Abstract
Introduction
Men have higher risk for hepatocellular carcinoma (HCC) than women. Pre liver transplant (LT) alpha fetoprotein (AFP) levels strongly predict post LT HCC recurrence. Though women with HCC have higher AFP, the contribution of AFP level by gender to post LT HCC recurrence is unknown.
Material and methods
In this UNOS-based, retrospective cohort study we investigate sex differences in HCC recurrence among LT recipients with MELD exception between 2006–2010. Covariates include race, disease etiology, co-morbidities, AFP at listing and LT, tumor burden, loco-regional therapy, and donor risk index. HCC recurrence was assessed by competing risks regression.
Results
Of the eligible cohort (n = 5,002) included 3,872 men and 1,130 women. HCC recurred in 258 men (7%) and 66 women (6%). Median listing AFP was higher in women than men (14 vs. 11 ng/dL, p < 0.001). While no sex difference in overall HCC recurrence was detected (HR 0.9, 95% CI 0.7–1.2, p = 0.38), there was a strong interaction between gender and AFP on recurrence risk (p = 0.02). HCC recurrence was nearly three times higher in women (OR 4.2, 95% CI 2.2–8.2, p < 0.001) than men (OR 1.5, 95% CI 1.1–2.1, p = 0.02) with AFP at LT between 101–500 ng/dL.
Conclusion
This study reveals novel sex differences in post LT HCC recurrence, which was nearly three times higher in women than men with high AFP at LT. Pre-LT AFP levels appear to carry a different prognosis in women than men, and a subset of female LT recipients may benefit from more intensive HCC surveillance after LT.
Keywords: Liver cancer, Women, Sex differences, Liver transplant, Recurrence
INTRODUCTION
Gender differences in the incidence of hepatocellular carcinoma (HCC) are evident, with male:female ratios ranging from 2:1 to 4:1.1,2 These trends also apply to noncirrhotic patients with chronic HBV, for which HCC surveillance in this setting is recommended starting at age 40 years in Asian men compared to 50 years in Asian women.3 While men do have increased risk factors for HCC, such as tobacco and alcohol use, increased HCC risk in men may be related to male sex hormones, with androgen-receptors involved in the activation of hepatocarcinogenic genes.4
For patients transplanted for HCC, elevated alpha fetoprotein (AFP) is an established risk factor for post LT recurrence, though the cutoff values that confer increased risk of post LT recurrence vary widely.5,6 Interestingly, data from an Italian cohort noted higher AFP levels in women treated for HCC than men, although no overall survival differences were identified.7 This study included multiple modalities of HCC treatment, and was not powered to assess sex differences in outcomes among patients that underwent liver transplantation.
Although pre transplant AFP is strongly predictive of post-transplant HCC recurrence,6,8 no studies have evaluated the impact of sex differences in AFP level on risk of post-transplant HCC recurrence. Using the large United Network for Organ Sharing (UNOS) database we aimed to identify potential sex differences in AFP values at listing and LT, and to assess whether there exists sex differences in post LT HCC recurrence by pre LT AFP.
MATERIAL AND METHODS
Study population
This is a retrospective cohort study based in the United Network of Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) database. Eligible patients included men and women over 18 years of age that received a liver transplant between January 2006 and September 2010. All patients were transplanted with hepatocellular carcinoma meeting policy 3.6.4.4 criteria (Stage T2), with receipt of HCC MELD exception points. Patients undergoing re-transplantation were excluded. This study was approved by the University of California, San Francisco Institutional Review Board.
Predictors/Outcomes
AFP by gender was the primary predictor, with AFP levels at LT defined as the last available value prior to transplant. AFP was assessed as both a continuous and categorical predictor, the latter in an effort to identify AFP thresholds that may differentially predict risk of HCC recurrence risk. The primary outcome was HCC recurrence after liver transplant defined as an HCC-related death or diagnosis of HCC recurrence. Recurrences were determined by physician review (JPR) of primary and contributory causes of death and indication of recurrence in the malignancy follow-up data. This methodology was previously used for identification of recurrent HCC,9 and these data were validated to exclude systematic differences by center in under- and over-reporting HCC recurrence.10
Covariates
Covariates with biologically plausible associations with HCC recurrence were considered. These included: age at listing and transplant, race/ethnicity, disease etiology, time on the waitlist, metabolic risk factors including diabetes and body mass index (BMI), pre LT tumor burden assessed by cross sectional imaging within 3 months prior to LT, loco-regional therapy (LRT) including chemoembolization and ablation, and the donor risk index (DRI).11
Statistical analysis
Patient characteristics were compared using χ2, t and Wilcoxon rank sum tests, when appropriate. Risk of HCC recurrence by sex was assessed while accounting for competing risks by:12
Calculating the cumulative incidence of HCC recurrence and
By estimating the subhazard ratio from the Fine and Gray competing risks model.
Post-LT patient follow-up in these time-to-event analyses ended at the date of HCC recurrence (event), date of death due to other causes (competing risk), or date of last follow-up (censored). Interactions between gender and AFP were first assessed in the regression model using AFP as a continuous predictor, then at various AFP thresholds. Models were then performed separately for men and women. Analyses were performed using StataIC 11, Stata Corp, College Station, TX.
RESULTS
The eligible cohort included 1,130 women and 3,872 men transplanted with MELD exception for HCC. There were racial differences between men and women with women less likely to be Caucasian (62.6 vs. 68.9%, p < 0.001) and more likely to be African American (10.5 vs. 8.5%, p = 0.03). Women were less likely to have cirrhosis due to alcohol or hepatitis C, but more likely to have nonalcoholic steatohepatitis (NASH) than men (7.8 vs. 3.3%, p < 0.001). Women were more likely to be obese, with 15.5% of women vs. 10.0% of men having a BMI ≥ 35 (p < 0.001). Longer waitlist time of > 120 days was also more common in women than men (37.3 vs. 33.7%, p = 0.02). Median AFP at LT was higher in women (14 ng/dL, IQR 6–60) than men (11 ng/dL, IQR 5–41), p < 0.001, and women were more likely to have AFP levels > 100 ng/dL at listing (21.4 vs. 16.2%, p < 0.001) and at time of LT (18.9 vs. 14.4%, p < 0.001). Despite higher median AFP, women were more likely than men to small tumors, with 14.3% of women vs. 12.1% of men having lesions < 2 cm (p = 0.04) (Table 1).
Table 1.
Cohort characteristics.
| Variable | Men (n = 3,872) | Women (n = 1,130) | p value |
|---|---|---|---|
| Mean age at LT (±SD) | 57.0 (6.9) | 58.6 (7.7) | < 0.001 |
| Race, n (%): | |||
| Caucasian | 2,666 (68.9) | 707 (62.6) | < 0.001 |
| African American (AA) | 328 (8.5) | 119 (10.5) | 0.03 |
| Hispanic | 517 (13.4) | 167 (14.8) | 0.2 |
| Asian | 322 (8.3) | 113 (10) | 0.08 |
| Diabetes, n (%): | 1,084 (28.3) | 308 (27.3) | 0.51 |
| Body mass index, n (%): | |||
| < 25 | 965 (24.9) | 357 (31.6) | < 0.001 |
| 25–29.9 | 1,627 (42) | 358 (31.7) | < 0.001 |
| 30–34.9 | 890 (23.0) | 233 (20.6) | 0.1 |
| ≥ 35 | 389 (10.1) | 180 (16.0) | < 0.001 |
| Primary disease etiology, n (%): | |||
| Alcohol | 367 (9.5) | 55 (4.9) | < 0.001 |
| HBV | 242 (6.3) | 48 (4.2) | 0.01 |
| HCV | 2,464 (63.9) | 634 (56.1) | < 0.001 |
| NASH | 127 (3.3) | 88 (7.8) | < 0.001 |
| Days on waitlist > 120 days, n (%): | 1,303 (33.7) | 421 (37.3) | 0.02 |
| Tumor Burden at LT, n (%): | |||
| > 1 tumor all < 2cm | 468 (12.1) | 162 (14.3) | 0.04 |
| 1 tumor ≥ 2cm | 3,165 (81.7) | 912 (80.7) | 0.43 |
| 2–3 tumors ≥ 2cm | 239 (6.2) | 56 (5.0) | 0.13 |
| AFP at listing, n (%): | |||
| ≤ 100 | 3,245 (83.8) | 888 (78.6) | < 0.001 |
| 101–500 | 427 (11.0) | 149 (13.2) | 0.046 |
| > 500 | 200 (5.2) | 93 (8.2) | < 0.001 |
| Last available AFP prior to LT, n (%): | |||
| ≤ 100 | 3,307 (85.4) | 916 (81.1) | < 0.001 |
| 101–500 | 384 (9.9) | 144 (12.7) | 0.006 |
| > 500 | 181 (4.7) | 70 (6.2) | 0.04 |
| Locoregional therapy, n (%): | 2,162 (55.8) | 618 (54.7) | 0.5 |
| Donor Risk Index, median (IQR) | 1.5 (1.1–1.7) | 1.4 (1.2–1.8) | < 0.001 |
Median post LT follow-up for women and men was 750 and 754 days, respectively. During this time, HCC recurred in a total of 66 (5.8%) women and 258 (6.7%) men resulting in similar 5-year cumulative incidence of recurrence of 7.4% (95% CI 5.7–9.4) and 9.1% (95% CI 7.9–10.4), respectively, (p = 0.38). In patients with AFP at LT ≤ 100 ng/dL, the 5-year post LT cumulative incidence of HCC recurrence in women was 6.0% (95% CI 4.3–8.2), or n = 40 women compared to 8.3% (95% CI 6.9–9.8), or n = 186 men (p = 0.15). However, in patients with AFP at LT between 101–500 ng/dL the recurrence rate in women was 17.0% (95% CI 11.0–24.2), or n = 22 compared to 9.9% (95% CI 7.0–13.5), or n = 34 men (p = 0.03). Evaluation of sex differences in HCC recurrence for AFP > 500 ng/dL at LT was not performed due to the limited number of recurrence events (n = 4) in these women.
On multivariate analysis of HCC recurrence, adjusting for race, disease etiology, waitlist time, tumor size and number, and LRT, there was a significant interaction between gender and continuous levels of AFP at LT (p < 0.001). AFP cutoffs that may differentially predict post LT recurrence in men and women were therefore explored. The greatest difference in recurrence risk by sex was identified at AFP levels of 101–500 ng/dL compared to < 100 at LT (Figure 1). HCC recurrence risk was nearly three times higher in women (OR 4.2, 95% CI 2.2–8.2, p < 0.001) than men (OR 1.5, 95% CI 1.1–2.1, p = 0.02), among recipients with AFP at LT between 101–500 ng/dL compared to ≤ 100 (Table 2).
Figure 1.
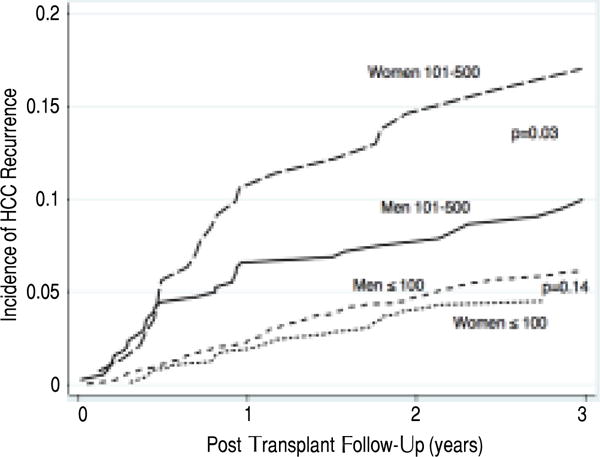
Increased risk of post LT HCC recurrence in women compared to men with AFP at LT between 101–500 ng/dL.
Table 2.
Multivariate analysis of factors associated with post LT HCC recurrence in men and women.
| Covariate | Men HR (95% CI) | p value | Women HR (95% CI) | p value |
|---|---|---|---|---|
| African American (AA) vs. non-AA | 0.77 (0.48–1.24) | 0.28 | 0.20 (0.03–1.44) | 0.11 |
| Alcohol vs. hepatitis C (HCV)* | 0.19 (0.07–0.52) | 0.001 | 0.97 (0.29–3.22) | 0.96 |
| Waitlist time > 120 days | 0.53 (0.33–0.86) | 0.01 | 0.52 (0.23–1.21) | 0.13 |
| Tumor Burden at LT (ref > 1 tumor, all < 2 cm): | ||||
| 1 tumor ≥ 2 cm | 1.45 (0.96–2.18) | 0.08 | 2.33 (0.93–5.9) | 0.07 |
| 2–3 tumors ≥ 2 cm | 1.62 (0.89–2.95) | 0.12 | 2.43 (0.65–9.11) | 0.19 |
| Loco-regional therapy (LRT) vs.no LRT | 1.24 (0.96–1.61) | 0.09 | 2.28 (1.32–3.94) | 0.003 |
| Pre LT AFP 101–500 vs. ≤ 100 | 1.50 (1.06–2.13) | 0.02 | 4.21 (2.17–8.18) | < 0.001 |
All disease etiologies were includedin the model using HCV as reference, with risk difference only identifiedin alcohol versus HCVamong men.
The mechanism leading to differential AFP levels in men and women is unknown. Therefore as an exploratory, ad hoc analysis, we sought to assess the potential influence of hormonal status in women on the observed sex differences in AFP by comparing median AFP before and after ages 45 and 50 years as surrogates of menopausal age. The 50 year age cutoff was selected based on the mean menopausal age of approximately 51 years in the United States,13 and a lower age cutoff of 45 years was also investigated due to known pre-mature cessation of menses in women with chronic liver disease.14,15 Median AFP was higher in women over 45 years (15 vs. 6 ng/dL, p = 0.03) with no differences in men at this age cutoff (13 vs. 12 ng/dL, p = 0.7). There were no significant differences detected in median AFP in men or women above or below age 50 years (p values = 0.7).
DISCUSSION
The current study is an exploratory analysis revealing novel sex differences in the risk of post-transplant HCC recurrence by AFP at LT. Although no sex differences were identified for overall recurrence, HCC recurrence was nearly three times higher in women than men among recipients with high AFP at LT (101–500 ng/dL). Importantly, this finding cannot be attributed to spurious associations related to multiple comparisons, as significant sex differences in HCC recurrence risk were apparent in models assessing AFP as a continuous measure.
In a large Italian cohort, Faranati, et al. found no significant impact of gender on HCC-related outcomes.7 Unlike the current study, modalities of HCC treatment were considered, and the specific risk of transplant-related events was not investigated. Similar to our findings, these authors did identify higher AFP values in women compared to men with HCC. The mechanism behind the observed differences in AFP by gender is not clear, though may relate to differential sex hormone profiles. Interestingly, in our post-hoc analysis on reproductive age, women over 45 years (compared to less than 45) actually had higher AFP values, arguing against a clear-cut association between reproductive age and AFP that could be supported by these data. This exploratory analysis was not powered to assess the effect of more extreme age cutoffs, such as median AFP levels in women < 40 years versus > 50–55 years for example, which could reveal more pronounced AFP differences. Granular data including sex hormone levels would help to clarify whether increased estrogens, or conversely, lower levels of androgens, may be associated with pre transplant AFP level.
There is growing awareness of the unique risk of HCC in patients with non-alcoholic steatohepatitis (NASH). Unlike most chronic liver diseases, for which HCC uncommonly develops in the absence of cirrhosis, up to 50% of NASH-related HCC develops in patients with earlier stages of hepatic fibrosis.16,17 Features of the metabolic syndrome, such as insulin resistance and obesity, are common in NASH, and are known to be independent risk factors of HCC.18,19 Purported mechanisms linking metabolic risk factors with HCC include increased reactive oxygen species, increased free fatty acid deposition in the liver, and subsequent activation of cytokines involved in hepatic cell apoptosis and regeneration.20 In the current study we did find that women with HCC were more likely to have NASH as compared to men. Though history of diabetes was similar between sexes, women were more likely to have BMI ≥ 35. Too few patients in the current study were transplanted for NASH cirrhosis to assess for gender differences in HCC recurrence within that population. Dedicated NASH cohorts may help to clarify sex differences in risk of HCC recurrence in these patients, and to further assess potential compounding effects of metabolic syndrome in men and women.
We identified marked interactions between gender and AFP on risk of post LT HCC recurrence, although causation cannot be established. We were constrained by lack of explant data, and future studies investigating gender differences in HCC would benefit from correlation between pre LT AFP, explant pathology, and recurrence events in men and women. There were few recurrence events in women transplanted with AFP at LT of > 500 ng/dL, although the robust data on recurrence events in women transplanted with AFP levels between 101–500 ng/dL enabled meaningful analyses of gender differences in this group. Though exploratory in nature, these data reveal novel sex differences in risk of post LT recurrence by AFP in men and women and provide a basis for further investigation into the implications of AFP by sex on tumor biology.
In conclusion, this study shows that women transplanted with HCC have higher overall AFP levels than men, as well as higher likelihood of having elevated AFP levels of ≥ 100 and > 500 ng/dL prior to transplant. While no sex differences were identified in overall risk of HCC recurrence, marked sex differences were identified among recipients with AFP levels between 101–500 ng/dL, with risk of HCC recurrence nearly three times higher in women than men. Pre-LT elevated AFP levels appear to carry a different prognosis in women than men, and a subset of female LT recipients may benefit from more intensive HCC surveillance after LT.
ABBREVIATIONS
- AFP
alpha fetoprotein
- BMI
body mass index
- DRI
donor risk index
- HCC
hepatocellular carcinoma
- LRT
loco-regional therapy
- LT
liver transplant
- NASH
non alcoholic steatohepatitis
- UNOS
United Network of Organ Sharing
References
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar M, Watt KD, Terrault N, Berenguer M. Outcomes in liver transplantation: does sex matter? J Hepatol. 2015;62:946–55. doi: 10.1016/j.jhep.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng H, Cheng AS, Tsang DP, Li MS, Go MY, Cheung YS, Zhao GJ, et al. Cell cycle-related kinase is a direct androgen receptor-regulated gene that drives beta-catenin/T cell factor-dependent hepatocarcinogenesis. J Clin Invest. 2011;121:3159–75. doi: 10.1172/JCI45967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945–51. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987–99. doi: 10.1111/j.1365-2036.2012.05060.x. [DOI] [PubMed] [Google Scholar]
- 7.Farinati F, Sergio A, Giacomin A, Di Nolfo MA, Del Poggio P, Benvegnu L, Rapaccini G, et al. Is female sex a significant favorable prognostic factor in hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2009;21:1212–8. doi: 10.1097/MEG.0b013e32831a86f8. [DOI] [PubMed] [Google Scholar]
- 8.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–94 e3. doi: 10.1053/j.gastro.2012.05.052. quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 9.Samoylova ML, Dodge JL, Yao FY, Roberts JP. Time to transplantation as a predictor of hepatocellular carcinoma recurrence after liver transplantation. Liver Transpl. 2014;20:937–44. doi: 10.1002/lt.23902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samoylova ML, Dodge JL, Vittinghoff E, Yao FY, Roberts JP. Validating posttransplant hepatocellular carcinoma recurrence data in the United Network for Organ Sharing database. Liver Transpl. 2013;19:1318–23. doi: 10.1002/lt.23735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783–90. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 12.Fine J, Gray R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 13.National Institutes of Health. 2015 Available at: URL. [Google Scholar]
- 14.Cieloszyk K, Hartel D, Moskaleva G, Schoenbaum EE. Effects of hepatitis C virus infection on menopause status and symptoms. Menopause. 2009;16:401–6. doi: 10.1097/gme.0b013e318186d7cf. [DOI] [PubMed] [Google Scholar]
- 15.Valimaki M, Pelkonen R, Salaspuro M, Harkonen M, Hirvonen E, Ylikahri R. Sex hormones in amenorrheic women with alcoholic liver disease. J Clin Endocrinol Metab. 1984;59:133–8. doi: 10.1210/jcem-59-1-133. [DOI] [PubMed] [Google Scholar]
- 16.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, et al. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–33. doi: 10.1016/j.cgh.2011.01.023. quiz e50. [DOI] [PubMed] [Google Scholar]
- 17.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–73. doi: 10.1001/jama.2015.5370. [DOI] [PubMed] [Google Scholar]
- 18.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 19.El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–8. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 20.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]


