Summary
Loss of expression of liver fatty acid binding protein (LFABP) by immunohistochemistry has been shown to be characteristic of a subset of hepatocellular adenomas (HCAs) in which HNF1A is inactivated. Transformation to hepatocellular carcinoma is thought to be a very rare phenomenon in the HNF1A-inactivated variant of HCA. However, we recently observed 2 cases at our institution, 1 definite hepatocellular carcinoma and 1 possible hepatocellular carcinoma, with loss of LFABP staining, raising the possibility that LFABP down-regulation may be associated with hepatocellular carcinogenesis. Our aim was to evaluate hepatocellular carcinomas arising in various backgrounds and with varying degrees of differentiation for loss of LFABP staining. Twenty total cases of hepatocellular carcinoma were examined. Thirteen cases arose in a background of cirrhosis due to hepatitis C (n = 8) or steatohepatitis (n = 5); 7 cases arose in a noncirrhotic background, with 2 cases arising within HNF1A-inactivated variant HCA and 2 cases arising within inflammatory variant HCA. Complete loss of expression of LFABP was seen in 6 of 20 cases, including 2 cases of hepatocellular carcinoma arising within HNF1A-inactivated variant HCA. Thus, loss of staining for LFABP appears to be common in hepatocellular carcinoma and may be seen in well-differentiated hepatocellular carcinoma. Therefore, LFABP loss should not be interpreted as evidence for hepatocellular adenoma over carcinoma, when other features support a diagnosis of hepatocellular carcinoma. The findings raise consideration for a role of HNF1A inactivation in hepatocellular carcinogenesis, particularly in less differentiated tumors.
Keywords: Liver fatty acid binding protein, Hepatocellular carcinoma, Hepatocellular adenoma, HNF1A, Well differentiated hepatocellular neoplasm
1. Introduction
Hepatocellular carcinoma (HCC) is the most common malignant primary tumor in the liver with most cases arising in the setting of chronic liver disease, often in patients with cirrhosis. Common etiologic associations include cirrhosis, viral hepatitis (specifically in chronic hepatitis B and C infection), aflatoxin B1, hereditary hemochromatosis, alcohol, and fatty liver disease. HCC arising outside the setting of cirrhosis has been particularly noted in patients with metabolic syndrome, emphasizing that multiple mechanisms of disease likely account for progression to HCC.
Hepatocellular adenoma (HCA), on the other hand, is a benign liver tumor, often seen in the setting of oral contraceptive use. Recent molecular studies have allowed for subclassification of HCAs [1–7]. One subtype, accounting for approximately 30% to 40% of HCAs, is defined by biallelic inactivating mutations in HNF1A (on chromosome 12q), which encodes hepatocyte nuclear factor 1α [7,8]. Hepatocyte nuclear factor 1α belongs to the hepatocyte nuclear factor family of proteins and is a key transcription factor known to be involved in the control of hepatocyte differentiation as well as glucose and lipid metabolism in the liver [9–11]. HNF1A-inactivated HCAs are characterized by marked steatosis, but steatosis can be seen in other subtypes of HCA. Steatosis may also be present in other well-differentiated (WD) hepatocellular lesions (ie, focal nodular hyperplasia and WD HCC) that may be considered in a differential diagnosis with HCA, especially on core biopsy. Further analysis has shown that expression of liver fatty acid binding protein (LFABP), which is normally expressed at high levels within hepatocyte cytoplasm and involved in fatty acid trafficking, is down-regulated in HNF1A-inactivated HCAs [8]. This down-regulation of LFABP can be demonstrated by immunohistochemical methods, with loss of hepatocyte staining for LFABP reported in all HNF1A-inactivated HCAs [2].
The risk of transformation of HCA to HCC is highest in the β-catenin–mutated subtype of HCAs but was originally described as a rare phenomenon in HNF1A-inactivated HCAs [7,12]; thus, the molecular mechanisms of carcinogenesis may be distinct in this scenario. Early studies of LFABP expression in HCC considered LFABP as a potential positive marker of HCC [13]. FABP1 (the murine homolog of LFABP) down-regulation has been demonstrated in a murine model of hepatocellular carcinogenesis [14], and hnf1a-deficient mice have been shown to develop liver enlargement associated with increased hepatocyte proliferation, with some cases associated with hepatocyte dysplasia [10,15,16]. However, there has been no systematic study of LFABP staining in HCC. In this study, we evaluated a range of HCC differentiation (well to poorly differentiated) arising in various clinical settings (eg, hepatitis C, steatohepatitis, HCA) for LFABP loss.
2. Materials and methods
2.1. Study population
The cases (n = 20) included in this study (Table 1) were selected from our institutional archives with the goal of selecting cases with a representative mix of background liver disease and with a spectrum of HCC differentiation. Thirteen cases (65%) of HCC arose in a background of cirrhosis due to hepatitis C (n = 8) or steatohepatitis (n = 5). Seven cases (35%) of HCC arose in a noncirrhotic background. Two of these cases (cases 1 and 2) had WD HCCs arising within HNF1A-inactivated variant HCAs; 1 case (case 1) had 2 separate HCCs, both arising within separate HCAs. Two other cases had HCC arising within inflammatory variant HCA (cases 3 and 9). Differentiation of HCC ranged from well to poorly differentiated, with a few cases demonstrating 2 distinct regions of differentiation (ie, well to moderately differentiated or moderately to poorly differentiated, as designated) (Table 2). The diagnosis of HCC was established using a combination of clinical, imaging, and histopathologic findings, including histochemical staining for reticulin, performed on formalin-fixed, paraffin-embedded tissue using standard methodology. This research was approved by the University of California, San Francisco, Institutional Review Board.
Table 1.
Cases selected for study
| Case | Age | Sex | Cirrhosis | Background liver disease |
|---|---|---|---|---|
| 1 | 65 | F | No | Multiple HNF1A-inactivated HCAs |
| 2 | 19 | F | No | Multiple HNF1A-inactivated HCAs |
| 3 | 29 | F | No | Inflammatory HCA |
| 4 | 67 | F | Yes | Hepatitis C |
| 5 | 61 | M | Yes | Steatohepatitis |
| 6 | 48 | M | Yes | Steatohepatitis |
| 7 | 53 | M | Yes | Hepatitis C |
| 8 | 56 | M | Yes | Hepatitis C |
| 9 | 40 | F | No | Inflammatory HCA |
| 10 | 36 | M | No | Mild steatosis |
| 11 | 65 | F | No | None |
| 12 | 59 | M | No | None |
| 13 | 52 | M | Yes | Hepatitis C |
| 14 | 68 | F | Yes | Steatohepatitis |
| 15 | 59 | F | Yes | Steatohepatitis |
| 16 | 59 | M | Yes | Hepatitis C |
| 17 | 48 | M | Yes | Hepatitis C |
| 18 | 71 | M | Yes | Steatohepatitis |
| 19 | 53 | M | Yes | Hepatitis C |
| 20 | 59 | F | Yes | Hepatitis C |
Abbreviations: HCA, hepatocellular adenoma; F, female; M, male.
Table 2.
LFABP expression in hepatocellular carcinoma
| Case | Degree of HCC differentiation | LFABP expression |
|---|---|---|
| 1 | WD | − |
| 2 | WD | − |
| 3 | WD | + |
| 4 | WD | + |
| 5 | WD | + |
| 6 | WD | + |
| 7 | WD | + |
| 8 | WD | + |
| 9 | WD | + |
| 10 | WD-MD | + |
| 11 | WD-MD | − |
| 12 | MD | + |
| 13 | MD | + |
| 14 | MD | + |
| 15 | MD | + |
| 16 | MD | − |
| 17 | MD-PD | + |
| 18 | PD | + |
| 19 | PD | − |
| 20 | PD | − |
Abbreviations: HCC, hepatocellular carcinoma; LFABP, liver fatty acid binding protein; WD, well differentiated; MD, moderately differentiated; PD, poorly differentiated.
2.2. Immunohistochemical staining
Immunohistochemical staining for LFABP (rabbit polyclonal antibody, 1:50 dilution; Abcam, Cambridge, United Kingdom) was performed on formalin-fixed, paraffin-embedded tissue using standard methodology on the Leica BOND platform (Leica Biosystems, Nussloch, Germany), with appropriate positive and negative controls. Only complete loss of staining for LFABP throughout the tumor was considered negative (Table 2, “−”); all other staining intensities and patterns were considered positive (Table 2, “+”).
3. Results
Overall, 6 of 20 HCCs demonstrated complete loss of staining for LFABP (6/20, 30%). The LFABP staining intensity and pattern in HCC varied from complete loss of staining throughout the tumor, to patchy loss, to weak patchy staining, to diffusely strong positive staining. Only complete loss of staining for LFABP throughout the tumor was considered negative (summarized in Table 2). Of the WD HCC cases (n = 9), 2 cases showed complete loss of staining for LFABP (cases 1 and 2). In case 1, 2 WD HCCs were observed, both arising in separate HNF1A-inactivated HCAs; both WD HCCs in this case showed complete loss of staining for LFABP (Fig. 1). Of note, this patient’s partial hepatectomy specimen contained a third nodule, which was also an HNF1A-inactivated HCA, without associated HCC. In case 2, 1 WD HCC was seen, arising in an HNF1A-inactivated HCA, with complete loss of staining for LFABP in both the HCC and HCA regions. The remainder of the WD HCCs showed intact staining for LFABP (eg, case 4 in Fig. 2A and B). Complete loss of LFABP staining was also demonstrated in 1 of 2 well to moderately differentiated HCC (case 11, Fig. 2C and D), 1 of 5 moderately differentiated HCC (case 16, Fig. 2E and F), and 2 of 3 poorly differentiated HCC (eg, case 20, Fig. 2G and H). Focal variable LFABP loss (overall considered positive/intact) was noted in moderately to poorly differentiated HCC (n = 1 of 1 moderately to poorly differentiated HCC; case 17, Fig. 2I and J).
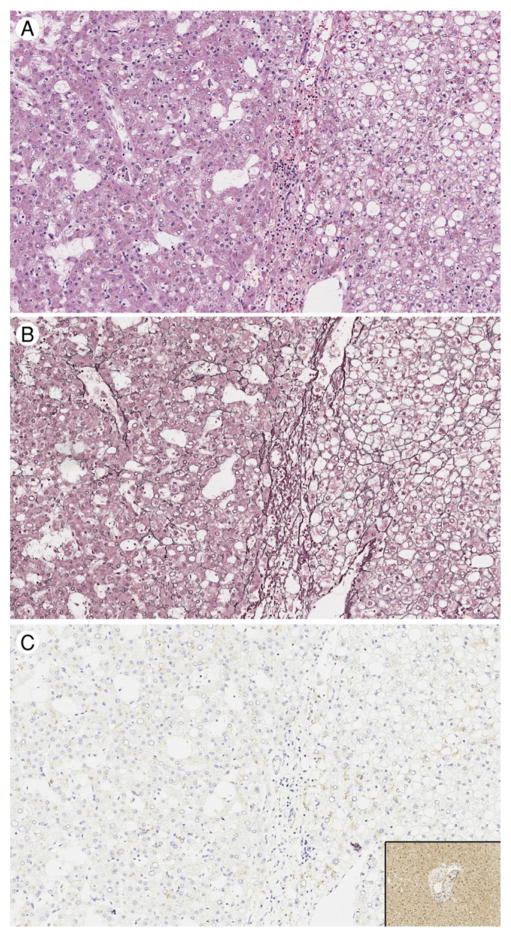
Fig. 1.
Case 1: Well-differentiated hepatocellular carcinoma (HCC) arising in HNF1A-inactivated variant hepatocellular adenoma (HCA). A, The right-hand portion of each panel shows the HNF1A-inactivated HCA portion of the tumor, whereas the left-hand portion of each panel shows the HCC portion of the tumor, hematoxylin and eosin, original magnification ×200. B, Loss of staining for reticulin is seen in the HCC component (left), ×200. C, Complete loss of staining for LFABP is seen in both components, compared to the intact staining seen in the background uninvolved liver parenchyma (inset), ×200.
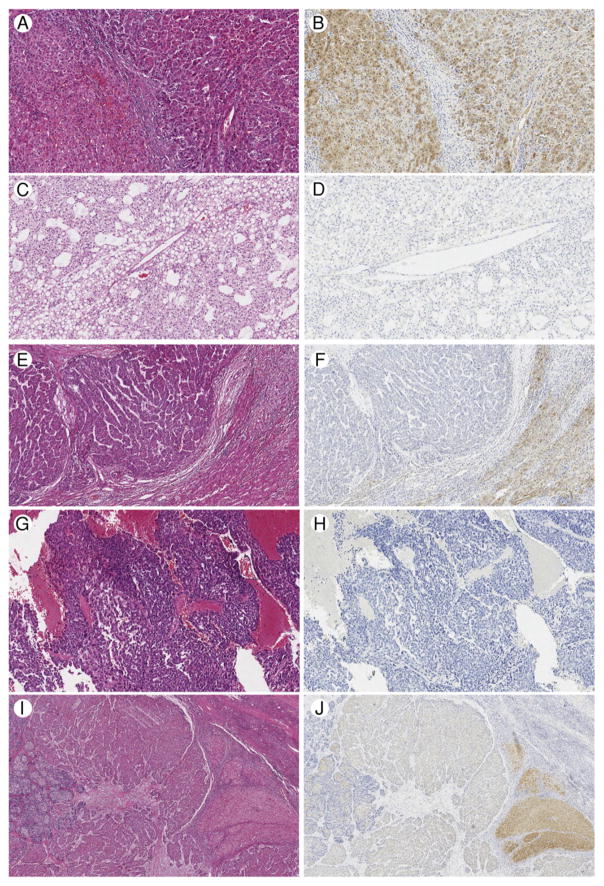
Fig. 2.
Spectrum of LFABP staining. A, Case 4, uninvolved hepatic parenchyma on left with well-differentiated hepatocellular carcinoma (HCC) on right, hematoxylin and eosin (H&E), ×100. B, Case 4, diffuse, strong LFABP staining in uninvolved hepatic parenchyma and HCC, ×100. C, Case 11, representative area of well to moderately differentiated HCC, H&E, ×100. D, Case 11, complete loss of LFABP staining, ×100. E, Case 16, moderately differentiated HCC on left, uninvolved hepatic parenchyma on right, H&E, ×100. F, Case 16, complete loss of LFABP staining in HCC with diffuse, strong LFABP staining in uninvolved hepatic parenchyma, ×100. G, Case 20, representative area of poorly differentiated HCC, H&E, ×100. H, Case 20, complete loss of LFABP staining, ×100. I, Case 17, Moderately to poorly differentiated HCC, H&E, ×40. J, Case 17, patchy, variably intense LFABP staining, ×40. A small portion of uninvolved hepatic parenchyma is seen at the right, with the most intense staining for LFABP.
4. Discussion
Recent studies have shed light on the diagnosis and subclassification of HCAs, providing practical tools, particularly immunohistochemistry, that may be used in the daily practice of surgical pathology. HCA subclassification aids clinical decision making, as it provides a means to stratify HCA cases by risk of transformation to HCC. The risk of transformation is thought to be greatest for those HCA harboring β-catenin mutations [7]. In addition to the β-catenin–mutated variant of HCA, some cases of inflammatory variant HCA have also been shown to harbor mutations in β-catenin [17]. In contrast, the risk of transformation to HCC is thought to be exceedingly low for HNF1A-inactivated HCA, although previous molecular studies have reported biallelic inactivation of HNF1A in rare WD HCC [3]. In our study, 22% of WD HCC (2/9) showed loss of staining for LFABP. Both of these cases are of particular note as they represent examples of WD HCC arising within HNF1A-inactivated HCAs.
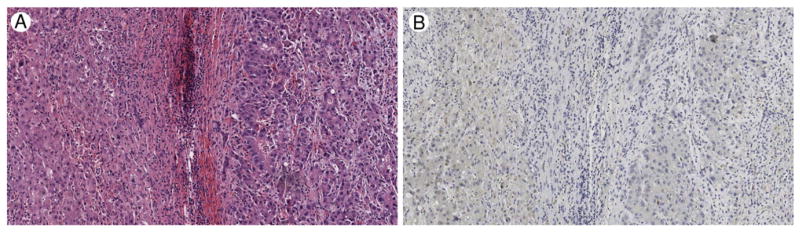
Although loss of staining for LFABP is thought to be a highly sensitive and specific finding in HNF1A-inactivated HCAs, our study also shows that LFABP loss is commonly seen in HCC, even outside the setting of preexisting HNF1A-inactivated HCAs (4/18). Our findings thus highlight the potential pitfall of using LFABP immunohistochemistry as the sole means of diagnosing a lesion as HNF1A-inactivated HCA. As seen in this study, loss of staining for LFABP can be seen in WD HCCs. Therefore, LFABP loss should not be interpreted as evidence of HCA over HCC when other features support a diagnosis of HCC. In addition, as others have reported [18], interpretation of LFABP staining can be problematic, especially in the absence of background liver for comparison and when LFABP staining intensity is weak. For example, case 14 shows very weak staining for LFABP in moderately differentiated HCC (Fig. 3), which can only be interpreted in comparison to background uninvolved liver parenchyma, which, in such cases, not uncommonly also shows weak staining for LFABP.
Fig. 3.
Moderately differentiated hepatocellular carcinoma (HCC), case 14. A, Uninvolved hepatic parenchyma on left with HCC on right, hematoxylin and eosin, ×100. B, Both the HCC and uninvolved hepatic parenchyma show diffuse, but weak, LFABP staining, ×100.
Given that a greater proportion of moderately and poorly differentiated HCC showed complete loss of LFABP (Table 2), it is possible that LFABP loss may be associated with these morphologic changes and thus may represent a pathway involved in HCC differentiation/carcinogenesis, as well as in variant HCA tumorigenesis. It should be noted that, in previous reports, β-catenin–inactivating mutations appeared “exclusive of HNF1A mutations,” although they could be found in combination with GP130 or GNAS mutations seen in the inflammatory variant of HCA [4]. Further investigation is needed to understand the biologic significance of focal loss of LFABP staining in hepatocellular neoplasms.
Abbreviations
- HCA
hepatocellular adenoma
- HCC
hepatocellular carcinoma
- LFABP
liver fatty acid binding protein
- WD
well differentiated
Footnotes
Disclosures: The authors have no conflict of interest to declare.
References
- 1.Bioulac-Sage P, Laurent-Puig P, Balabaud C, Zucman-Rossi J. Genetic alterations in hepatocellular adenomas. Hepatology. 2003;37:480. doi: 10.1053/jhep.2003.50058. author reply 480–481. [DOI] [PubMed] [Google Scholar]
- 2.Bioulac-Sage P, Rebouissou S, Thomas C, et al. Hepatocellular adenoma subtype classification using molecular markers and immunohistochemistry. Hepatology. 2007;46:740–8. doi: 10.1002/hep.21743. [DOI] [PubMed] [Google Scholar]
- 3.Bluteau O, Jeannot E, Bioulac-Sage P, et al. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312–5. doi: 10.1038/ng1001. [DOI] [PubMed] [Google Scholar]
- 4.Nault JC, Bioulac-Sage P, Zucman-Rossi J. Hepatocellular benign tumors-from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888–902. doi: 10.1053/j.gastro.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 5.Nault JC, Zucman Rossi J. Molecular classification of hepatocellular adenomas. Int J Hepatol. 2013;2013:315947. doi: 10.1155/2013/315947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zucman-Rossi J. Genetic alterations in hepatocellular adenomas: recent findings and new challenges. J Hepatol. 2004;40:1036–9. doi: 10.1016/j.jhep.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515–24. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 8.Pelletier L, Rebouissou S, Paris A, et al. Loss of hepatocyte nuclear factor 1alpha function in human hepatocellular adenomas leads to aberrant activation of signaling pathways involved in tumorigenesis. Hepatology. 2010;51:557–66. doi: 10.1002/hep.23362. [DOI] [PubMed] [Google Scholar]
- 9.Rebouissou S, Imbeaud S, Balabaud C, et al. HNF1alpha inactivation promotes lipogenesis in human hepatocellular adenoma independently of SREBP-1 and carbohydrate-response element-binding protein (ChREBP) activation. J Biol Chem. 2007;282:14437–46. doi: 10.1074/jbc.M610725200. [DOI] [PubMed] [Google Scholar]
- 10.Servitja JM, Pignatelli M, Maestro MA, et al. Hnf1alpha (MODY3) controls tissue-specific transcriptional programs and exerts opposed effects on cell growth in pancreatic islets and liver. Mol Cell Biol. 2009;29:2945–59. doi: 10.1128/MCB.01389-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelletier L, Rebouissou S, Vignjevic D, Bioulac-Sage P, Zucman-Rossi J. HNF1alpha inhibition triggers epithelial-mesenchymal transition in human liver cancer cell lines. BMC Cancer. 2011;11:427. doi: 10.1186/1471-2407-11-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bioulac-Sage P, Laumonier H, Sa Cunha A, Balabaud C. Hepatocellular adenomas. Liver Int. 2009;29:142. doi: 10.1111/j.1478-3231.2008.01884.x. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Watanabe K, Ono T. Immunohistochemical demonstration of liver fatty acid-binding protein in human hepatocellular malignancies. J Pathol. 1990;161:79–83. doi: 10.1002/path.1711610113. [DOI] [PubMed] [Google Scholar]
- 14.Elchuri S, Naeemuddin M, Sharpe O, Robinson WH, Huang TT. Identification of biomarkers associated with the development of hepatocellular carcinoma in CuZn superoxide dismutase deficient mice. Proteomics. 2007;7:2121–9. doi: 10.1002/pmic.200601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pontoglio M, Barra J, Hadchouel M, et al. Hepatocyte nuclear factor 1 inactivation results in hepatic dysfunction, phenylketonuria, and renal Fanconi syndrome. Cell. 1996;84:575–85. doi: 10.1016/s0092-8674(00)81033-8. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Sauer B, Gonzalez FJ. Laron dwarfism and non–insulin-dependent diabetes mellitus in the Hnf-1alpha knockout mouse. Mol Cell Biol. 1998;18:3059–68. doi: 10.1128/mcb.18.5.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pilati C, Letouze E, Nault JC, et al. Genomic profiling of hepatocellular adenomas reveals recurrent FRK-activating mutations and the mechanisms of malignant transformation. Cancer Cell. 2014;25:428–41. doi: 10.1016/j.ccr.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Bioulac-Sage P, Cubel G, Balabaud C, Zucman-Rossi J. Revisiting the pathology of resected benign hepatocellular nodules using new immunohistochemical markers. Semin Liver Dis. 2011;31:91–103. doi: 10.1055/s-0031-1272837. [DOI] [PubMed] [Google Scholar]