Abstract
Purpose
Because Intimate partner violence (IPV) may disproportionately impact women’s quality of life (QOL) when undergoing cancer treatment, women experiencing IPV were hypothesized to have (a) more symptoms of depression or stress and (b) lower QOL as measured with the Functional Assessment of Cancer Therapy (FACT-B) and Functional Assessment of Chronic Illness Therapy-Spiritual Well-being (FACIT-SP) Scales relative to those never experiencing IPV.
Methods
Women, ages 18–79, who were included in one of two state cancer registries from 2009–2015 with a recent incident, primary, invasive biopsy-confirmed cancer diagnosis were recruited and asked to complete a phone interview, within 12 months of diagnosis. This interview measured IPV by timing (current and past) and type (physical, sexual, psychological), socio-demographics, and health status. Cancer registries provided consenting women’s cancer stage, site, date of diagnosis, and age.
Results
In this large cohort of 3,278 women who completed a phone interview, 1,221 (37.3%) disclosed lifetime IPV (10.6% sexual, 24.5% physical, and 33.6% psychological IPV). Experiencing IPV (particularly current IPV) was associated with poorer cancer-related QOL defined as having more symptoms of depression and stress after cancer diagnosis and lower FACIT-SP and FACT scores than women not experiencing IPV and controlling for confounders including demographic factors, cancer stage, site and number of comorbid conditions. Current IPV was more strongly associated with poorer QOL. When compared with those experiencing past IPV (and no IPV), women with cancer who experienced current IPV had significantly higher depression and stress symptoms scores and lower FACIT-SP and FACT-G scores indicating poorer QOL for all domains. While IPV was not associated with being diagnosed at a later cancer stage, current IPV was significantly associated with having more than one comorbid physical conditions at interview (adjusted rate ratio = 1.35; 95% confidence interval: 1.19–1.54) and particularly for women diagnosed with cancer when <55 years of age.
Conclusions
Current and past IPV were associated with poorer mental and physical health functioning among women recently diagnosed with cancer. Including clinical IPV screening may improve women’s cancer-related quality of life.
Keywords: spouse abuse, partner violence, cancer registries, Quality of Life (QOL), survivorship, epidemiology
Precis
In this large cohort of 3,278 women recruited from two statewide cancer registries, 37.3% disclosed lifetime intimate partner violence (IPV). Both current (7.9%) and IPV experienced in the past were associated with greater symptoms of stress and depression and poor cancer-related quality of life as measured by Functional Assessment of Cancer Therapy scales.
Introduction
Intimate partner violence (IPV) has mental [1] and physical [2] health effects yet little research has investigated the impact of IPV on cancer-related quality of life (QOL) during cancer treatment and recovery [3, 4]. As defined by the Centers for Disease Control and Prevention, intimate partner violence (IPV) includes physical violence, sexual violence, stalking and psychological aggression (including coercive acts) by a current or former intimate partner [5]. Based on lifetime prevalence estimates, one in four women (25%) have experienced severe physical violence by an intimate partner in their lifetimes. [6] When the IPV definition was expanded to additionally include sexual IPV and stalking behaviors by a partner, 35.9% of women disclosed these forms of IPV in their lifetime and among women with cervical cancer (n=103), the lifetime prevalence rate of IPV was twice that of women never having had cancer (62.1%). [7]
Women with cancer may be more likely to have a history of IPV for the following reasons. IPV has consistently been associated with increased self-perceived stress including symptoms of post-traumatic stress disorder (PTSD) among women with [4] and without cancer [8]. Convincing epidemiologic and clinical evidence now links chronic stress, depression and cancer progression [9–11]. Stressed individuals are more likely to smoke, excessively consume alcohol, and become obese; all three stress responses are associated with chronic inflammation which may influence cancer risk. [9] Both IPV and child abuse have been associated with smoking, alcohol abuse and obesity. [6, 12–13] Stress was also found to mediate or explain the observed effect of IPV on cervical neoplasia risk [14]. Thus, stress may directly or indirectly be associated with an increased prevalence of IPV among those with cancer.
Having experienced IPV may additionally exacerbate a woman’s ability to cope with and recover from a new cancer diagnosis. IPV may influence cancer survivorship through delays in diagnosis or cancer treatment, reducing QOL [3] during treatment or recovery, and ultimately affect survival. While IPV may [15–17] or may not [18–19] be associated with receiving cancer screening at recommended intervals, IPV has been associated with not receiving follow-up care for pre-invasive disease [20] and with delays in receipt of care for invasive cancer [21]. IPV may impact women’s ability to obtain cancer treatment because those currently experiencing IPV are less likely to have health insurance, are more likely to live in poverty, and fewer transportation options [13, 22]. Given the chronic nature of IPV, its associated stress [8], and partner isolation behaviors, women experiencing IPV, either currently or in the past, have limited social support networks [23] to cope with cancer if diagnosed. Women experiencing IPV are more likely to have comorbid conditions or disabilities [24] which may limit their ability to receive recommended cancer care if diagnosed. Further, women compromised by cancer treatment may be at higher risk of IPV. Depression has been associated with increased cancer mortality [25]; women experiencing IPV have consistently higher depression [26] and PTSD [8] rates than women not experiencing this violence. Johnson & Pieters [27] have recently summarized existing literature which characterized the influence of IPV among women with cancer; stress, fear and depression were commonly identified symptoms. This review [27] provides additional evidence that current (or past) IPV may further affect the lives of women with cancer by negatively impacting their self-perceived quality of life in the months following their cancer diagnosis.
In an earlier analyses among 553 women diagnosed with cancer [4], lifetime IPV was significantly associated with more symptoms of both depression and self-perceived stress at diagnosis. Further, IPV was associated with lower scores indicating poorer cancer quality of life within the domains of social / family and emotional functioning as measured within subscales of the Functional Assessment of Cancer Therapy scale. This prior analyses had limited power and therefore could not thoroughly investigate the impact of 1) IPV timing (current versus past alone or never experiencing IPV) and 2) specific IPV forms (physical, sexual, psychological IPV) on cancer-related QOL outcomes. Additionally, this prior analyses did not yet have data to correlate IPV status and stage at cancer diagnosis or to characterize the effect that cancer treatment may have on the association between IPV and cancer-related QOL.
In this current analysis using the completed sample of 3,278 women recently diagnosed with cancer, we sought to determine the role of IPV, by timing and form, on cancer-related quality of life [QOL] as defined by how well women function within the 12–18 months following a cancer diagnosis. Cancer-related QOL was assessed using standard measures of women’s perception of their ability function in their lives within the realms of physical, work/life, emotional, social/family, and spirituality domains. Perceived stress and symptoms of depression were additional measures of cancer-related QOL. Our primary hypothesis was that lifetime IPV, and particularly current IPV, would be associated with (a) reporting more symptoms of depression or stress, and (b) having lower cancer-related quality of life during cancer treatment and recovery. While not a measure of QOL, we additionally hypothesized that IPV would be associated with (c) being diagnosed at a later cancer stage, (d) having more comorbid conditions at diagnosis as secondary hypotheses.
Method
Participant Recruitment
Women, aged 18–79, diagnosed as an incident and primary case of any form of cancer in the prior 12 months and included in either the Kentucky Cancer Registry (KCR) or the North Carolina Central Cancer Registry (NCCR) Rapid Case Ascertainment program were eligible for this study. Dates for recruitment ranged from 2009 to 2015. Breast, colorectal, cervical and gynecologic cancers were the focus of recruitment yet we opened recruitment in Kentucky to any form of invasive cancer reported. KCR contacted eligible women by mail and phone to ensure that only those providing consent were contacted by research staff while NCCR staff provided contact information directly to study researchers. Physicians were informed that their patients’ were eligible for this study. We did not contact patients whose physician provided a reason their patient should not be contacted (i.e. dementia, death, or too ill to participate). Women opted to participate (or not) using an enclosed card addressed to research staff.
Eligible women received a letter inviting them to participate in the study. In this letter women were told that they were eligible for this study because they had been diagnosed with a cancer therefore included in their state’s cancer registry. The research was referred to as the “life stresses, family and partner support and cancer care for women” study. The letter and phone script additionally indicated that the study’s goal was “to understand more about how life stresses including violence may affect women’s cancer care and ability to recover from cancer. Most question are about your health, life stresses, access to health care, and ways you and your family or partner may make decisions about your care”. Because we focused on both current and lifetime IPV, interviewers were trained to remind women who consented to the study that questions about partner abuse were part of the interview. Interviewers asked all women whether they currently felt safe to complete a phone interview. Interviewers specifically asked whether women were alone or if someone might listen in on the call. If women did not feel safe to complete an interview when contacted, interviewers rescheduled these interviews. For all calls, interviewers provided a “safe word” to use to end the call if needed.
Trained research staff at the University of Kentucky Survey Research Center (SRC) conducted a structured phone interview; the average time to complete this interview ranged from 30 to 45 minutes. Those completing the interview received a $10.00 incentive. This study was approved by the IRB at the University of Kentucky, protocol number 09-0685-F1V and an NIH Certificate of Confidentiality was granted (MD-09-007).
Lifetime and Current Physical, Sexual, Psychological IPV Measures
Information to describe IPV occurrence by type (physical, sexual, psychological) and timing (current or past) was obtained from participants [4]. Early in the interview women were asked about their current intimate relationships. An intimate relationship was described as a romantic, dating, marital, or other intimate relationship with a husband, boyfriend or girlfriend. If a woman was not in a current relationship, the interviewer asked about a relationship at cancer diagnosis. Women were asked if they had experienced specific partner behaviors (IPV items), and, if so, whether the partner was their current partner or prior partner.
An abbreviated form of questions based on the Conflict Tactic Scale [28] was used to measure physical and sexual IPV. Physical violence items were designed to be inclusive of a range of physically aggressive partner behaviors and were organized by severity. Three items asked whether a partner a) shoved, grabbed, pushed, pinched, slapped, shook, or threw something that was not done in a playful manner, 2) hit you with a fist, kicked, punched, bit, slapped hard, threw, dragged, hit with an object, or used any other type of physical aggression that could cause injuries, and 3) pointed a weapon at you, beat, choked or attempted to strangle, burned, or used a weapon or other dangerous object that might otherwise hurt you. The two sexual violence items asked whether a partner had 1) ever insisted on sexual activity that you did not want to do such as insisting on having sex when your partner wanted it, not taking no for an answer, insisting on a particular sex act that you did not want to engage in, and 2) physically forced you to have sex or to engage in sexual activities. The response options were yes or no for all 5 items. In this sample, the Cronbach’s ɑ for the 3 physical IPV items was 0.88 and were 0.68 for the 2 sexual IPV items.
Two complementary measures of psychological abuse were used: the Measure of Psychologically Abusive Behaviors (MPAB) [29–30] and the Women’s Experience with Battering (WEB) Scale [31–32]. The MPAB measured severe psychologically abusive partner behaviors while the WEB measured how the woman felt in the relationship due to partner behaviors which may be physically, sexually or psychologically abusive.
The original 42-item MPAB had excellent internal consistency (Cronbach's ɑ = 0.97) and validity as measured by its ability to distinguish those in distressed intimate relationships (criterion group) from those in non-distressed relationships (Sensitivity = 0.725; Specificity 0.628) [30]. For this current study, an abbreviated measure exclusively assessed partner control and intimidation. Note that items included more than one partner behaviors and thus span many of the items within the original MPAB control and intimidation subscales [29]. The intimidation items were: has any partner 1) embarrassed you in public on purpose, yelled or screamed, put you down, called you mean names or treated you as an inferior, and 2) used threatening behaviors toward you, harmed or destroyed your personal things of value, harmed pets, or threatened to harm your family, children, or friends to scare you. The MPAB control items were: has any partner 1) used behaviors to control you such as getting upset if you made even small decisions, dictated your personal choice like what you wore, made major decisions without you, acted upset to make you restrict your behaviors around others, tried to keep you from interacting with members of the opposite sex, or accused you of having an affair; 2) done any of the following to control you: ignored important events, withheld affection, refused to speak to you, acted upset or threatened to end the relationship, or threatened to commit suicide until you did what they wanted; and 3) tried to keep track of you at all times, or keep you away from your family or friend (Examples were: made you report on your whereabouts or activities, listened in on your phone calls, read your email or mail when you did not what them to). Response options were yes or no for all 5 items. In this sample, the Cronbach’s ɑ for the 5-item MPAB was 0.842. Answering yes to any of the 5 items indicated psychological abuse by either a current or past partner (separately queried).
In prior research, the 3-item WEB had excellent internal consistency (Cronbach’s ɑ = 0.88–0.97) [31] and good validity based on ability to distinguish women who were and were not currently battered [32]. The following 3 of the original 10 WEB items were used: 1) your partner makes you feel like you have no control over your life, no power, no protection, 2) you hid the truth about your relationship from others because you are afraid not to, and 3) your partner can scare you without laying a hand on you. The 3 items were asked separately for a current partner and for a prior intimate partner. Response options ranged from strongly disagree to strongly agree. In this sample, the 3-item WEB had good internal consistency (Cronbach’s ɑ = 0.81). A response of agree or strongly agree for any of the three WEB items or yes to any of the 5 MPAB items was used to indicate psychological IPV by a current or past partner.
Data from the IPV items described above were used to create indicators of IPV timing and lifetime IPV form (sexual, physical, psychological IPV). Each IPV form was included in separate models as dichotomous variables.
Cancer Quality of Life (QOL) Measures
Cancer registry staff provided stage at diagnosis as localized (stage I) through distant spread (IV). Stages III and IV (regional extension beyond the immediate tumor region and distant) were grouped as late stage while earlier stage was defined to include both localized and regional with direct expansion to the immediate tumor region.
Information to characterize the remaining cancer outcomes was obtained during phone interviews. The number of comorbid conditions was defined as the count of seven physical conditions: heart disease, diabetes, irritable bowel syndrome, fibromyalgia, stroke, or liver disease. Participants were asked whether a physician had diagnosed each condition; response options were yes or no. A dichotomous indicator was created to indicate more than 1 comorbid condition (>1 versus 0–1 conditions).
Five items from the 18-item Brief Symptom Inventory (BSI) [33] were used to measure depressive symptoms experienced since the woman’s cancer diagnosis; scores ranged from 0–5 (Cronbach ɑ=0.804). The included items, all with yes versus no responses, were: has there been a period of at least two straight weeks in which most of the time you 1) were down, depressed or felt hopeless, 2) experienced very little interest or pleasure in doing things, 3) had difficulty sleeping and eating (not as a result of any medical treatment), 4) felt no energy, difficulty concentrating, or feelings of worthlessness, and 5) since your cancer diagnosis were you told by a medical doctor or mental health professional that you were depressed?
Three items from the 14-item Perceived Stress Scale [34] were used to measure patients’ perception of their stress in the month prior to the interview and the first 2–3 months after the cancer diagnosis. The three items were: how often have you felt 1) that you were unable to control the important things in your life, 2) confident about your ability to handle your personal problems (reversed), and 3) that difficulties were piling up so high that you could not overcome them. Response options were ranged from never to very often. Scores ranged from 0–12 for the each of the two time frames and internal consistency for the abbreviated measure was acceptable (Cronbach’s ɑ=.673).
The first 12 items from the Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale (FACIT-Sp) were used to measure a spiritual dimensional of QOL. [35] Response options range from not at all (=0) to very much (4) and measure how well the statement describes how the woman has felt over the past 7 days. Scores ranged from 2–36 and internal consistency was good (Cronbach’s ɑ=0.817). Example FACIT-SP items were: I have trouble feeling peace of mind; my life lacks meaning and purpose; I am able to reach down deep into myself for comfort (reversed); and I find strength in my faith or spiritual beliefs (reversed). Higher scores indicate poorer spiritual QOL.
Four subscales from the Functional Assessment of Cancer Therapy–General Cancer questionnaire [36] (FACT–G) were used and measured physical functioning (7 items: Range 0–21; Cronbach’s ɑ=.817; Example items included: I have a lack of energy, nausea, pain, feel ill), social/family functioning (7 items: Range 0–21; Cronbach’s ɑ=.763; Example items included: I get support from my family, friends; I feel close to my partner; I am satisfied with my sex life), emotional functioning (6 items: Range 0–18; Cronbach’s ɑ=.755; Example items included: I feel nervous; sad; worry about dying), and work/life functioning (7 items: Range 0–21; Cronbach’s ɑ=.793; Example items included: I am able to work (or do housework); enjoy life; I am content with my quality of life right now). The four subscales were summed to create a 27-item total score with high scores indicating poorer QOL (Range: 10–81; Cronbach’s ɑ=0.909) for the total and subscale scores; response options were the same as those for the FACIT-Sp.
Demographic attributes of participants were obtained directly from women or through data available from cancer registries. See Table 1 for the frequency distributions (number and % of all participants) and response options for all relevant variables including IPV, childhood sexual abuse, demographic factors, cancer sites, stage at diagnosis and number of comorbid conditions.
Table 1.
Frequency of Intimate Partner Violence (IPV) and demographic characteristics of women recently diagnosed with cancer and participating in this study (n=3278)
| # | % | |
|---|---|---|
| Lifetime IPV by form | ||
| Any IPV | 1,221 | 37.3 |
| By form | ||
| Sexual IPV | 346 | 10.6 |
| Physical IPV | 802 | 24.5 |
| Psychological IPV | 1,099 | 33.6 |
| Psychological IPV ‘alone’ | 344 | 10.5 |
| IPV Timing | ||
| Current | 258 | 7.9 |
| Past NOT current | 963 | 29.4 |
| No IPV | 2,057 | 62.8 |
| Current IPV by form** | ||
| Current Sexual IPV | 26 | 1.1* |
| Current Physical IPV | 54 | 2.2* |
| Current Sexual or Physical IPV | 66 | 2.0 |
| Current Psychological IPV | 243 | 7.4 |
| Childhood sexual abuse3 | ||
| Yes | 354 | 10.8% |
| No | 2924 | 89.2% |
| Demographic Factors | ||
| Age at diagnosis | ||
| <45 | 441 | 13.5% |
| 45–54 | 810 | 24.7% |
| 55–64 | 1174 | 35.8% |
| 65-79 | 853 | 26.0% |
| Number of Children | ||
| 3+ | 1026 | 31.3% |
| 2 | 1208 | 36.9% |
| 1 | 633 | 19.3% |
| 0 | 411 | 12.5% |
| Monthly Family Income1 | ||
| <$1,000 | 387 | 11.8% |
| $1,000–$1,999 | 716 | 21.8% |
| $2,000–$2,999 | 561 | 17.1% |
| $3,000–$3,999 | 430 | 13.1% |
| $4,000–$4,999 | 459 | 14.0% |
| ≥$5000 | 725 | 22.1% |
| Highest educational attainment2 | ||
| < High school graduate | 305 | 9.3% |
| High school graduate or received GED | 984 | 30.0% |
| Some college, vocational degree or Associates degree | 575 | 17.5% |
| College graduate | 455 | 13.9% |
| Professional or post baccalaureate training/degree | 959 | 29.3% |
| Race / Ethncity | ||
| Non-Hispanic White | 2972 | 90.7 |
| All other race or ethnicity minority groups | 306 | 9.3 |
| Lived in an Appalachian region at diagnosis | ||
| Yes | 972 | 29.7% |
| No | 2306 | 70.3% |
| Lived in North Carolina at diagnosis | ||
| Yes (Lived in North Carolina) | 883 | 26.9% |
| No (Lived in Kentucky) | 2395 | 73.1% |
| Smoking History | ||
| Current Smoker | 394 | 12.0% |
| Former Smoker | 1063 | 32.4% |
| Never Smoker | 1820 | 55.6 |
| Had Private Medical Insurance at diagnosis | ||
| Yes | 1904 | 58.1% |
| No | 1374 | 41.9% |
| Married at diagnosis | ||
| Yes | 2051 | 62.6% |
| No | 1227 | 37.4% |
| Cancer Site | ||
| Breast | 1954 | 59.6 |
| Colorectal | 288 | 8.8 |
| Head, neck, lung | 201 | 6.1 |
| Endometrial | 154 | 4.7 |
| Leukemia / Lymphoma | 148 | 4.5 |
| Thyroid | 135 | 4.1 |
| Cervical | 95 | 2.9 |
| Ovarian | 83 | 2.5 |
| Melanoma and other non squamous cell skin cancer | 81 | 2.5 |
| Bladder or kidney cancer | 80 | 2.4 |
| Other GI | 33 | 1.0 |
| Brain, Bone, Connective Tissue | 26 | 0.8 |
| Stage at Diagnosis (abbreviated) | ||
| Localized | 2060 | 62.9 |
| 2 | 355 | 10.8 |
| 3 | 583 | 17.8 |
| 4 | 280 | 8.5 |
| Number of comorbid physical conditions | ||
| 0 | 1417 | 43.2 |
| 1 | 1100 | 33.6 |
| 2 | 468 | 14.3 |
| 3 | 182 | 5.5 |
| 4 | 82 | 2.5 |
| 5 or more | 29 | 0.9 |
| By Physical Health Condition | ||
| Heart Disease | 389 | 11.9 |
| Diabetes | 636 | 19.4 |
| Irritable Bowel Syndrome (Frequent diarrhea) | 585 | 17.8 |
| Fibroids | 306 | 9.3 |
| Stroke or TIA | 172 | 5.2 |
| Liver disease(s) | 88 | 2.7 |
Statistical Analysis
The frequency of IPV and demographic characteristics of the sample were provided on Table 1 and, in Table 2, the correlates of IPV (lifetime and current) were assessed using two-sided chi-square tests for categorical variables. These unadjusted associations were used to determine potential confounders for multivariable analyses. Demographic factors associated with lifetime IPV (any form) were considered potential confounders and included in subsequent modeling.
Table 2.
Rate of Current and Lifetime IPV Prevalence within strata of demographic and cancer characteristics among women with cancer
| Rate of Current and Lifetime IPV by Form and within Demographic or Risk Factor Strata | ||||||||
|---|---|---|---|---|---|---|---|---|
| Current a IPV c | Lifetime b IPV c | |||||||
| # in Strata |
Any Form |
Sexual or Physical |
Psychological | Any Form | Sexual | Physical | Psychological | |
| Childhood sexual abuse c,d |
n=258 | n=66 | n=243 | n=1221 | n=346 | n=802 | n=1099 | |
| Yes | n=354 | 17.7% | 6.4% | 15.7% | 74.0% | 37.8% | 59.3% | 66.2% |
| No | n=2924 | 7.3% | 1.7% | 6.9% | 34.6% | 8.7% | 22.0% | 31.2% |
| χ2 | df p value |
28.19 1 <.0001 |
20.54 1 <.0001 |
21.32 1 <.0001 |
127.06 1 <.0001 |
172.33 1 <.0001 |
144.50 1 <.0001 |
104.83 1 <.0001 |
| Age at diagnosis e | ||||||||
| <45 | n=441 | 8.9% | 3.4% | 8.2% | 47.4% | 14.5% | 29.5% | 44.9% |
| 45–54 | n=810 | 10.5% | 2.6% | 10.2% | 43.1% | 12.4% | 28.1% | 39.0% |
| 55–64 | n=1174 | 7.9% | 1.8% | 7.4% | 37.3% | 10.1% | 24.9% | 33.7% |
| 65–79 | n=853 | 4.9% | 1.1% | 4.5% | 26.3% | 7.4% | 17.6% | 22.2% |
| χ2 | df p value |
18.46 3 .0004 |
9.9 3 .02 |
19.90 3 .0002 |
74.82 3 <.0001 |
19.36 3 .0002 |
33.84 3 <.0001 |
85.63 3 <.0001 |
| Number of Children c | ||||||||
| 3+ | n=1026 | 6.9% | 1.2% | 6.9% | 39.1% | 11.5% | 24.5% | 33.3% |
| 2 | n=1208 | 7.1% | 2.1% | 6.7% | 36.8% | 10.3% | 22.8% | 32.9% |
| 1 | n=633 | 8.2% | 2.5% | 7.3% | 35.3% | 9.1% | 23.3% | 32.8% |
| 0 | n=411 | 8.5% | 1.8% | 8.3% | 39.0% | 12.1% | 26.9% | 34.9% |
| χ2 | df p value |
1.76 3 NS |
3.03 3 NS |
1.94 3 NS |
3.93 3 NS |
5.63 3 NS |
5.06 3 NS |
1.26 3 NS |
| Monthly Family Income c | ||||||||
| <$1,000 | n=387 | 10.1% | 1.3% | 9.8% | 45.1% | 16.1% | 32.5% | 39.1% |
| $1,000–$1,999 | n=716 | 6.7% | 3.1% | 6.3% | 39.3% | 12.2% | 29.1% | 34.4% |
| $2,000–$2,999 | n=561 | 9.5% | 2.5% | 8.8% | 39.1% | 10.7% | 24.7% | 34.6% |
| $3,000–$3,999 | n=430 | 7.5% | 1.2% | 7.2% | 38.5% | 10.5% | 24.2% | 35.7% |
| $4,000–$4,999 | n=459 | 7.4% | 2.4% | 6.1% | 31.5% | 8.1% | 19.9% | 28.5% |
| ≥$5000 | n=725 | 7.2% | 1.2% | 7.2% | 32.6% | 7.6% | 18.1% | 30.9% |
| χ2 | df p value |
6.58 5 NS |
9.86 5 NS |
7.25 5 NS |
25.72 5 .0001 |
24.0 5 .0002 |
43.34 5 <.0001 |
14.37 5 .01 |
| Highest educational attainment c | ||||||||
| < HS graduate |
n=305 | 9.5% | 2.6% | 8.9% | 35.5% | 11.2% | 27.1% | 30.3% |
| HS grad or GED |
n=984 | 7.4% | 1.7% | 6.9% | 35.7% | 10.1% | 23.9% | 31.5% |
| Some college |
n=575 | 7.2% | 2.1% | 6.8% | 40.8% | 12.7% | 27.6% | 37.3% |
| College grad |
n=455 | 9.9% | 2.6% | 9.5% | 43.4% | 13.0% | 29.4% | 39.7% |
| Post baccalaureate |
n=959 | 7.3% | 1.8% | 6.9% | 34.4% | 8.5% | 20.0% | 31.5% |
| χ2 | df p value |
4.84 4 NS |
2.18 4 NS |
4.77 4 NS |
15.18 4 .004 |
10.46 4 .03 |
20.58 4 .0004 |
16.19 4 .003 |
| Lived in an Appalachia at diagnosis e | ||||||||
| Yes | n=972 | 8.5% | 2.4% | 8.2% | 36.4% | 11.3% | 25.0% | 33.0% |
| No | n=2306 | 7.6% | 1.9% | 7.1% | 37.6% | 10.3% | 24.2% | 33.8% |
| χ2 | df p value |
.708 1 NS |
.80 1 NS |
1.16 1 NS |
.44 1 NS |
.68 1 NS |
.242 1 NS |
.186 1 NS |
| Race / Ethnicity c | ||||||||
| Non- Hispanic White |
n=2972 | 8.1% | 2.0% | 7.6% | 37.5% | 10.5% | 24.4% | 34.2% |
| Any other |
n=306 | 6.3% | 2.0% | 5.9% | 34.8% | 11.2% | 24.6% | 27.5% |
| χ2 | df p value |
1.24 1 NS |
0.004 1 NS |
1.14 1 NS |
.89 1 NS |
.12 1 NS |
.005 1 NS |
5.43 1 .02 |
| Smoking History c | ||||||||
| Current | n=394 | 13.5% | 2.5% | 12.7% | 49.7% | 17.6% | 35.5% | 44.1% |
| Former | n=1064 | 7.7% | 2.3% | 7.5% | 41.5% | 12.7% | 29.2% | 37.7% |
| Never | n=1821 | 6.8% | 1.8% | 6.3% | 32.1% | 7.8% | 19.3% | 28.8% |
| χ2 | df p value |
20.07 2 <.0001 |
1.48 2 NS |
19.53 2 <.0001 |
54.91 2 <.0001 |
40.05 2 <.0001 |
65.03 2 <.0001 |
45.87 2 <.0001 |
| Had Private Medical Insurance at diagnosis e | ||||||||
| Yes | n=1904 | 7.9% | 2.1% | 7.4% | 37.5% | 9.2% | 22.5% | 34.7% |
| No | n=1374 | 7.9% | 2.0% | 7.5% | 36.7% | 12.5% | 27.0% | 32.0% |
| χ2 | df p value |
.002 1 NS |
.02 1 NS |
.009 1 NS |
.21 1 NS |
8.80 1 .003 |
8.53 1 .004 |
2.56 1 NS |
| Married at diagnosis c | ||||||||
| Yes | n=2051 | 8.6% | 1.9% | 8.2% | 30.5% | 7.8% | 18.4% | 27.8% |
| No | n=1227 | 6.7% | 2.1% | 6.2% | 48.6% | 15.3% | 34.7% | 43.3% |
| χ2 | df p value |
3.99 1 .06 |
.15 1 NS |
4.46 1 .02 |
107.42 1 <.0001 |
45.59 1 <.0001 |
109.95 1 <.0001 |
80.96 1 <.0001 |
| Cancer Site e | ||||||||
| Breast | n=1954 | 7.2% | 1.3% | 6.6% | 35.4% | 9.6% | 22.7% | 31.5% |
| Colorectal | n=288 | 6.9% | 1.4% | 6.6% | 40.4% | 7.3% | 24.3% | 35.9% |
| Head, neck, lung |
n=201 | 7.5% | 2.5% | 7.0% | 39.8% | 11.9% | 28.5% | 37.8% |
| Endometrial | n=154 | 11.0% | 4.6% | 11.1% | 31.8% | 9.1% | 21.4% | 29.2% |
| Leukemia or Lymphoma |
n=148 | 12.2% | 4.7% | 11.5% | 35.8% | 15.5% | 27.0% | 33.1% |
| Thyroid | n=135 | 6.7% | 3.0% | 6.7% | 42.2% | 15.6% | 27.4% | 40.0% |
| Cervical | n=95 | 13.7% | 2.1% | 13.7% | 57.9% | 23.2% | 42.1% | 52.6% |
| Ovarian | n=83 | 4.8% | 0.0% | 4.8% | 30.1% | 6.0% | 20.5% | 27.7% |
| Melanoma f | n=81 | 6.2% | 3.7% | 4.9% | 38.3% | 8.6% | 23.5% | 37.0% |
| Bladder or kidney |
n=80 | 16.3% | 6.3% | 15.0% | 50.0% | 17.5% | 35.0% | 40.0% |
| Other GI |
n=33 | 6.1% | 3.0% | 6.1% | 33.3% | 15.2% | 27.3% | 33.3% |
| Brain, Bone, Connective Tissue |
n=26 | 11.5% | 7.7% | 11.5% | 46.2% | 11.5% | 26.9% | 42.3% |
| χ2 | df p value |
22.18 11 .04 |
30.99 11 .002 |
23.14 11 .03 |
34.58 11 .0005 |
36.31 11 .0003 |
29.06 11 .0004 |
30.15 11 .0003 |
Current IPV means the current partner or partner at diagnosis was the perpetrator
Lifetime IPV includes physical, sexual or psychological partner violence at any time as an adult, > 18 years of age
Data available through phone interview with women
Child sexual abuse defined as an affirmative (yes) answer to this question “Before age 18, did anyone ever physically force or attempt to physically force you to do any sexual activity against your will?” Yes, no, or refused as response options.
Data available from the State Cancer Registry
Melanoma and other forms of skin cancer, excluding squamous cell
IPV, stage at diagnosis, and number of comorbid physical conditions: While the association between IPV and QOL was the primary hypothesized association, analyses to address the association between IPV and later stage and number of comorbid conditions was conducted first because both outcomes may confound the associations between IPV and the QOL measures. Multivariable logistic regression (Proc Genmod link=log dist=bin) was used to estimate the adjusted outcome prevalence rate by IPV strata and the adjusted prevalence rate ratios and 95% confidence intervals [CI] for associations between IPV and stage (later versus earlier) and number of comorbid conditions (2 or more versus 0–1 condition). Separate models were run for these two outcomes and IPV timing (current, past alone, no IPV) and by IPV form (physical, sexual, psychological). Because psychological IPV frequently co-occurs with either sexual or physical IPV, an addition measure of psychological IPV ‘alone’ was created to compare those experiencing psychological IPV yet neither sexual nor physical IPV.
IPV and cancer-related QOL: Because QOL outcomes were correlated, Multiple Analysis of Co-Variance (MANCOVA) was used. The Wilks’ Lambda Statistic for models was significant and indicated correlations between outcomes and the appropriateness of MANCOVA analyses (p<.001 for all models). Mean scores for each QOL outcome (symptoms of depression, stress, FACIT-SP and Fact-G total and by subscale) were presented by the IPV exposure groups used in logistic regression (described above). These models were additionally adjusted for both stage and number of comorbid conditions because both were correlated with QOL outcomes.
Because a younger age at diagnosis may modify the impact of IPV cancer outcomes (later stage, comorbid conditions, and QOL outcomes), both logistic and MANCOVA analyses were repeated within age groups (<55, 55–64 and 65 and older) where the IPV timing measure was used (current, past, no IPV) independent of IPV form. Lastly, MANCOVA subanalyses were conducted to additionally adjust for cancer treatment received because cancer treatment influences QOL; these data were available only for Kentucky participants.
All analyses were conducted using SAS 9.3 or 9.4.
Results
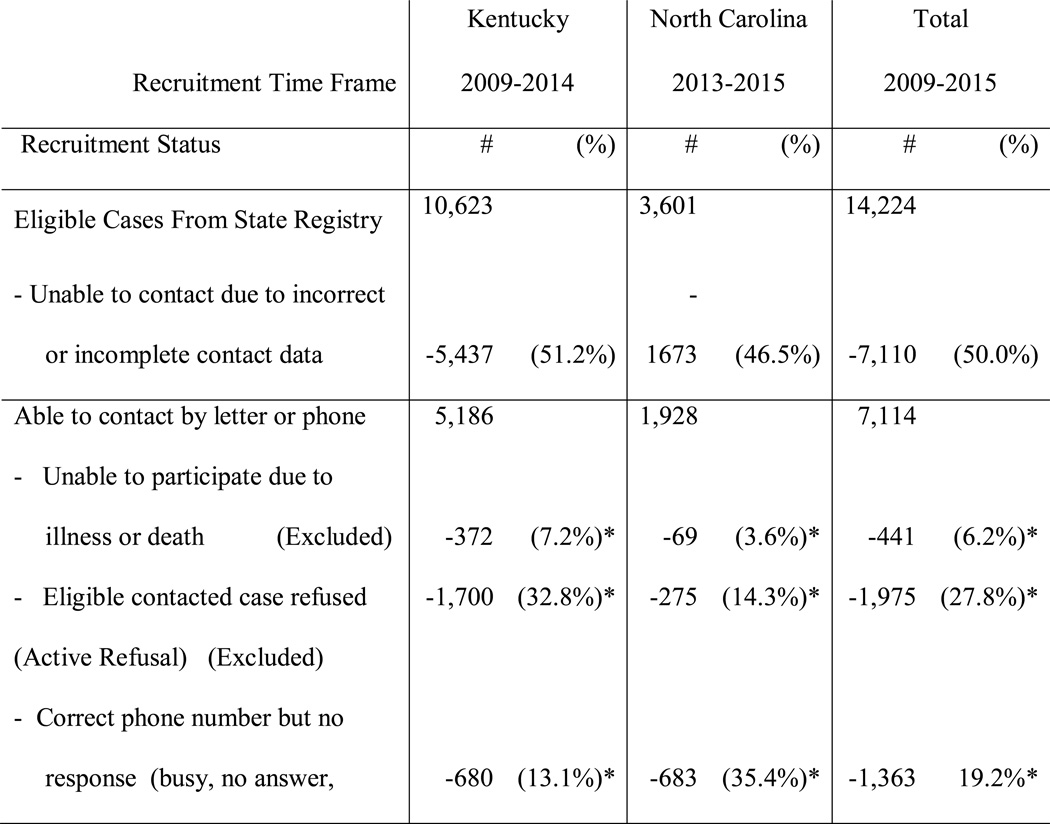
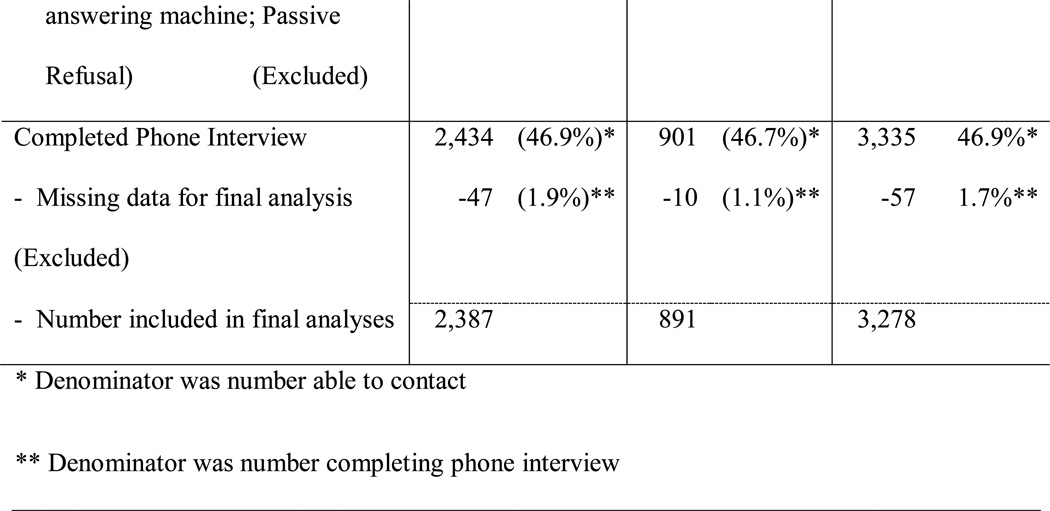
Figure 1 provides a diagram for recruitment by cancer registry. Briefly, of 14,224 eligible (age 18–79) participants, 7,114 were able to be contacted, and 3,335 completed a phone interview. The final analytic sample included 3,278 who provided complete data for the relevant QOL outcomes, IPV measures, and demographic factors. The 57 participants with missing IPV data (n=49) or other demographic factors (n=8) were excluded for data analyses.
Figure 1.
Case Recruitment by State Cancer Registry
Despite differences in how the two registries allowed access to participants, the overall proportion of cases completing the survey, among those staff were able to contact, was very similar in Kentucky (46.9%; 2,434/5186) and North Carolina (46.7%; 901/1928). No differences were noted between those who completed the survey based on Appalachian residence (χ2=2.54NS) as a proxy for socioeconomic status. Women diagnosed with breast cancer were more likely to participate than women diagnosed with other cancers (χ2 =10.65 p=.001).
Briefly, 37.3% of the participants disclosed experiences consistent with lifetime IPV: 33.6% scored as ever experiencing psychological IPV, 24.5% as physical IPV and 10.6% as sexual IPV [Table 1]. Almost 8% were experiencing IPV by a current partner (2.0% as sexual or physical IPV and 7.4% as current psychological IPV, unadjusted rates).
Adjusted prevalence rates of current IPV were significantly higher among the 354 women who had also experienced childhood sexual abuse (17.7%) compared with the 2924 never experiencing childhood sexual abuse (7.3%) (See Table 2). Current IPV was also higher among younger women, current smokers and those with cervical, kidney or bladder cancers. Similar patterns were observed for lifetime IPV and childhood sexual violence, younger age, being a current smoker, and by cancer site. The following differences between IPV timing and demographic factors deserved mention. In contract with current IPV, lifetime IPV was associated with being lower income, no having private medical insurance (for sexual and physical IPV) and not being currently married. Neither current nor lifetime IPV prevalence rates differed by number of children, Appalachian residence, or race or ethnicity. IPV prevalence was not associated with less education; women with some college or a college degree had higher lifetime IPV rates.
Subsequent modeling was adjusted for the following factors associated with lifetime IPV: childhood sexual abuse, age at diagnosis, monthly family income, current smoking status, current marital status, and cancer site. Education and insurance were not included in multivariable models because both were highly correlated with income and marital status. Given modest differences in recruitment by registry, any indicator of state was included in all models.
Results from Logistic Regression (Table 3)
Table 3.
IPV by form and timing and Cancer Stage and Number of Comorbid Conditions: Adjusted Rate Ratio [RR] and 95% Confidence Intervals [CI]
| Later Stage at Diagnosis (n=863) | More than 1 comorbid condition at diagnosis (n=761) |
||||
|---|---|---|---|---|---|
| IPV by Timing |
# in IPV strata |
# (Adjusted a %) Late stage within IPV strata |
Adjusted a Rate Ratio (RR) (95% CI) |
# (Adjusted a %) >1 comorbid conditions within IPV strata |
Adjusted a Rate Ratio (RR) (95% CI) |
| Current IPV | 258 | 66 (22.0%) | 1.04 (0.91–1.18) | 78 (25.1%) | 1.35 (1.19–1.54) |
| By age | |||||
| <55 | 124 | 33 (21.8%) | 1.02 (0.83–1.50) | 24 (19.2%) | 1.63 1.26–2.12) |
| 55–64 | 92 | 25 (22.7%) | 0.97 (0.79–1.20) | 36 (28.8%) | 1.30 (1.09–1.93) |
| 65+ | 42 | 8 (24.3%) | 1.22 (0.92–1.62) | 18 (28.5%) | 1.21 (0.94–1.56) |
| Past IPV ‘Alone’ | 963 | 270 (21.8%) | 1.02 (0.85–1.23) | 251 (20.4%) | 1.10 (0.89–1.35) |
| By age | |||||
| <55 | 434 | 125 (21.4%) | 1.13 (0.78–1.42) | 96 (14.2%) | 1.21 (0.82–1.78) |
| 55–64 | 347 | 96 (21.4%) | 0.92 (0.67–1.25) | 108 (23.7%) | 1.08 (0.79–1.46) |
| 65+ | 182 | 49 (18.7%) | 0.94 (0.56–1.58) | 47 (25.4%) | 1.08 (0.70–1.67) |
| No IPV | 2057 | 528 (21.6%) | 1.00 (REF) | 429 (18.6%) | 1.00 (REF) |
| By age | |||||
| <55 | 693 | 181 (21.8%) | 1.00 (REF) | 88 (11.7%) | 1.00 (REF) |
| 55–64 | 735 | 208 (23.3%) | 1.00 (REF) | 181 (22.1%) | 1.00 (REF) |
| 65+ | 629 | 139 (19.9%) | 1.00 (REF) | 160 (23.5%) | 1.00 (REF) |
| Current IPV by Form | |||||
| Sexual or Physical IPV | 66 | 18 (18.6%) | 0.85 (0.57–1.26) | 22 (27.4%) | 1.33 (0.96–1.84) |
| Psychological IPV | 243 | 61 (20.3%) | 0.92 (0.74–1.15) | 73 (26.8%) | 1.32 (1.09–1.59) |
| Psychological IPV ‘alone’ | 192 | 47 (21.0%) | 0.95 (0.74–1.22) | 56 (26.5%) | 1.31 (1.06–1.61) |
| No Current IPV | 3020 | 798 (22.0%) | 1.00 (REF) | 680 (20.3%) | 1.00 (REF) |
| Lifetime IPV by Form | |||||
| Any | 1221 | 335 (21.6%) | 1.03 (0.91–1.16) | 329 (24.1%) | 1.28 (1.14–1.45) |
| Sexual | 346 | 95 (22.1%) | 1.01 (0.84–1.21) | 116 (27.8%) | 1.41 (1.22–1.64) |
| Physical | 802 | 218 (22.2%) | 1.01 (0.89–1.16) | 233 (25.0%) | 1.31 (1.15–1.49) |
| Psychological IPV | 1099 | 298 (21.7%) | 0.99 (0.88–1.12) | 295 (24.0%) | 1.25 (1.11–1.42) |
| Psychological ‘alone’ | 344 | 95 (20.3%) | 1.02 (0.85–1.23) | 74 (20.4%) | 1.09 (0.89–1.35) |
Adjusted prevalence rates and ratio for age (in years at diagnosis), family monthly income (1=<$1000, 2=$1000–1999, 3=$2000–$2999, 4=$3000–3999,5=$4000–4999, 6=≥$5000), cancer site (included in models as 1=cancers for which an early detection screening is widely available (i.e., breast, colorectal or cervical cancers) versus 0=all other cancer forms), current smoking status (1= current smoker, 0=current non-smoker), Cancer Registry State (1= North Carolina, 0= Kentucky), current marital status (1=currently married, 0=all other marital status)
Later Stage
After adjusting for the above noted factors, among the 258 women with cancer who reported current IPV, 22.0% were diagnosed with a later cancer stage compared with 21.8% of 963 women with past IPV ‘alone’, and 21.6% of 2,057 reporting no IPV. Neither current IPV nor specific IPV forms were associated with being diagnosed at a later cancer stage. Age at diagnosis did not modify the association (Table 3).
>1 Comorbid physical condition
Current IPV was associated with having two or more comorbid physical conditions. Among the 258 women with cancer who reported current IPV, 25.18% had more than one comorbid physical health condition compared with 20.4% among past IPV alone and 18.6 of women experiencing no IPV. Current IPV was associated with a 35% increased prevalence rate of more than one comorbid conditions at diagnosis (aRR = 1.35; 95% CI = 1.19–1.54) while past IPV alone was not associated with having >1 comorbid condition. Subanalyses by age revealed similar patterns, for current IPV being associated with having more comorbid conditions and particularly for those under 55 age at cancer diagnosis (aRR = 1.63). All form of current IPV and all lifetime IPV forms except psychological IPV alone were associated with having more than one comorbid conditions (Table 3).
Results from MANCOVA (Table 4) Quality of Life Outcomes
Table 4.
Current and Lifetime Intimate Partner Violence (IPV) and Measures of Cancer-Related Quality of Life Indicators (Symptoms of Depression, Stress, FACIT-Sp and FACT-G and FACIT-SP): Mean Score (Standard Error) by IPV form (MANCOVA)
| Mean Score (Standard Error) for Cancer-Related Quality of Life (QOL) Indicator by IPV timing and form IPV Timinga |
|||
|---|---|---|---|
| Cancer QOL Indicators |
No IPV | Past IPV Alone | Current IPV |
| MANCOVA Model 1c,d |
(n=2057) | (n=963) | n=258 |
| Depressive symptoms g | 1.58 (0.06)REF | 2.25 (0.07)f | 2.72 (0.11)f,g |
| By Age at Diagnosis | |||
| <55 (n=1251) | 1.77 (0.10)REF | 2.59 (0.11)f | 2.79 (0.16)f, g |
| 55–64 (n=1174) | 1.56 (0.09)REF | 2.10 (0.11)f | 2.67 (0.18)f, g |
| 65+ (n=853) | 1.24 (0.11)REF | 1.76 (0.15)f | 2.67 (0.25)f, g |
| Perceived Stress Scale h | |||
| Within 2 month after diagnosis | 4.16 (0.10)REF | 5.09 (0.12)f | 5.43 (0.20)f |
| By Age at Diagnosis | |||
| <55 (n=1251) | 4.78 (0.18) REF | 5.90 (0.19)f | 6.02 (0.29)f |
| 55–64 (n=1174) | 4.17 (0.17)REF | 5.16 (0.20)f | 5.56 (0.33)f |
| 65+ (n=853) | 3.25 (0.21)REF | 3.67 (0.28) | 4.34 (0.48)f |
| Stress, in month before interview | 3.09 (0.09)REF | 3.77 (0.10)f | 4.92 (0.16)f, g |
| By Age at Diagnosis | |||
| <55 (n=1251) | 3.54 (0.15)REF | 4.16 (0.16)f | 5.23 (0.25)f, g |
| 55–64 (n=1174) | 2.98 (0.15)REF | 3.79 (0.17)f | 4.64 (0.28)f, g |
| 65+ (n=853) | 2.61 (0.17)REF | 3.15 (0.23)f | 5.03 (0.40)f, g |
| MANCOVA Model 2 i | |||
| -FACIT-Sp j | 31.17 (0.17)REF | 30.01 (0.21) | 27.69 (0.33)f, g |
| By Age at Diagnosis | |||
| <55 (n=1251) | 30.43 (0.30)REF | 29.21 (0.30)f | 27.14 (0.50)f, g |
| 55–64 (n=1174) | 30.43 (0.30)REF | 30.36 (0.34)f | 28.14 (0.56)f, g |
| 65+ (n=853) | 32.09 (0.32)REF | 30.98 (0.44)f | 27.78 (0.75)f, g |
| -FACT-G Total (Summed) Scalek | 63.10 (0.40)REF | 59.03 (0.47)f | 53.94 (0.75)f, g |
| By Age at Diagnosis | |||
| <55 (n=1251) | 61.46 (0.71)REF | 57.07 (0.76)f | 52.35 (1.16)f, g |
| 55–64 (n=1174) | 63.09 (0.66)REF | 59.22 (0.76)f | 55.22 (1.25)f, g |
| 65+ (n=853) | 65.58 (0.72)REF | 61.48 (0.93)f | 54.58 (1.67)f, g |
| MANCOVA Model 3 l by -FACT Subscalesm | |||
| Physical Functioning | 13.65 (0.15)REF | 12.55 (0.18)f | 11.81 (0.29)f, g |
| Work/Life Functioning | 17.9 (0.13)REF | 16.02 (0.15)f | 15.27 (0.25)f, g |
| Emotional Functioning | 13.97 (0.12)REF | 13.07 (0.14)f | 11.80 (0.23)f, g |
| Social Functioning | 18.39 (0.11)REF | 17.39 (0.13)f | 15.07 (0.21)f, g |
| MANCOVA Model 4o | |||
| -FACIT-Sp | 31.33 (0.17)REF | 30.01 (0.21)f | 28.08 (0.35)f, g |
| -FACT-G Sum Scale | 64.98 (0.40)REF | 59.03 (0.47)f | 56.17 (0.84)f, g |
| MANCOVA Model 5 p by FACT-G Subscales | |||
| Physical Functioning | 15.07 (0.16)REF | 13.45 (0.19)f | 13.25 (0.32)f |
| Work/Life Functioning | 17.40 (0.13)REF | 16.12 (0.15)f | 15.69 (0.27)f, g |
| Emotional Functioning | 13.85 (0.12)REF | 12.27 (0.14)f | 11.77 (0.26)f, g |
| Social Functioning | 18.66 (0.11) | 17.05 (0.13)f | 15.63 (0.23)f, g |
| Lifetime IPV by formb | ||||
|---|---|---|---|---|
| Cancer QOL Indicators | Any form | Sexual | Physical | Psychological |
| MANCOVA Model 1c, d | N=1221 | N=346 | N=802 | N=1099 |
| Depressive symptoms g | 2.35 (0.06)f | 2.58 (0.10)f | 2.36 (0.07)f | 2.39 (0.06)f |
| Perceived Stress Scale h | ||||
| Within 2 month after diagnosis | 5.17 (0.11)f | 5.39 (0.18)f | 5.21 (0.13)f | 5.23 (0.12)f |
| In the month before interview | 4.04 (0.10)f | 4.34 (0.15)f | 4.10 (0.11)f | 4.11 (0.10)f |
| MANCOVA Model 2 i | ||||
| -FACIT-Sp j | 29.49 (0.19)f | 29.16 (0.30)f | 29.31 (0.22)f | 29.34 (0.20)f |
| -FACT-G Total (Summed) Scale k | 57.86 (0.43)f | 55.89 (0.67)f | 57.55 (0.48)f | 57.46 (0.45)f |
| MANCOVA Model 3 l By FACT-G Subscales m | ||||
| Physical Functioning | 12.38 (0.17)f | 11.71 (0.26)f | 12.23 (0.19)f | 12.29 (0.17)f |
| Work/Life Functioning | 15.85 (0.14)f | 15.36 (0.22)f | 15.70 (0.16)f | 15.75 (0.15)f |
| Emotional Functioning | 12.78 (0.13)f | 12.44 (0.20)f | 12.82 (0.15)f | 12.67 (0.14)f |
| Social Functioning | 16.86 (0.12)f | 16.37 (0.19)f | 16.80 (0.14)f | 16.73 (0.13)f |
| MANCOVA Model 4 o | ||||
| -FACIT-Sp | 29.70 (0.19)f | 29.03 (0.32)f | 29.47 (0.22)f | 29.62 (0.20)f |
| -FACT-G Total Scale | 59.59 (0.44)f | 57.18 (0.75)f | 59.18 (0.53)f | 59.32 (0.46)f |
| MANCOVA Model 5p By FACT-G Subscales | ||||
| Physical Functioning | 13.73 (0.17)f | 13.03 (0.29)f | 13.60 (0.20)f | 13.62 (0.18)f |
| Work/Life Functioning | 16.16 (0.14)f | 15.52 (0.24)f | 15.98 (0.17)f | 16.10 (0.15)f |
| Emotional Functioning | 12.61 (0.13)f | 12.29 (0.23)f | 12.64 (0.16)f | 12.56 (0.14)f |
| Social Functioning | 17.09 (0.12)f | 16.33 (0.21)f | 16.95 (0.15)f | 17.04 (0.13)f |
IPV by a current partner comparisons made as follows: Any Current IPV form (sexual, physical, or psychological IPV) versus No Lifetime IPV and relative to Past IPV ‘alone’
Lifetime IPV by any partner comparisons made as follows: Any Lifetime IPV form (sexual, physical, or psychological IPV by a current) versus No Lifetime IPV; Sexual IPV versus No Lifetime IPV, Physical IPV versus No Lifetime IPV; Psychological IPV versus No Lifetime IPV
All MANCOVA models were adjusted for age (in years at diagnosis), family monthly income (1=<$1000, 2=$1000–1999, 3=$2000–$2999, 4=$3000–3999,5=$4000–4999, 6=≥$5000), cancer type (included in models as 1=cancers for which an early detection screening is widely available (i.e., breast, colorectal or cervical cancers) versus 0=all other cancer forms), current smoking status (1= current smoker, 0=current non-smoker), Cancer Registry State (1= North Carolina, 0= Kentucky), current marital status (1=currently married, 0=all other marital status), stage at diagnosis (1=localized, 2=regional, direct extension 3=regional, 4= metastasis), and number of comorbid physical health conditions, and number of days between cancer diagnosis and interview
MANCOVA model 1 includes the following three outcomes as dependent variables: symptoms of depression, perceived stress within 2 months after cancer diagnosis and stress in the prior month before phone interview.
Depression symptoms measured with an abbreviated version of the Brief Symptoms Inventory: 5 items, Cronbach’s ɑ =0.804, Range 0=5; Unadjusted Mean = 1.67)
compares mean scores with no IPV as referent group; statistically significant at p<.01
compares mean scores with past IPV as referent group; statistically significant at p<.01
Symptoms of stress measured with 3 items of the 4 item Perceive Stress Scale (PSS) asked for two time frames within 2 months following cancer diagnosis (Cronbach’s ɑ =0.673; Range 0=12; Unadjusted Mean = 4.51, and at in the month prior to the phone interview (Cronbach’s ɑ =0.653); Range 0–12; Unadjusted Mean =3.23)
MANCOVA model 2 includes the following two outcomes as dependent variables: FACT-G and FACIT-SP
Functional Assessment of Chronic Illness Therapy-Spiritual Well-being (FACIT-Sp) Psychometrics: 12 items, Cronbach’s ɑ=0.816; Range 2–36; Unadjusted Mean=31.34.
Functional Assessment of Cancer Therapy – General (FACT-G): four subscales were summed to create a total score Psychometrics: 27 items; Cronbach’s α=0.905, Range 10–81; Unadjusted Mean=64.00.
MANCOVA model 3 includes the following four FACT-G subscales as correlated dependent variables: physical, social/family, emotional, and work/life functioning
FACT-G subscales and psychometrics: physical functioning (7 items, Range 0–21; Mean=14.38; Cronbach’s ɑ=0.817), work/life functional status (7 items: Range 0–21; M=17.30, Cronbach’s α=.793), emotional functioning (6 items: Range 0–18; M=14.02, Cronbach’s α=.755), and social/family functioning (7 items: Range 0–21; M=18.28, Cronbach’s α=.763)
FACT- cognitive functioning included for a subsample of women (n=881 surveyed in 2008–2009) Psychometrics: 12 items, Range 0–12; Mean=10.18; Cronbach’s ɑ=0.915. This outcome alone was included in a ANOVA model.
MANCOVA Model 4 includes the following two outcomes as dependent variables: FACT-G and FACIT-SP and treatment received was included additionally included as a covariate (chemotherapy, radiation, surgery). This analysis was restricted to the 2380 women recruited from the Kentucky Cancer Registry because treatment data was available for these women.
MANCOVA Model 5 includes the following four FACT-G subscales (physical, work-life, emotional and social functioning). The model additionally adjusts for treatment received among the 2387 women recruited from the Kentucky Cancer Registry because treatment data was available for these women.
Symptoms of Depression and Stress
Adjusted depressive symptom scores were highest for those experiencing current IPV, intermediate for those experiencing IPV in the past and lowest for those never experiencing IPV (p<.0001). The differences between current and past IPV were significantly different for depression score (p<.001). Lifetime IPV independent of IPV form was also associated with higher depression scores (p<.0001). Note: these analyses were additionally adjusted for cancer stage and number of comorbid conditions
As anticipated, perceived stress scores were higher surrounding diagnosis than at interview (~12 months after diagnosis). IPV was also linked with reporting more symptoms of perceived stress both at diagnosis and in the month before study interview. Women currently experiencing IPV had higher stress scores for both time frames relative to those experiencing past IPV and those never experiencing IPV. Subanalyses by age confirmed the pattern of greater depressive and stress symptoms among those with current relative to past alone or no IPV; younger women (<55) with current PV had consistency higher stress at diagnosis symptoms. All forms of IPV were associated with having more symptoms of stress.
FACIT-Sp and FACT-G Scores
Both current and past ‘alone’ IPV were associated with lower FACIT-Sp and FACT-G scores (p<.0001), however current IPV was additionally associated with the lowest FACIT-Sp and FACT-G total and subscale scores compared with those experiencing IPV only in a past relationship (p<.001). Subanalyses by age group indicated that while both past and current IPV were consistently associated with lower FACT-G scores, the difference in impact appeared to be somewhat greater for young women (age <55) experiencing current IPV as evidenced by a nine point lower FACT-G scores among women disclosing current IPV (52.35) relative to those with no IPV (61.46). All forms of lifetime IPV were associated with lower FACIT-Sp and FACT-G total and subscale scores indicating poorer spiritual well-being and functioning in the domains of emotional, work/life, physical and social QOL after a cancer diagnosis.
Discussion
As hypothesized, IPV was associated with having poorer cancer-related quality of life (as measured by lower FACT-G and FACIT-Sp scores and having more symptoms of depression and stress). These findings are consistent with an earlier report (n=553) which observed lifetime IPV to be associated with increased symptoms of stress and depression and lower FACT and FACIT-SP scores [4]. The current, larger study (n=3,278) was appropriately powered to detect differences in IPV timing and form and, in the current analysis, women experiencing IPV by a current partner were most affected as evidenced by greater self-reported symptoms of stress and depression and poorer FACIT and FACT scores relative to those experiencing IPV in the past or those never experiencing IPV. In contrast with the prior analysis [4], all lifetime IPV forms (sexual, physical and psychological IPV) were associated with poorer QOL. To our knowledge, this large population-based cohort of women recently diagnosed with cancer represents one of the first to estimate the frequency of IPV by timing and forms and the impact of this violence on cancer-related quality of life.
In contrast with the prior analysis [4], in this analysis with a larger sample (n=3278) a significant increased rate of having more than one comorbid conditions was observed for women experiencing IPV by a current partner relative to those never experiencing IPV and past IPV alone. In contrast with our secondary hypotheses, neither current nor past IPV were associated with being diagnosed at a later stage. Recent research suggests that specific partner controlling or interfering behaviors were associated with being diagnosed at a later stage cancer stage (p<.01). [38] These partner controlling or interfering behaviors were characterized as behaviors women experienced after cancer diagnosis and included actions that may impact women’s cancer care or ability to recover.
The emerging literature to describe the effect of IPV on receipt of cancer screening deserves mention here because screening reduces the risk of being diagnosed at a later cancer stage if positive screening results in appropriate follow-up. Findings from several such studies have been mixed [15–19] and suggest that IPV may not affect mammographic screening yet women experiencing IPV may be less likely to receive cervical cancer screening at recommended intervals [18]. Differences in socioeconomic status and access to health care of women at risk of cervical relative to breast cancer may explain these observed differences cancer screening patterns.
Because abbreviated measures of IPV were used in this study, a comparison of the current and lifetime IPV prevalence estimates reported here relative to prior studies is needed. Modesitt et al. [3] estimated current (2%) and lifetime sexual or physical IPV (37%) among 101 women recently diagnosed with cancer. In our larger sample of 3,278 women, very similar rates of current physical or sexual IPV were observed (66 or 2.0%) and when psychological IPV was excluded, our comparative lifetime sexual or physical IPV rate was 26.8% (877/3278).
Strengths of this study include use of the same interview protocol for all participants in both cancer registries and use of outcomes measures with strong psychometric properties, limiting measurement bias. Confounding bias was unlikely to explain these findings given measurement and adjustment for regional, relationship and individual covariates. Sampling from population-based cancer registries improved study power and sample representativeness. Because QOL differs by cancer site and stage, analyses assessing the association between IPV timing and form and QOL were adjusted for these potentially confounding factors.
Limitations in our methodology include defining IPV based entirely on women’s self-reports which may be biased. However, women are the ultimate authority on their own relationship experiences. Those completing phone interviews may still differ from those who did not on attributes we can and cannot measure (e.g., partner violence or other partner behaviors). We did not find differences in interview completion by State or Appalachian region, yet women with breast cancer relative to other cancers were somewhat more likely to complete an interview. The more likely scenario impacting selection bias may be that a larger proportion of women currently experiencing IPV were either too ill to be interviewed, refused, or could not be contacted than women without this experience. If women currently experiencing IPV were disproportionately not included and these women had poorer cancer outcomes then our resulting measures of association would be biased toward the null; we would have included fewer women experiencing IPV with poorer cancer outcomes.
IPV was posited to affect cancer-related QOL through increasing perceived stress. Both current and lifetime IPV were associated with increased stress symptoms after a cancer diagnosis. Finding that current and past IPV were associated with both depression and persistent stress (in the month prior to study interview) supports the posited mechanism for IPV’s impact on the range of cancer-related QOL indicators measured. Recent findings support a complimentary mechanism by which IPV may affect cancer-related QOL and potentially survivorship. The partner interfering or controlling behaviors in cancer-care (PIB-C) was found to be associated being diagnosed at a later cancer stage and with increased stress, depression, and poorer QOL indicators. Finding specific partner behaviors as measured with the PIB-C [38] and, in the current study, as partner controlling or intimidating behaviors may directly or indirectly influence access to recommended cancer care or women’s ability to recover from cancer treatment.
Finding an effect of current IPV whether sexual, physical or psychological, on cancer-related quality of life has direct implications for those caring for cancer patients. A range of IPV screening tools exist and have been evaluated for efficacy [37]. Selected screening tools have been used in clinical trials to effectively identify current IPV [39–40] thus establishing feasiblity of large scale IPV screening. Informed by Institute of Medicine recommendations [41], the US Department of Health and Human Services now requires screening for domestic violence, including IPV, as part of an annual preventive care visit for reproductive aged women. US Preventive Services Task Force also identified the efficacy for IPV interventions to reduce the health impact of IPV [42]. Our findings suggest that screening for current (and past) IPV may benefit women newly diagnosed with cancer because many of this group have ever (37%) or are currently (8%) experiencing IPV. If appropriately asked in clinical settings, women will disclose IPV, particularly when they are ready [43]. Taken together this body of research indicates the value of IPV screening and the potential efficacy of clinical IPV screening to reduce the impact of this common and readily identifiable form of violence. Others have argued for incorporating IPV screening within oncology care [44] yet additional evidence-based intervention research is needed to determine the efficacy of IPV screening in oncologic settings to improve cancer-related QOL.
Acknowledgments
Funding Acknowledgement: This paper reflects results obtained from a study funded by NIH 5R01MD004598.
Footnotes
Disclosure Statement No competing financial interests exist.
Contributor Information
Ann L. Coker, University of Kentucky, Endowed Chair, Center for Research on Violence Against Women.
Diane R. Follingstad, University of Kentucky, Director and Endow Chair, Center for Research on Violence Against Women.
Lisandra S. Garcia, University of Kentucky, College of Medicine, Department of Obstetrics and Gynecology.
Heather M. Bush, Endowed Professor, Center for Research on Violence Against Women.
References
- 1.Logan TK, Walker R, Jordan CE, et al. Women and victimization: Contributing factors, interventions, and implications. Washington, DC: American Psychological Association; 2006. [Google Scholar]
- 2.Coker AL, Williams C, Follingstad D, et al. Psychological, reproductive and maternal health, behavioral and economic impact. In: White JW, Koss MP, Kazdin AE, editors. Violence Against Women and Children: Consensus, Critical Analyses, and Emergent Priorities. Volume I: Mapping the Terrain. Washington, DC: American Psychological Association; 2011. pp. 265–284. [Google Scholar]
- 3.Modesitt SC, Gambrell AC, Cottrill HM, et al. Adverse impact of a history of violence for women with breast, cervical, endometrial, or ovarian cancer. Obstet Gynecol. 2006;107:1330–1336. doi: 10.1097/01.AOG.0000217694.18062.91. [DOI] [PubMed] [Google Scholar]
- 4.Coker AL, Follingstad D, Garcia LS, et al. Association of intimate partner violence and childhood sexual abuse with cancer-related well-being in women. J Womens Health. 2012;21:1180–1188. doi: 10.1089/jwh.2012.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Breiding MJ, Basile KC, Smith SG, Black MC, Mahendra RR. Intimate Partner Violence Surveillance: Uniform Definitions and Recommended Data Elements, Version 2.0 ( http://www.cdc.gov/violenceprevention/pdf/ipv/intimatepartnerviolence.pdf) Atlanta (GA): National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2015. [Google Scholar]
- 6.Black MC, Basile KC, Breiding MJ, et al. The National Intimate Partner and Sexual Violence Survey (NIDVS): 2010 Summary Report. Atlanta, GA: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 7.Coker AL, Hopenhayn C, DeSimone CP, Bush HM, Crofford L. Violence against women raises risk of cervical cancer. J Women’s Health. 2009;18(8):1179–1185. doi: 10.1089/jwh.2008.1048. [DOI] [PubMed] [Google Scholar]
- 8.Golding JM. Intimate partner violence as a risk factor for mental disorders: a meta-analysis. J Fam Viol. 1999;14:99–132. [Google Scholar]
- 9.Baum A, Trevino LA, Dougall AJL, editors. Stress and cancers. New York: NY: Springer; 2011. [Google Scholar]
- 10.Satin JR, Linden W, Phillips MJ. Depression as a predictor of disease progression and mortality in cancer patients: A meta-analysis. Cancer. 2009;115:5349–5361. doi: 10.1002/cncr.24561. [DOI] [PubMed] [Google Scholar]
- 11.Denaro N, Tomasello L, Russi EG. Cancer and stress: what's matters? from epidemiology: the psychologist and oncologist point of view. J Cancer Ther Res. 2014;3:1–11. [Google Scholar]
- 12.Afifi TO, MacMillan HL, Boyle M, Cheung K, Taillieu T, Turner S, Sareen J. Child abuse and physical health in adulthood. Health Reports. 2016;27(3):10–18. [PubMed] [Google Scholar]
- 13.Hathaway JE, Mucci LA, Silverman JG, et al. Health status and health care use of Massachusetts women reporting partner abuse. Am J Prev Med. 2000;19:302–307. doi: 10.1016/s0749-3797(00)00236-1. [DOI] [PubMed] [Google Scholar]
- 14.Thananowan N, Vongsirimas N. Factors mediating the relationship bweteen intimate partner violence and cervical cancer among Thai women. J Interpers Violence. 2014:1–17. doi: 10.1177/0886260514556108. [DOI] [PubMed] [Google Scholar]
- 15.Farley MS, Golding JA, Minkoff JR. Is a history of trauma associated with a reduced likelihood of cervical cancer screening? J Fam Practice. 2002;51:827–831. [PubMed] [Google Scholar]
- 16.Loxton D, Powers J, Schofield M, et al. Inadequate cervical cancer screening among midaged Australian women who have experienced partner violence. Prev Med. 2009;48:184–188. doi: 10.1016/j.ypmed.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi S, Rovi S, Vega M, Johnson MS, Ferrante J, Chen P-H. Intimate Partner Violence and Cancer Screening among Urban Minority Women. J Am Board Fam Med. 2010;23(3):343–353. doi: 10.3122/jabfm.2010.03.090124. [DOI] [PubMed] [Google Scholar]
- 18.Brown MJ, Weitzen S, Lapane Association between intimate partner violence and preventive screening among women. J Women’s Health. 2013;22(11):947–952. doi: 10.1089/jwh.2012.4222. Epub 2013 Jun 29. [DOI] [PubMed] [Google Scholar]
- 19.Lemon SC, Verhoek-Oftedahl W, Donnelly EF. Preventive healthcare, use, smoking, and alcohol use among Rhode Island women experiencing intimate partner violence. J Women’s Heath. 2002;11(6):555–562. doi: 10.1089/152460902760277912. [DOI] [PubMed] [Google Scholar]
- 20.Coker AL, Bond SM, Pirisi LA. Life stressors are an important reason for women discontinuing follow-up care for cervical neoplasia. Cancer Epidem Biomar. 2006;15:321–325. doi: 10.1158/1055-9965.EPI-05-0148. [DOI] [PubMed] [Google Scholar]
- 21.Martino MA, Balar A, Cragun JM, et al. Delay in treatment of invasive cervical cancer due to intimate partner violence. Gyn Onc. 2005;99:507–509. doi: 10.1016/j.ygyno.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Kimerling R, Alvarez J, Pavao J, et al. Unemployment Among Women Examining the Relationship of Physical and Psychological Intimate Partner Violence and Posttraumatic Stress Disorder. J Interpers Violence. 2009;24:450–463. doi: 10.1177/0886260508317191. [DOI] [PubMed] [Google Scholar]
- 23.Coker AL, Smith PH, Thompson MP, et al. Social support protects against the negative effects of partner violence on mental health. J Womens Health. 2002;11:465–476. doi: 10.1089/15246090260137644. [DOI] [PubMed] [Google Scholar]
- 24.Curry MA, Hassouneh-Phillips D, et al. Abuse of women with disabilities - An ecological model and review. Violence Against Wom. 2001;7:60–79. [Google Scholar]
- 25.Pinquart M, Duberstein PR. Depression and cancer mortality: a meta-analysis. Psychol Med. 2010;40:1797–1810. doi: 10.1017/S0033291709992285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JC, Kub J, Belknap RA, et al. Predictors of depression in battered women. Viol Victims. 1997;3:271–293. [Google Scholar]
- 27.Johnson W, Pieters HC. Intimate partner violence among women diagnosed with cancer. Cancer Nursing. 2016;39(2):87–96. doi: 10.1097/NCC.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 28.Straus MA, Hamby SL, Boney-McCoy S, Sugarman DB. The Revised Conflict Tactics Scales: Development and Preliminary Psychometric Data. J Fam Issues. 1996;17:283–316. [Google Scholar]
- 29.Follingstad DR. A measure of severe psychological abuse normed on a nationally representative sample. J Interpersonal Viol. 2011;26:1194–1214. doi: 10.1177/0886260510368157. [DOI] [PubMed] [Google Scholar]
- 30.Follingstad DR, Coker AL, Lee E, Willimas CM, Bush HM, Mendiondo MM. Validity and Psychometric properties of the measure of psychologically abusive behaviors among young women and women in distressed relationships. Violence against women. 2015;21(7):875–896. doi: 10.1177/1077801215584070. [DOI] [PubMed] [Google Scholar]
- 31.Bonomi AE, Anderson ML, Reid RJ, Carrett D, Fishman PA, Rivara FP, Thompson RS. Intimate partner violence in older women. The Gerontologist. 2007;47(1):34–41. doi: 10.1093/geront/47.1.34. [DOI] [PubMed] [Google Scholar]
- 32.Smith PH, Earp JA, DeVellis R. Measuring battering: Development of the Women’s Experience With Battering (WEB) Scale. Women’s Health. 1995;1:273–288. [PubMed] [Google Scholar]
- 33.Derogatis LR, Lazarus L. Brief symptom inventory-18. Administration, Scoring and procedures manual. 2001 [Google Scholar]
- 34.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 35.Peterman AH, Fitchett G, Brady MJ, et al. Measuring spiritual well-being in people with cancer: The functional assessment of chronic illness therapy-spiritual well-being scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 36.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy Scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 37.Rabin RF, Jennings JM, Campbell JC, et al. Intimate Partner Violence Screening Tools. Am J Prev Med. 2009;36:439–445. doi: 10.1016/j.amepre.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coker AL, Follingstad DR, Garcia LS, Bush HM. Partner interfering behaviors affecting cancer quality of life. Psycho-oncology. 2016 Jun 1; doi: 10.1002/pon.4157. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacMillan HL, Wathen CN, Jamieson E, et al. Screening for Intimate Partner Violence in Health Care Settings: A Randomized Trial. JAMA. 2009;302:493–501. doi: 10.1001/jama.2009.1089. [DOI] [PubMed] [Google Scholar]
- 40.McFarlane JM, Groff JY, O'Brien JA, et al. Secondary Prevention of Intimate Partner Violence: A Randomized Controlled Trial. Nurs Res. 2006;55:52–61. doi: 10.1097/00006199-200601000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Institute of Medicine. Clinical Preventive Services for Women: Closing the Gaps. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 42.Moyer VA, Force UPST. Screening for Intimate Partner Violence and Abuse of Elderly and Vulnerable Adults: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2013;158:1–28. doi: 10.7326/0003-4819-158-6-201303190-00588. [DOI] [PubMed] [Google Scholar]
- 43.McFarlane J, Malecha A, Gist J, et al. An intervention to increase safety behaviors of abused women - Results of a Randomized clinical trial. Nurs Res. 2002;51:347–354. doi: 10.1097/00006199-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Cesario SK, McFarlane J, Nava A, et al. Linking cancer and intimate partner violence: the importance of screning women in the Oncology setting. Clin J Oncol Nurs. 2013;18:65–73. doi: 10.1188/14.CJON.65-73. [DOI] [PubMed] [Google Scholar]