlled cup-horn device at 14°C for 5 min to disrupt any possible aggregates. Temperature control is essential to ensuring that sonication does not lead to peptide denaturation/aggregation.
4. Flash freeze substrate solutions in small (usually 20μL) aliquots on dry ice and store at −80°C.
6. ESTABLISHING AN INDUCIBLE AND FLUOROGENIC INTRAMEMBRANE PROTEASE ASSAY
Although it is likely that our inducible and real-time assay can be applied to most, if not all, active intramembrane proteases, conditions must be carefully and deliberately set up to reap the benefits of this assay system. In fact, two broad variables require careful consideration.
To date, all intramembrane proteases that have been analyzed are very slow enzymes, usually taking minutes (and even hours) to cleave each substrate molecule (Dickey et al., 2013; Kamp et al., 2015; Bolduc et al., 2016). As such, even trace ‘invisible’ amounts of contaminating cellular proteases can create severe interference when establishing a protease assay with such slow enzymes. Secondly, many intramembrane proteases are dependent on a specific membrane environment to recapitulate true features of their activity. As such, testing different membrane characteristics (e.g. lipid composition, fluidity, thickness, curvature) is ultimately important. However, a good starting point is forming liposomes from natural lipid extracts from various organisms or tissues.
6.1 Co-Reconstitution of Intramembrane Protease and Substrate
As a starting point with a new intramembrane protease, we recommend a simple experiment to evaluate reconstitution efficiency, quenching of uncleaved substrate fluorescence by the liposomes, specificity of any detected protease activity, and effect of pH on protease activity. This can be achieved with a simple set of four reconstitution reactions: the wildtype intramembrane protease assayed at pH 7.4 and at pH 4.0, and its catalytic mutant assayed at pH 7.4 (in the absence or presence of proteinase K as a positive cleavage control). For acid proteases, elevated pH instead of pH 4 is likely to be required; pH 8.5 works well in reversibly inactivating the aspartyl intramembrane protease γ-secretase (Bolduc et al., 2016).
To evaluate reconstitution efficiency, a small proportion of the enzyme/substrate/liposome mixture is taken before dilution, and after resuspension of the proteoliposome pellet after ultracentrifugation, and analyzed by gel analysis (Fig. 4A). Because these are slow enzymes, it is essential that an inactive version of the target intramembrane protease (harboring a catalytic residue mutation) be purified and analyzed in parallel (Fig. 4B). Even if no other interfering enzymes prove detectable, it is conceivable that time-dependent protein interactions could de-quench the substrate without proteolysis actually occurring. This potential ‘background’ can also be revealed using the catalytic mutant protein analyzed in parallel. Next, analyzing the reaction at pH 4 (Fig. 4C) will evaluate whether the pH conditions are sufficient to hold the intramembrane protease inactive (which has to be analyzed by gel, because the fluorophore is not fluorescent at pH 4 even if the proteolytic product is released from liposomes). The proteinase K control (added after resuspending the substrate-containing proteoliposomes) serves as a positive control for proteolysis generating a fluorescence signal (Fig. 4B) (and gel analysis further verifies that cleavage by proteinase K is juxtamembrane relative to intramembrane for GlpG, Fig. 4C). Finally, at the conclusion of the real-time reads, a spectral scan of the inactive mutant reaction following incubation before and after adding detergent will reveal how effectively the lipids quench non-cleaved substrate fluorescence (Fig. 3B).
Plate reader settings are another important consideration, but it is difficult to offer meaningful guidelines because many different instrument manufacturers and models exist. Nevertheless one point warrants emphasis: we do not use automatic gain settings, but instead manually set the photomultiplier tube (PMT) gain (to between 60 and 70 on a BioTek Synergy H4 instrument) based on prior experience or preliminary trials. This helps to ensure that relative fluorescence units (RFUs) are consistent and comparable across experiments and enzymes.
1. Mix 4-5 pmoles of DDM-solubilized and purified GlpG protease or its catalytically inactive mutant (S201A+H254A) with a 100-fold excess (~400 pmoles) of FITC-labeled peptide substrate (prepared as described above) in a 1 mg/mL solution of E. coli liposomes in 50mM NaOAc, pH 4.0 and 150mM NaCl (30μL final reaction volume). The final detergent concentrations with our GlpG preparations are typically 0.15-0.2mM DDM and 0.9mM sarcosine. Take a small (e.g. 3μL) sample of this ‘pre-reconstitution’ mixture for gel analysis.
2. After incubation for 10 min at room temperature, dilute the reaction mixture 20-fold in low salt, low pH buffer (12.5mM NaOAc, pH 4.0, and 37.5mM NaCl), thereby diluting both detergents well below their critical micelle concentrations to facilitate co-reconstitution of the protease and substrate. Incubate the diluted solution at room temperature for 10 minutes.
3. Separate the proteoliposomes, which are collected in the pellet, from the detergent, which is discarded with the supernatant, by ultracentrifugation at 600,000 × g for 30 min at room temperature. Dilution followed by ultracentrifugation is the most rapid method for detergent removal, thereby minimizing the amount of time the reaction components are maintained at low pH.
4. Resuspend the proteoliposome pellets in 75μL neutral reaction buffer (50mM Tris, pH 7.4, 150mM NaCl, and 1mM DTT) or, as a control for pH inactivation of the wildtype enzyme, in 75μL low pH buffer (50mM NaOAc, pH 4.0 and 150mM NaCl). Note that resuspension of proteoliposomes requires extensive but gentle pipetting (being careful to avoid generating bubbles, which interfere with the assay). Take a ‘post-reconstitution’ gel sample to allow calculation of reconstitution efficiency (see 6.2). After resuspension in neutral buffer, supplement one of the two reactions containing the catalytically inactive mutant with a small amount (2.5 pmoles) of proteinase K as a positive control for fluorescence signal generation.
5. For each reaction, transfer a 50μL volume into a separate well of an optical-bottom, black 384-well plate (Nunc) that has been pre-warmed and maintained at 37°C in a digital heatblock (VWR). Seal plates with polyester microplate sealing film (Axygen Scientific) to prevent evaporation.
6. Read fluorescence each minute over a 30 minute time-course at 37°C using a Synergy H4 microplate reader (Biotek) with optics configured to read from the bottom of the plate, exciting at 480 ± 20 nm and detecting emission at 520 ± 20 nm, and an appropriate (60-70) manual PMT gain setting.
6.2 Gel Analysis of Reconstitution Efficiency and Reaction Products
Several factors affect the efficiency of protein reconstitution into the liposome membrane, including excess detergent or incomplete pelleting. As such, the reconstitution efficiency needs to be evaluated directly by gel electrophoresis, and optimized if low.
An important consideration when developing any protease activity assay based on the generation of a fluorogenic signal is to verify ‘activity’ using a direct method to detect proteolysis. Electrophoretic separation of substrate and product is an excellent way to ensure that the fluorescence signal detected truly corresponds to product formation (Fig. 4C). This is particularly relevant when analyzing a new enzyme or substrate, or in relating the decreased fluorescence signal in the presence of a potential inhibitor to a decrease in substrate processing.
1. At the end of a protease reaction time course (or with timepoints analyzed in parallel, see 6.4), quench a sample of each reaction by the addition of an equal volume of 2X LDS sample buffer supplemented with 1/10 volume of 10X sample reducing agent (Life Technologies). Samples taken pre- and post-reconstitution are similarly treated.
2. Heat samples at 70°C for 10 min and then load 5-10μL on a 12% BOLT Bis-Tris gel (Life Technologies).
3. Resolve full-length FITC-TatA substrate from the smaller proteolytic cleavage product by electrophoresis at 150V for 40min or until the dye front is near the bottom of the gel.
4. Perform fluorescence imaging of the gel on a Typhoon FLA 9500 imager (GE Healthcare) using the 473nm (blue) laser to detect the FITC-labeled substrate. GlpG protease is detected by staining the gel with Krypton infrared protein stain followed by scanning in the 700nm channel of an Odyssey infrared scanner (Li-Cor Biosciences).
5. Quantify bands corresponding to the full-length substrate and proteolytic cleavage product using ImageQuant TL (GE Healthcare) or similar densitometry software, and GlpG levels using Image Studio software (Li-Cor Biosciences).
6.3 Extended Progress Curve Analysis
Two characteristics of the reconstituted substrate must be examined in detail before a kinetic analysis can be performed: whether all of the substrate is cleavable, and whether there is a substrate orientation bias (addressed in 6.4). A significant consideration for any proteolytic system is whether all of the substrate is accessible to the enzyme for cleavage. This is most readily examined by extending the progress curves until all of the substrate has had a chance to be cleaved (for slow enzymes like intramembrane proteases this may require overnight incubation). A sub-population of substrate that cannot be cleaved could result from substrate aggregation, trapping inside the liposome lumen, or highly biased intramembrane protease reconstitution orientation bias (which we have never observed). Such a sub-population cannot interact productively with the enzyme and it would therefore be incorrect to include all of the substrate in calculating KM.
1. Incubate a larger intramembrane protease reconstitution reaction for extended reaction time at 37°C, and remove and quench a series of aliquots (taken from several hours to overnight) by addition to an equal volume of 2X LDS reducing sample buffer. Heat and electrophoretically resolv the terminated reactions (as described above). Observing cleavage proceeding to completion may require using more enzyme in the reaction than is appropriate for kinetic assays.
2. Perform fluorescence imaging using a Typhoon fluorescence imager (as described) and quantify cleavage products for the time course of extended reactions. Reactions in which the full-length substrate is essentially undetectable and only a low molecular weight band corresponding to the rhomboid cleavage product is visible provides evidence of complete turnover of the FITC-TatA substrate. Conversely, a plateau of cleavage where full-length, uncut substrate remains unchanged with increasing time suggests that a sub-population of the substrate may be refractory to cleavage by the protease.
6.4 Substrate Orientation
A second key consideration is substrate orientation in proteoliposomes, since non-random substrate orientation, although rare, is possible and could affect the actual concentration of substrate that the intramembrane protease experiences. This we assess by labeling a C-terminal cysteine residue with a membrane-impermeable IRdye-maleimide. (relative to labeling in the presence of detergents that solubilize the liposomes and allow complete substrate labeling). Following gel electrophoresis, this analysis revealed that half of the FITC-TatA molecules are positioned in the liposomes with the N-terminus facing in, indicating a lack of orientation bias (Dickey et al., 2013).
1. To address whether there is substrate orientation-bias in proteoliposomes, incubate reconstituted FITC-TatA resuspended in DTT-free buffer (50mM Tris, pH 7.4, 150mM NaCl) with a 10-fold molar excess (~4000 pmoles) of membrane-impermeable IRDye 800CW maleimide (Li-Cor Biosciences) for 2 hours at RT, protected from light, in either the absence or presence of 0.2% sarcosine detergent.
2. Quench labeling reactions by the addition of an equal volume of 2X Tricine sample buffer and 1/10 volume of NuPAGE sample reducing agent (Life Technologies), and load 5μL samples on a 16% Tricine gel (Life Technologies) followed by electrophoresis at 120V for about 2h or until the dye front has reached the bottom of the gel.
3. Detect IRDye-labeled FITC-TatA in the 800nm channel using an Odyssey infrared imaging system and quantify with Image Studio software (Li-Cor Biosciences).
4. The amount of substrate labeled with the membrane-impermeable dye in the absence of detergent corresponds to those molecules with a dye-accessible carboxy-terminus facing outside the proteoliposome. This amount divided by the total amount of substrate labeled (in the presence of 0.2% sarcosine) gives the fraction of substrates with outward-facing carboxy-termini and if this fraction is close to one half, there is no orientation bias.
6.5 Further Characterization of the Experimental System
While the series of experiments described above should establish the minimal requirements for an inducible and real-time fluorescence assay strategy, ultimately additional controls should be conducted to characterize the system in greater detail. These include verifying the time-course of product generation by electrophoresis (see 6.2), fluorescence generation being blocked in the presence of a known inhibitor and/or by cleavage-site substrate mutations, and mass spectrometry analysis of the cleaved fragments to verify that they correspond to the bona fide protease cleavage site. Finally, any minor effect of the pH switch on the enzyme, if any, should be evaluated for effects on protein structure, stability, and/or enzyme activity. Examples of these analyses with GlpG and experimental methods required for their execution have been published in Dickey et al., 2013.
7. REAL-TIME KINETIC ANALYSIS OF MEMBRANE-IMMERSED PROTEOLYSIS
7.1 Steady-State Kinetic Analysis of Proteolysis in Membranes
A final requirement before a kinetic experiment can be conducted is to determine the appropriate concentration of enzyme to use in the reactions. In general, the amount of enzyme should be titrated so that <10% of the substrate is cleaved in the timespan in which the initial reaction velocities will be extracted (generally between 4 and 14 minutes for GlpG).
We will next delineate how the kinetic parameters of KM, Vmax, and kcat are calculated from the assay data. The readout for our real-time intramembrane protease assay is fluorescence signal intensity (in RFU), which increases linearly with time to give a reaction rate in RFU/min. To derive the kinetic parameter of Vmax, the signal in RFU must be converted to pmoles of substrate. This can be achieved in a number of ways, but perhaps the most convenient is quantifying the fluorescence signal that is produced relative to a series of known concentrations of pure FITC (to define a standard curve). Linear regression of the resulting plot provides a conversion factor for expressing the initial rates measured in the assay in pmoles/min. The catalytic rate (kcat) for a particular enzyme can be calculated from the Vmax parameter by determining the concentration of active protease in the assay. In the case of GlpG, active-site labeling by an activity-based covalent inhibitor proceeded to completion, indicating that the entirety of enzyme present in the assay is in an active conformation (Dickey et al., 2013).
Steady-state kinetic analysis also provides an approximation of enzyme affinity for substrate, reflected in the kinetic constant, KM. Unlike soluble proteases that diffuse through the three-dimensional milieu of the cytoplasm, intramembrane proteases and their substrates are constrained within the plane of the membrane, thereby limiting them to diffusion in two dimensions. As such, the concentration of substrate at which an intramembrane protease is half saturated cannot be described accurately in typical molarity units, but instead, is expressed as a molar fraction of substrate compared to lipids (mole percent). The KM of GlpG for FITC-TatA of ~0.2 mole%, when considered in the context of total protein content of less than 2 mole% for the E. coli inner membrane (Schnaitman, 1970a, b), indicates a very weak affinity of rhomboid proteases for their substrates.
KM measurements rely on knowing the actual (not just intended) substrate concentration in each reaction. Two experimental factors must be incorporated into calculations of substrate concentration. The first is reconstitution efficiency, which is measured by gel analysis of pre- and post-reconstitution samples and typically ranges from 85-95% for FITC-TatA in E. coli lipids, while the second is substrate orientation (described in 6.3). Reconstitution efficiency must be determined empirically for each kinetic experiment as it changes the ‘effective’ concentration of substrate used to calculate KM. Substrate orientation does not vary between experiments. The substrate concentration is therefore the intended substrate mole% multiplied by the reconstitution efficiency achieved at each substrate concentration in that experiment and divided by two (for unbiased substrate orientation).
1. Prepare serial dilutions of the FITC-TatA substrate in 50mM Tris, pH 7.4, 150mM NaCl, 1mM DTT, and 0.2% (w/v) sodium dodecanoyl sarcosine.
2. Co-reconstitute each substrate dilution into E. coli liposomes with a limiting amount (4-5pmol) of GlpG protease at low pH (as described), and take a sample of this ‘pre-reconstitution’ mixture for gel analysis.
3. During ultracentrifugation, prepare a series of FITC concentration standards, from a 2.5μM working stock in reaction buffer by performing five 2-fold serial dilutions and aliquot 50μL of each concentration in duplicate into wells of the 384-well reaction plate.
4. After collection by ultracentrifugation at 600,000 × g for 30 min at room temperature, resuspend the pelleted proteoliposomes in neutral reaction buffer (50mM Tris, pH 7.4, 150mM NaCl, and 1mM DTT), and take a ‘post-reconstitution’ gel sample to allow calculation of reconstitution efficiency (as a percent of the pre-reconstitution sample).
5. Rapidly transfer 50μL of the resuspended and neutralized reactions to a 384-well plate that is incubated at 37°C in a digital heatblock (VWR), and rapidly transfer the sealed plate to a fluorescence plate reader whose sample chamber has been prewarmed to 37°C. Maintaining temperature while performing these steps quickly is important, because changes in temperature will result in changes in reaction velocity (perceived as acceleration with the first few time points as the plate reaches 37°C if it was allowed to cool). Read fluorescence each minute over a 30 minute time-course at 37°C (as described above).
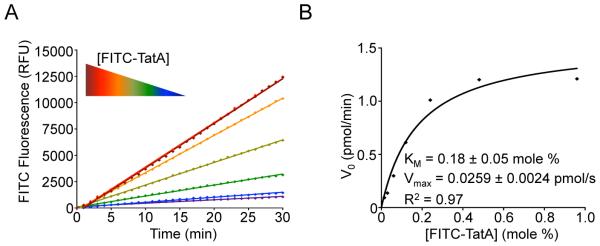
6. Plot raw fluorescence intensity readings (in RFU) against time, and obtain initial reaction rates (V0) from the slope of a linear model (y = mx + b) (Fig. 5A). We typically use rates measured every minute between 4 and 14 minutes.
Figure 5.
Kinetic analysis of membrane-immersed proteolysis. (A) Real-time reaction time courses showing an increase in reaction rate with increasing substrate concentration. Note that the two highest concentrations of substrate (black and red points/lines overlay, indicating enzyme saturation by substrate). (B) A plot of initial velocities against FITC-TatA substrate concentration (in mole percent) is modeled well by a Michaelis-Menten enzyme kinetics model, allowing KM and Vmax kinetic constants to be extracted for GlpG intramembrane proteolysis.
7. Convert initial velocity rates in RFU/min to pmoles/min by standardizing the fluorescence signal generated in the assay to a series of FITC fluorophore standards that are read in parallel wells.
8. Plot the converted reaction rates in pmoles/min against effective substrate concentration (in mole%) to generate a curve that can be fit using the Michaelis-Menten equation (y=Vmax•x/(KM+x) to derive KM and Vmax kinetic constants (Fig. 5B). To assess whether there is cooperativity, the data can be fit to a Hill-modified Michaelis-Menten equation (y=Vmax•xh/(KMh+xh). If the resulting Hill coefficient is significantly greater than 1, positive cooperativity may exist, although its basis could be technical (not related to the enzyme itself).
7.2 Intramembrane Protease Inhibition Assay
Intramembrane proteases, having evolved independently from their soluble counterparts, differ in their catalytic mechanism and, as a consequence, their means of inhibition. For instance, rhomboid intramembrane serine proteases are resistant to many classical serine protease inhibitors (except at extreme concentrations that force inhibition of even unrelated proteases), with one useful exception being isocoumarins (Urban et al, 2001; Urban & Wolfe, 2005; Lemberg et al, 2005). 7-amino-4-chloro-3-methoxy-isocoumarin (JLK6) has served as an effective tool for the inhibition and labeling of the E. coli rhomboid protease, GlpG, in detergent systems (Urban & Wolfe, 2005; Vinothkumar et al., 2010; Dickey et al., 2013).
Our inducible reconstitution system allows potential intramembrane protease inhibitors to be evaluated in the more relevant context of the membrane, without pre-incubation of enzyme and inhibitor. Under our assay conditions, JLK6 inhibition of FITC-TatA cleavage by GlpG in proteoliposomes is an order of magnitude less efficient than in detergent micelles (Fig. 6A), underscoring the importance of evaluating inhibitors in the more natural membrane environment. Moreover, our ability to measure kinetic rates allows inhibition mechanisms and efficacies to be determined. For example, steady-state kinetic analysis in the presence of a substrate-mimicking peptide aldehyde inhibitor reveals a non-competitive mode of inhibition for the GlpG intramembrane serine protease (Fig. 6B), indicative of two distinct enzyme-substrate complexes (Cho et al., 2016).
1. Co-reconstitute GlpG protease into E. coli liposomes with 25-1600 pmoles of FITC-TatA substrate at low pH (as described).
2. After ultracentrifugation, resuspend the proteoliposome pellet in 50μL control reaction buffer (50mM Tris, pH 7.4, 150mM NaCl, and 1mM DTT) or with the same buffer containing the desired concentrations of inhibitor. The enzyme is neutralized in the presence of both substrate and inhibitor, thereby allowing for their potential competition, as would be the case in the natural cellular environment.
3. Read fluorescence every minute over a 30 minute time-course at 37°C and determine initial reaction rates for each substrate concentration in the absence or presence of inhibitor.
4. Plot initial velocity rates (V0) in RFU/min against substrate concentration (expressed as mole %) to generate Michaelis-Menten curves for each concentration of inhibitor (Fig. 6B).
5. Perform nonlinear regression analysis using the R environment or Graphpad Prism (or similar graphing software) to determine the best model for the mode of inhibition, and derive the Ki value for the inhibitor. For the example illustrated here (Fig. 6B), data was fit to models for either non-competitive inhibition: Vmaxinh=Vmax/(1+[I]/Ki); y= Vmaxinh•x/(KM+x) or competitive inhibition: KMObs= KM•(1+[I]/Ki); y= Vmax•x/(KMObs+x).
8. SUMMARY
Intramembrane proteases, by virtue of their requirement for membranous or detergent environments, are more challenging to study than their soluble cousins, particularly with respect to steady-state kinetic analyses. We employ a reversible pH switch from low pH, which maintains the protease in a stable but inactive state during the reconstitution process, to neutral pH to initiate proteolysis. Fluorescence quenching of the substrate’s FITC label is a fortuitous consequence of reconstitution that brings it in close proximity to the membrane and allows detection of fluorescent proteolytic fragments as they are released from the membrane vicinity. The linear progress curves measured can be used to derive steady-state kinetic constants for proteolysis and inhibition. Since all known forms of proteolysis are sensitive to pH, this strategy should prove widely applicable to any intramembrane proteases that have well defined substrates and can be detergent solubilized and purified in active form. Such analyses promise to provide a powerful toolkit for elucidating the unique catalytic mechanisms of this unusual class of enzymes.
ACKNOWLEDGEMENTS
We are grateful to our former lab mate and friend Dr Seth Dickey for his contribution to assay development. This work was supported by NIH grants 2R01AI066025 and R01AI110925.
REFERENCES
- Akiyama Y, Kanehara K, Ito K. RseP (YaeL), an Escherichia coli RIP protease, cleaves transmembrane sequences. EMBO Journal. 2004;23(22):4434–4442. doi: 10.1038/sj.emboj.7600449. http://dx.doi.org/10.1038/sj.emboj.7600449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RP, Urban S. Architectural and thermodynamic principles underlying intramembrane protease function. Nature Chemical Biology. 2012;8(9):759–768. doi: 10.1038/nchembio.1021. http://dx.doi.org/10.1038/nchembio.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RP, Urban S. Cytosolic extensions directly regulate a rhomboid protease by modulating substrate gating. Nature. 2015;523(7558):101–105. doi: 10.1038/nature14357. http://dx.doi.org/10.1038/nature14357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. Journal of Molecular Biology. 1964;8(5):660–668. doi: 10.1016/s0022-2836(64)80115-7. http://dx.doi.org/10.1016/S0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- Barzykin AV, Tachiya M. Reaction kinetics in microdisperse systems with exchange. Journal of Physical Chemistry. 1994;98(10):2677–2687. http://dx.doi.org/10.1021/j100061a027. [Google Scholar]
- Ben-Shem A, Fass D, Bibi E. Structural basis for intramembrane proteolysis by rhomboid serine proteases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):464–466. doi: 10.1073/pnas.0609773104. http://dx.doi.org/10.1073/pnas.0609773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc DM, Montagna DR, Gu Y, Selkoe DJ, Wolfe MS. Nicastrin functions to sterically hinder γ–secretase-substrate interactions driven by substrate transmembrane domain. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(5):E509–518. doi: 10.1073/pnas.1512952113. http://dx.doi.org/10.1073/pnas.1512952113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Ye J, Rawson RB. Regulated intramembrane proteolysis: a control mechanism conserved from bacteria to humans. Cell. 2000;100(4):391–398. doi: 10.1016/s0092-8674(00)80675-3. http://dx.doi.org/10.1016/S0092-8674(00)80675-3. [DOI] [PubMed] [Google Scholar]
- Cho S, Dickey SW, Urban S. Crystal structures and inhibition kinetics reveal a two-stage catalytic mechanism with drug design implications for rhomboid proteolysis. Molecular Cell. 2015;61(3):329–340. doi: 10.1016/j.molcel.2015.12.022. http://dx.doi.org/10.1016/j.molcel.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398(6727):518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Annaert W. Novel research horizons for presenilins and gamma-secretases in cell biology and disease. Annual Review of Cell and Developmental Biology. 2010;26:235–260. doi: 10.1146/annurev-cellbio-100109-104117. http://dx.doi.org/10.1146/annurev-cellbio-100109-104117. [DOI] [PubMed] [Google Scholar]
- Dickey SW, Baker RP, Cho S, Urban S. Proteolysis inside the membrane is a rate-governed reaction not driven by substrate affinity. Cell. 2013;155(6):1270–1281. doi: 10.1016/j.cell.2013.10.053. http://dx.doi.org/10.1016/j.cell.2013.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. Journal of Cell Biology. 2010;191(5):933–942. doi: 10.1083/jcb.201008084. http://dx.doi.org/10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Winkler E, Trambauer J, Ebke A, Fluhrer R, Steiner H. Intramembrane proteolysis of β–amyloid precursor protein by γ–secretase is an unusually slow process. Biophysical Journal. 2015;108(5):1229–1237. doi: 10.1016/j.bpj.2014.12.045. http://dx.doi.org/10.1016/j.bpj.2014.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch LN, Ginalski K, Grishin NV. Site-2 protease regulated intramembrane proteolysis: sequence homologs suggest an ancient signaling cascade. Protein Science. 2006;15(1):84–93. doi: 10.1110/ps.051766506. http://dx.doi.org/10.1110/ps.051766506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin EV, Makarova KS, Rogozin IB, Davidovic L, Letellier MC, Pellegrini L. The rhomboids: a nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers. Genome Biology. 2003;4(3):R19.1–12. doi: 10.1186/gb-2003-4-3-r19. http://dx.doi.org/10.1186/gb-2003-4-3-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg MK, Menendez J, Misik A, Garcia M, Koth CM, Freeman M. Mechanism of intramembrane proteolysis investigated with purified rhomboid proteases. EMBO Journal. 2005;24(3):464–472. doi: 10.1038/sj.emboj.7600537. http://dx.doi.org/10.1038/sj.emboj.7600537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemberg MK, Freeman M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Research. 2007;17(11):1634–1646. doi: 10.1101/gr.6425307. http://dx.doi.org/10.1101/gr.6425307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieux MJ, Fischer SJ, Cherney MM, Bateman KS, James MNG. The crystal structure of the rhomboid peptidase from Haemophilus influenzae provides insight into intramembrane proteolysis. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):750–754. doi: 10.1073/pnas.0609981104. http://dx.doi.org/10.1073/pnas.0609981104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinoshima H, Glickman MS. Site-2 proteases in prokaryotes: regulated intramembrane proteolysis expands to microbial pathogenesis. Microbes and Infection. 2006;8(7):1882–1888. doi: 10.1016/j.micinf.2006.02.021. http://dx.doi.org/10.1016/j.micinf.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Manolaridis I, Kulkarni K, Dodd RB, Ogasawara S, Zhang Z, Bineva G, O’Reilly N, Hanrahan SJ, Thompson AJ, Cronin N, Iwata S, Barford D. Mechanism of farnesylated CAAX protein processing by the intramembrane protease Rce1. Nature. 2013;504(7479):301–305. doi: 10.1038/nature12754. http://dx.doi.org/10.1038/nature12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moin SM, Urban S. Membrane-immersion allows rhomboid proteases to achieve specificity by reading transmembrane segment dynamics. eLife. 2012;1:e00173. doi: 10.7554/eLife.00173. http://dx.doi.org/10.7554/eLife.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paslawski W, Lillelund OK, Kristensen JV, Schafer NP, Baker RP, Urban S, Otzen DE. Cooperative folding of a polytopic α-helical membrane protein involves a compact N-terminal nucleus and nonnative loops. Proceedings of the National Academy of Sciences of the United States of America, 2015;112(26):7978–83. doi: 10.1073/pnas.1424751112. http://dx.doi.org/10.1073/pnas.1424751112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawson RB, Zelenski NG, Nijhawan D, Ye J, Sakai J, Hasan MT, Chang TY, Brown MS, Goldstein JL. Complementation cloning of S2P, a gene encoding a putative metalloprotease required for intramembrane cleavage of SREBPs. Molecular Cell. 1997;1(1):47–57. doi: 10.1016/s1097-2765(00)80006-4. http://dx.doi.org/10.1016/S1097-2765(00)80006-4. [DOI] [PubMed] [Google Scholar]
- Rigaud JL, Levy D. Reconstitution of membrane proteins into liposomes. Methods in Enzymology. 2003;372:65–86. doi: 10.1016/S0076-6879(03)72004-7. http://dx.doi.org/10.1016/S0076-6879(03)72004-7. [DOI] [PubMed] [Google Scholar]
- Sakai J, Duncan EA, Rawson RB, Hua X, Brown MS, Goldstein JL. Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell. 1996;85(7):1037–1046. doi: 10.1016/s0092-8674(00)81304-5. http://dx.doi.org/10.1016/S0092-8674(00)81304-5. [DOI] [PubMed] [Google Scholar]
- Schnaitman CA. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. Journal of Bacteriology. 1970a;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman CA. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. Journal of Bacteriology. 1970b;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson LG, Strisovsky K, Clemmer KM, Bhatt S, Freeman M, Rather PN. Rhomboid protease AarA mediates quorum-sensing in Providencia stuartii by activating TatA of the twin-arginine translocase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):1003–1008. doi: 10.1073/pnas.0608140104. http://dx.doi.org/10.1073/pnas.0608140104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Lee JR, Freeman M. Drosophila rhomboid-1 defines a family of putative intramembrane serine proteases. Cell. 2001;107(2):173–182. doi: 10.1016/s0092-8674(01)00525-6. http://dx.doi.org/10.1016/S0092-8674(01)00525-6. [DOI] [PubMed] [Google Scholar]
- Urban S, Wolfe MS. Reconstitution of intramembrane proteolysis in vitro reveals that pure rhomboid is sufficient for catalysis and specificity. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):1883–1888. doi: 10.1073/pnas.0408306102. http://dx.doi.org/10.1073/pnas.0408306102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S. Making the cut: central roles of intramembrane proteolysis in pathogenic microorganisms. Nature Reviews Microbiology. 2009;7(6):411–423. doi: 10.1038/nrmicro2130. http://dx.doi.org/10.1038/nrmicro2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban S, Dickey SW. The rhomboid protease family: a decade of progress on function and mechanim. Genome Biology. 2011;12(10):231. doi: 10.1186/gb-2011-12-10-231. http://dx.doi.org/10.1186/gb-2011-12-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinothkumar KR, Strisovsky K, Andreeva A, Christova Y, Verhelst S, Freeman M. The structural basis for catalysis and substrate specificity of a rhomboid protease. EMBO Journal. 2010;29(22):3797–3809. doi: 10.1038/emboj.2010.243. http://dx.doi.org/10.1038/emboj.2010.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang Y, Ha Y. Crystal structure of a rhomboid family intramembrane protease. Nature. 2006;444(7116):179–180. doi: 10.1038/nature05255. http://dx.doi.org/10.1038/nature05255. [DOI] [PubMed] [Google Scholar]
- Weihofen A, Binns K, Lemberg MK, Ashman K, Martoglio B. Identification of signal peptide peptidase, a presenilin-type aspartic protease. Science. 2002;296(5576):2215–2218. doi: 10.1126/science.1070925. http://dx.doi.org/10.1126/science.1070925. [DOI] [PubMed] [Google Scholar]
- Wolfe MS. Intramembrane proteolysis. Chemical Reviews. 2009;109(4):1599–1612. doi: 10.1021/cr8004197. http://dx.doi.org/10.1021/cr8004197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398(6727):513–517. doi: 10.1038/19077. http://dx.doi.org/10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Wu Z, Yan N, Feng L, Oberstein A, Yan H, Baker RP, Gu L, Jeffrey PD, Urban S, Shi Y. Structural analysis of a rhomboid family intramembrane protease reveals a gating mechanism for substrate entry. Nature Structural and Molecular Biology. 2006;13(12):1084–1091. doi: 10.1038/nsmb1179. http://dx.doi.org/10.1038/nsmb1179. [DOI] [PubMed] [Google Scholar]