Abstract
Current strategies for engineering cardiovascular cells and tissues have yielded a variety of sophisticated tools for studying disease mechanisms, for development of drug therapies, and for fabrication of tissue equivalents that may have application in future clinical use. These efforts are motivated by the need to extend traditional two-dimensional (2D) cell culture systems into 3D to more accurately replicate in vivo cell and tissue function of cardiovascular structures. Developments in microscale devices and bioprinted 3D tissues are beginning to supplant traditional 2D cell cultures and pre-clinical animal studies that have historically been the standard for drug and tissue development. These new approaches lend themselves to patient-specific diagnostics, therapeutics, and tissue regeneration. The emergence of these technologies also carries technical challenges to be met before traditional cell culture and animal testing become obsolete. Successful development and validation of 3D human tissue constructs will provide powerful new paradigms for more cost effective and timely translation of cardiovascular tissue equivalents.
Keywords: Heart, Tissue Engineering, Stem Cells, Biomaterials, 3D Printing
Introduction
At the intersection of stem cell biology and tissue engineering resides enormous potential for patient-specific drug screening, disease modeling, and tissue equivalents that offer hope to treat some of the most devastating cardiovascular diseases. Tissue engineering comprises the optimization of three primary components: (i) the type or types of cells being implanted such as somatic cells, induced-pluripotent stem cells or embryonic stem cells derived cells, adult stem cells, cardiac progenitor cells), (ii) type of scaffolds supporting the cells (i.e. the mechanical cues provided to the cells), and (iii) type of small molecules, extra-cellular matrix (ECM), and growth factors conditioning the cells, (i.e. the chemical cues provided to the cells). In addition, the conditions (e.g. fluid flow, oxygenation, temperature) in which the construct is cultured can have a significant impact on its maturation, making the development of novel bioreactors a major part of tissue engineering. Bioreactors can be used to aid in the in vitro development of new tissue by providing biochemical and physical regulatory signals to cells and encourage differentiation and/or production of extracellular matrix prior to in vivo implantation1. Bioreactor technology is integral to the emergence of microfluidic lab-on-a-chip, organs-on-chips, and bioprinted 3D tissues. These techniques are emerging as a supplement to traditional 2D cell cultures and pre-clinical animal testing as the standard for drug and tissue development. This review summarizes the bioengineering basis for these technologies and how they are shaping the future of cardiovascular tissue engineering. We begin our review by defining microfluidic chip technology and its application to studying some basic mechanisms governing behavior of cardiovascular cells. We briefly discuss 3D organs-on-chips. We then focus on mainly on 3D tissue printing methods and the relationship to cardiovascular bioprinting with an emphasis on the fabrication of vascular networks. We further discuss bioprinting using cell spheroids and methods to manipulate spheroids to produce their own extracellular matrix without the use of natural or synthetic polymer scaffolding. Finally, we summarize what is already possible with these technologies and their limits as compared with more traditional cardiovascular tissue engineering methods.
3D On-Chip Technologies
During embryonic development, the fate specification of stem cells differentiation is regulated by the 3D microenvironment, in which not only a variety of biochemical factors, but also the biophysical signals are presented within the 3D extracellular matrices. These seamless signaling pathways, and spatial, temporal factors together dictate stem cell differentiation and maturation2.
Why 3D is Outpacing 2D in Cell Culture Technologies
Cell cultures were developed in the first half of the 20th century by Ross Harrison3. Despite the significant contributions and their demonstrated value in biomedical research, they are unable to recapitulate the tissue-specific functions of many differentiated cell types or accurately predict the in vivo effects of drugs. These limitations prompted development of more complex two-dimensional (2D) tissue culture models, such as those that incorporate multiple cell types or involve cell patterning. In the case of cardiomyocytes, paracrine signals from endoderm-like cells, endothelial, cardiac fibroblasts and other stromal cell types have been shown to support normal physiology and maturation of cardiomyocytes. Similarly, patterning of cell adhesion molecules or fabricating channels of appropriate microgeometry can promote cardiomyocyte function and alignment. However three-dimensional (3D) models are rapidly gaining favor as they have the capacity to better represent the structural and functional complexity of living tissues (Figure 1). The cost-benefit analysis of 3D versus 2D approaches for cardiovascular tissue engineering includes consideration of cell-cell and cell-matrix interactions, the ability to modulate culture stiffness to mimic that of the native heart with development or disease, the capacity to impose mechanical and electrical stimulation akin to that experienced in the heart, and the inclusion of perfusable vasculature to carry not only nutrients, but also relevant cytokines and other signaling molecules (Table 1, and 4). As one pertinent example, a recent study showed that cardiomyocytes maintained in 3D hydrogels composed of fibrin exhibit higher conduction velocities, longer sarcomeres and enhanced expression of genes involved in contractile function than 2D monolayers matched in age and purity of myocytes. For this reason, many 3D model systems for cardiomyocyte culture have emerged with the goal of optimizing scaffold formulation, supporting cell content, and electromechanical stimuli to promote cardiomyocyte maturation. The 3D models in use today, often termed “engineered heart tissue”, are more suitable than conventional or 2D cultures for studying the molecular basis of cardiac function and represent better disease models for studying signaling pathways and drug responsiveness (Figure 2). In 3D cultures, cells can be exposed to normal physical factors, such as mechanical tension/stress, compression or fluid shear stress, which affect tissue architecture, organ development and function. The absence of fluid flow in 2D tissue models also precludes the study of the interaction of cultured cells with circulating perfusion or the cytokines released.
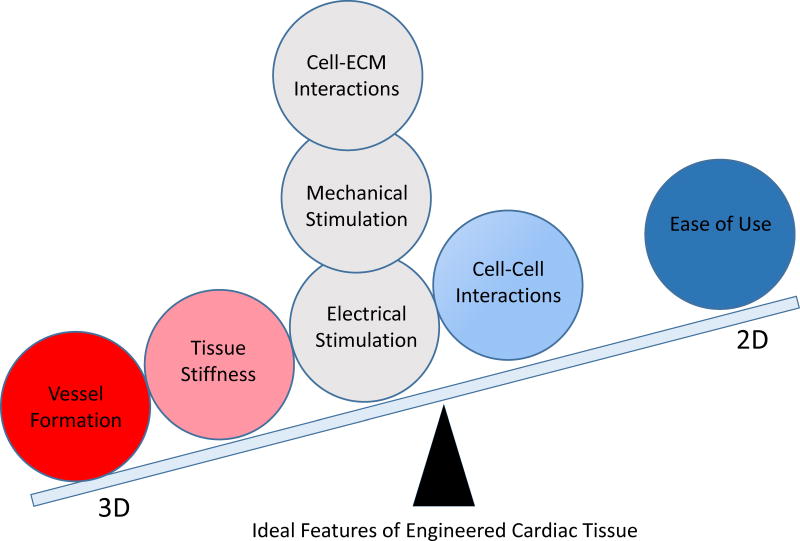
Figure 1.
Utility of the 3D relative to the 2D formats for cardiovascular tissue engineering applications. Red circle indicates the feature only feasible in 3D. Pink, gray and blue circles and their corresponding positions represent features compatible with both 2D and 3D systems, but more ideally achieved in the formats in closest proximity. Note, the overwhelming majority of ideal feature are best achieved in 3D and typically result in a more anatomic and physiologic representation of cardiac tissues. In particular, action potential, abundance of sarcomeric and sarcoplasmic proteins, quality of Frank-Starling behavior, force-frequency relationship, reaction to calcium, isoprenaline and carbachol have been found to be more akin to tissue response when assessed in 3D format.
Table 1.
Comparison of 3D and 2D Cardiac Culture Systems
| FEATURE | 3D Cardiovascular Tissues vs 2D Systems | |
|---|---|---|
| Implementation | Advantage | |
| Cell-Cell and Cell Matrix Interactions | Incorporation of multiple supportive cell types and formulations of extracellular matrix proteins | Feature can be tuned to reflect attributes of the native CV system with receptor engagement on all cell surfaces |
| Tissue Stiffness | Various biomaterials and associated processing steps can be employed to control tissue stiffness | Feature can be tuned to reflect that of developing, healthy or diseased tissue |
| Mechanical Strain | Strains can be imposed that allow auxotonic contraction | Feature supports alignment and maturation of cardiomyocytes |
| Electrical Stimulation | 3D engineered tissues can be paced at constant or increasing frequency | Feature supports alignment and maturation of cardiomyocytes |
| Vascularization | Only possible in 3D and being implemented in a range of forms | Feature provides nutrient perfusion and paracrine functions vital for cardiac homeostasis |
| Intercellular Electrical Coupling (Analytic) | Can be assessed by calcium handling or reporters for gap junctions in combination with confocal or mutiphoton laser scanning microscopy | Electrical and structural coupling are more accurately assessed in 3D where the coordinated effect of multiple Z planes can be included |
| Mature Cardiac Cell Function (Analytic) | Can be assessed by quality of physiological and pharmacological responses | Action potential, abundance of sarcomeric and sarcoplasmic proteins, quality of Frank-Starling behavior, force-frequency relationship, reaction to calcium, isoprenaline, carbachol found to be more akin to tissue response |
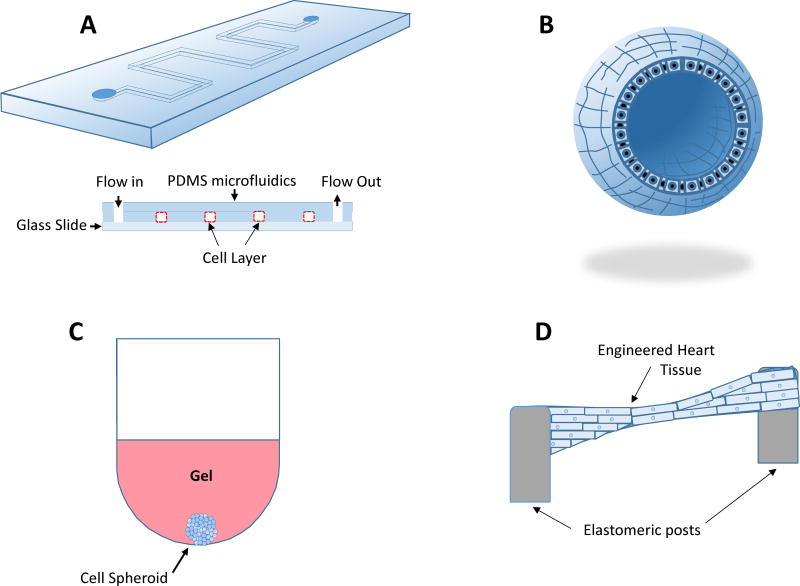
Figure 2.
In vitro testing of cells and tissues may occur in several ways. Microfluidic systems (A) have emerged as a tool for basic science studies of the effect of highly controlled fluid mechanical and solid mechanical forces on single cell types or co-cultures. Microfluidic systems are also gaining favor as a diagnostic tool and a platform for drug development. Organoid cultures (B) are described as organ buds grown in culture that feature realistic microanatomy and are useful as cellular models of human disease. These cultures have found utility in the study of basic mechanisms of organ-specific diseases. Spheroid cultures (C) feature sphere-shaped clusters of a single cell type or co-culture sustained in a gel or a bioreactor in order to interact with their 3D surroundings and are useful in testing drug efficacy and toxicity. (D) Engineered heart tissues are constructed by polymerizing an extracellular matrix-based gel containing cardiac cell types between two elastomeric posts or similar structures allowing auxotonic contraction of cardiomyocytes. This allows to mimic the normal conditions of the heart contracting against the hydrostatic pressure imposed by the circulation. This type of tissue construct has been used for testing toxicity of drugs and basic studies of muscle function and interplay between multiple cardiac cell types.
Microscale Devices: Transition to 3D Platforms
More recently, microscale devices have emerged to recapitulate some functional properties and features of minimal functional units of tissues of organs. These devices, sometimes called organs-on-chips, can harbor channels or reservoirs in which hydrogels support multiple cell types and are typically perfused by microfluidic vascular conduits designed to model human physiology in vitro. An organ-on-a-chip is a microfluidic cell culture device created with microchip manufacturing methods that contains continuously perfused chambers inhabited by living cells arranged to simulate tissue- and organ-level physiology44. These platforms provide a useful alternative to macroscale 3D model systems as they allow scaling down of reagents and cells and are also easily amenable to continuous perfusion, drug dosing and imposition of mechanical and electrical stimulation5–7,
Manufacturing of microscale devices often involves a replica molding technique wherein a liquid polydimethylsiloxane (PDMS) pre-polymer is cast into the mold and peeled off following polymerization. Critical to the success of microscale devices in this context is consideration of the channel or reservoir material. For decades polydimethylsiloxane (PDMS) was used as support material, but this material suffers two primary limitations: 1) evaporation due to permeability to water vapor, and 2) bulk absorption of hydrophobic components including protein8. To avoid these drawbacks, polystyrene, cyclo-olefin copolymer and Teflon have been implemented recently with success.
Microscale devices enable modeling and analyses of a whole variety of physiological processes. Such analyses are often not feasible for static 3D cultures or bioreactors. Another important advantage of the organ-on-chip devices is the potential to control cell patterning. Different cell types can be plated in the microchannel in distinct patterns or in direct juxtaposition on the same planar substrate. Tissue-tissue interfaces can be engineered by culturing two different cell types on opposite sides of a permeable (porous) membrane to model universal interactions between a vascular endothelium and parenchymal tissues. Furthermore, electromagnetic fields applied to chip-based tissue models, are capable of stimulating wound healing or contractile movements (pacing) of muscle tissues9.
Finally, integration of electronic microsensors, constructed by using microchip fabrication technologies, enables monitoring cell migration, fluid pressure or other factors of the artificial tissue/organ microenvironment. Modulation of flow rates or microfluidic channel sizes enables monitoring fluid shear stresses independently of physical and chemical gradients.
Organs-on-Chips
Utilization of induced pluripotent stem cells (iPSCs) for organs-on-chips and 3D tissue engineering holds potential for organ modeling in disease- as well as in a patient-specific manner. Studies using these cells may lead to the development of personalized ‘humans-on-chips’ systems with all cellular components derived from a patient. Over the past decade researchers have constructed organ-on-chip model systems for studying functions of different organs including kidney10–13, intestine14, 15, lung,16–18, liver19–23, heart9, 24, 25, smooth and striated muscle tissue26, fat27–29, bone30, marrow29, 31, cornea32, skin33, blood vessels 34–36, nerves37, 38 and even blood-brain barrier39, 40. However, due to the single cell type composition, many of the above systems cannot be considered organ models. Nonetheless, fluid flow and shear stress alone have been demonstrated by some of those microchip systems to have a profound structural and functional impact on cells. In addition, oxygen pressure variations applied to organs-on-chips allowed better recapitulation of hypoxia-caused diseases such as myocardial ischemia24 or vaso-occlusion in sickle-cell disease41 and facilitated drug screening.
The feasibility of integration of muscular cell layers into microfluidic chips9 or confined to microscale pillars opened perspectives for examining the contribution of fluid flow, tissue-tissue interactions, as well as mechanical and electrical signals to the development of cardiovascular diseases. A simple microfluidic model of cardiac ischemia/perfusion injury has been created by culturing primary porcine cardiomyocytes under variable oxygenation to mimic hypoxic conditions or hypoxia/normoxia transition24, 42.
Microscale devices with gas control and oxygen monitoring functionalities have also been used for culturing cardiac tissue and physical stimulation of cardiomyocyte contractile function43. Those cardiac model systems have been used for assaying loss of membrane potential and cytochrome C release as an early manifestation of apoptosis typically observed following ischemia reperfusion. This chip and related approaches have proven to be useful tools for inducing ischemia/reperfusion injury in primary cardiomyocytes and for determining the kinetics of apoptosis with cardiomyocyte loss24, 44.
Multiple on-chip models of angiogenesis and microvascular function have been reported. Those were primarily composed of microfluidically ported microchannels permeating an ECM stroma to create functional capillary networks with free sprouting potential in response to soluble gradients of angiogenic factors. The use of ECM to embed internal networks of microchannels filled with sacrificial materials, enabled independent cell seeding in either the channels (endothelial cells) or surrounding ECM (tumor cells, fibroblasts etc). Once functionally integrated into the existing vascular network, the newly-formed micro-vessels were perfused by connection to external flow via a gasket that also served to house the 3D ECM. Such models of angiogenesis allowed studying 3D morphogenetic processes, including the functional mechanism of angiogenesis inhibitors, and helped our understanding of how spatial diffusive gradients influence angiogenic sprouting45–47.
A more complex model of vascular networks, reported recently, was based on an electrical circuit design and involved an array of nearly identical human microtissues with interconnected vascular networks. The authors applied resistive circuit concepts to design pressure dividers in serially-connected micro-tissue chambers, thereby creating a controlled micro-physiological environment within fibrin scaffold-containing micro-chambers. This methodology enabled culturing a large array of micro-tissues with interconnected vascular networks for biological studies and applications such as drug development48.
Lining a fibronectin-coated polycarbonate membrane with human brain microvascular endothelium on one side and human astrocytes on the other allowed development of a human blood-brain-barrier-on-a-chip40. Another complex microfluidic system was developed as a neurovascular model of neuroinflammation by lining a porous membrane with rat brain microvascular endothelial cells on one side and a mixture of astrocytes, neurons and microglia, on the other. The cultured neural cells showed capability of building inhibitory and excitatory potentials, while engineered endothelium retained good barrier function and activated the adjacent microglia and astrocytes via TNF-α, analogous to neuroinfectious disease49.
3D Printing approach in tissue and organ engineering
To date, 3D printing techniques have been primarily used for fabrication of acellular 3D scaffolds and molds,50, 51 that in some cases could be subsequently filled up with live cells. A more advanced strategy of 3D printing, known as bioprinting, that allows printing tissue constructs by direct deposition of cells or cell aggregates, has also recently been explored52–54.
To reproduce structural architecture and functional characteristics of a natural tissue and ultimately whole organs with high degree of accuracy, engineered constructs have to embody several key components such as cells, ECM and vascular networks, which have to be assembled together with precise 3D-patterning using contemporary bioink deposition technologies. Perhaps the most important of the above components is the vasculature that provides nutrients, signaling molecules/factors and efficient clearance/excretion of metabolites (waste transport) to matrix-seeded cells. 3D tissue constructs without adequate vascularization quickly develop necrotic regions within a few hundred microns of the boundaries/edges of the construct55.
Bioprinting technology allows fabrication of biomimetic and even anatomical 3D structures by using patients’ images obtained using medical imaging technologies, e.g. computer tomography (CT) and magnetic resonance imaging (MRI). As one of the most advanced tissue/organ fabrication technologies, 3D printing employs automated processes and standardized materials as building blocks and enables creation of 3D objects from personalized computer-aided designs. Three dimensional printing, also referred to as additive manufacturing (AM) or solid free form fabrication has already been utilized by cardiovascular surgeons to fabricate personalized organ models for visualization of anatomical structures56. Personalized bioprinted models can better reflect structural abnormalities than traditional models or cadavers, and as a result can improve the choice of surgical approach and offer a platform to practice the procedures.
To precisely recapitulate complex objects 3D bioprinting technology utilizes a computer generated 3D design file created by virtually decomposing the shape of the object (obtained through medical imaging) into a series of 2D layers. The 3D bioprinter deposits bioinks (building blocks containing cells/biomaterials mixture or spheroids) in a layer-by-layer manner based on the design file. Each layer is then bonded to the previous layer to fabricate the 3D constructs. The layer-by layer bioprinting can be accomplished by different methods depending on the type of printed material57, 58.
There are four methods of 3D printing compatible with biopolymers typically used for generating scaffolds and/or ECM in tissue engineering applications: (i) selective laser sintering (SLS)59, (ii) stereolithography (SLA)60, (iii) fused deposition modeling (FDM)61, and (iv) pressure-based extrusion (PBE).52, 62 In brief, SLS uses a CO2 laser to locally “melt” a thin layer of powder to fuse particles into a solid object. SLA utilizes UV or visible light to trigger polymerization of a thin layer or a small focal volume of a photocrosslinkable resin-containing solution. FDM melts and extrudes a polymer through a nozzle onto a flat substrate to build up a 3D structure. PBE is based on a differential pressure, generated by a syringe pump or an upstream pressure reservoir, to drive the material through a nozzle. While SLS and SLA are typically faster printing methods than FDM and PBE, they both require expensive lasers and optics. SLS printing might require higher temperatures and thus is ideal for ceramics or metals, but could be prohibitive for bioprinting applications. SLA, although fast and straightforward, requires materials (typically plastics) compatible with photocurable chemistries. Although both FDM and PBE represent potentially simpler systems, they can be slower compared to other bioprinting methods used for cardiac tissue engineering.
3D printing for tissue engineering applications has the flexibility to use inks with or without cells. In the former case, scaffolds of complex geometries should be printed using only biocompatible materials. The technology of engineering artificial tissues by directly encapsulating cells as part of the ink (called ‘bioink’) during the printing process is known as bioprinting63, 64. While 3D printing, using routine methods for industrial applications, allows a direct use of commercial printers without modifications, bioprinting technologies may not be compatible with commercial printer use and would require custom-built printing devices and bio-compatible ink materials. Bioinks comprising cells suspended in hydrogels that serve as ECM, are currently being developed and used in cardiovascular tissue engineering to directly print implants in the form of myocardial tissue, heart valves, and coronary arteries (see below). Furthermore, 3D printing has the ability to integrate electronics into tissue-engineered constructs to provide additional functionality, such as sensing and actuation65–68.
The main technologies used for deposition and patterning of biological materials include laser-assisted printing, multiphoton excitation-based fabrication, inkjet printing and micro-extrusion (Table 2, Figure 3). The features of these technologies should be discussed in conjunction with the most important factors in 3D bioprinting, such as feature resolution, cell viability, and the biological materials used for printing69 (summarized in Table 2).
Table 2.
Features of the Current 3D Bioprinting Approaches
| Feature | 3D BIOPRINTING APPROACHES | |||
|---|---|---|---|---|
| Laser-Assisted | Multiphoton-Excitation | Inkjet | Micro-Extrusion | |
| Resolution | High | Very High | Medium | Low |
| Droplet size | > 20 μm | 300 nm – 3 μm | 50 – 300 μm | 100 μm – 1 mm |
| Printer speed | Medium (200–1600 μm/s) | Slow (1 mm2/hour) | Fast (1–10,000 droplets/second) | Medium (10–1000 μm/s) |
| Cell viability | High | Low | Medium | High |
| Cost | High | High | Low | Medium |
| Primary Advantage(s) | Single cell manipulation, no clogging associated with nozzles, wide viscosity range | Can print ECM exclusively, not dependent on high viscosity of bioink | Gradients can be generated by altering droplet size, low cost | High cell density can be used |
| Primary Disadvantage(s) | High cost, time consuming, technically challenging | Cells cannot be deposited with printing, end product mm small scale | Nozzle clogging, low droplet directionality | Limited number of biomaterials used to date |
| References | 70, 71 | 72 | 87–90 | 91 |
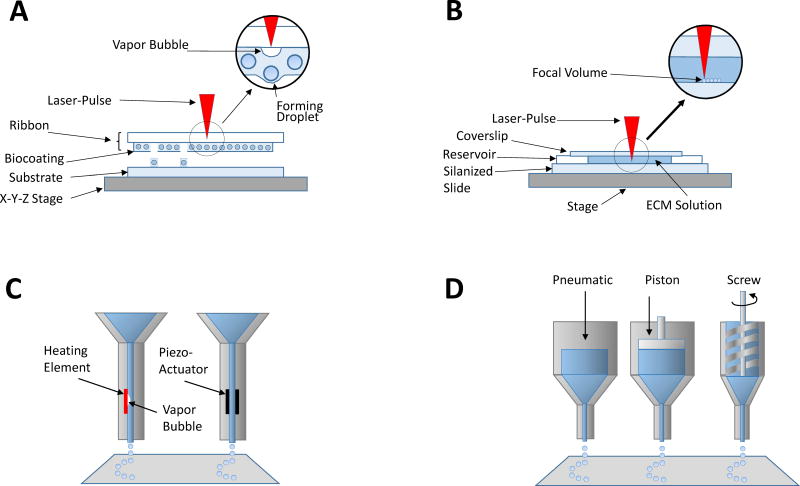
Figure 3.
Bioprinting is usually accomplished using a combination of gel and cells. Laser assisted bioprinting (A) using Laser-induced forward transfer (LIFT) relies on the focused energy of a laser onto an energy absorbing ribbon to induce bioink droplet formation. This technique is advantageous because it avoids the problem of clogging of the bioink nozzle that plagues other bioprinting techniques. Multiphoton excitation-based printing (B), is accomplished via photocrosslinking of proteins or polymers in the focal volume of the laser and excels in its high resolution and ability to polymerize many native proteins that do not form hydrogels spontaneously outside the body. Inkjet printing (C), one of the most common printing techniques, relies upon a vapor bubble or a piezoelectric actuator to displace material to extrude the bioink from a nozzle. Robotic dispensing (D) employs other mechanical means of displacing bioink under robotic control.
Laser-assisted bioprinting
Laser-assisted bioprinting (LAB) is based on laser-induced forward transfer (LIFT)70, 71 of the printed material/ink. Initially developed to transfer metals, LIFT technology has been successfully applied to biological material, such as peptides, DNA and cells. Although being less common than inkjet or micro-extrusion bioprinting, LAB is often used for tissue- and organ-engineering applications (Figure 3A). LAB functions using focused laser pulses on the absorbing layer of a ribbon, that has a donor transport support usually made from glass that is covered with a laser-energy-absorbing layer (e.g., gold or titanium), to generate a high-pressure bubble propelling a cell-containing material toward the collector substrate. The important advantage of LAB devices is that they do not use nozzle, which avoids clogging with cells or materials, the problem limiting performance of other bioprinting technologies. In addition, LAB is compatible with a range of viscosities (1–300 mPa/s) and can print mammalian cells with negligible effect on cell viability and function. LAB can deposit cells at high density (up to 108 cells/ml) with microscale resolution (1 cell per drop, minimum drop size is approximately 20 μm) and 5 kHz laser pulse repetition rate with overall printing speeds up to 16 cm/s. However, high LAB resolution requires fast ink gelation kinetics, which is hard to achieve, and practically leads to a relatively low overall flow rate. Besides, preparation of each individual ribbon, often required for each printed cell or hydrogel type, is time-consuming, costly and may be technically challenging for depositing multiple cell types. Accurate targeting and positioning cells can be difficult owing to the nature of the ribbon cell coating and may require cell-recognition scanning technology to enable the laser beam to select a single cell per pulse or using a ribbon with very high cell concentrations. The high cost of LAP systems is also a concern for basic tissue engineering research, although as is the case with most 3D printing technologies, these costs are rapidly decreasing (Table 2).
Multiphoton excitation (MPE)-based fabrication
Multiphoton excited (MPE) photochemistry can also be used to 3D print synthetic materials and native proteins72–74. This method is analogous to multiphoton laser scanning microscopy (MPLSM) in that the excitation, and thus the photochemistry, is restricted to the focal volume75–80 (Figure 3B). Previously, MPE fabrication technology was shown to crosslink layer-by-layer soluble and structural proteins into 3D matrices and fiber patterns with spatial fidelity of >85%. The crucial component of this approach was implementation of a new photochemistry81 allowing efficient crosslinking of all types of collagen (e.g. type I, II, and IV), laminin, fibronectin and other proteins with limited solubility82 into 3D structures without the use of synthetic polymers. Extensive studies have characterized several scaffold materials and investigated stem cell-ECM interactions within fabricated structures82–85. The major advantage of this rapid prototyping approach to CAD-guided MPE fabrication is that, in contrast to the best commercially available 3D printing technologies with 5–10 μm resolution limit,69 it allows fabrication of complex models with submicron-level resolution enabled by the MPE point spread function86. A primary drawback of this 3D printing modality is limited macroscale size. To date tissue fabrication is limited to microscopic fields of view. Bioprinting of larger structures thus would require merging fields of view and would result in structural “seams” significantly limiting printing speed, throughput and fidelity to the digital template.
Inkjet printing
Inkjet printers (also known as drop-on-demand printers) are the most commonly used printers for both non-biological and biological 3D printing applications. The key property of the inkjet technology is automated (computer-assisted) delivery of controlled volumes of liquid (ink) to predefined locations. Modified versions of commercially available 2D inkjet printers, where the cartridge ink was replaced with a biological material, and an electronically controlled z-axis elevator stage87 replaced paper, were the first 3D bioprinters used for tissue fabrication applications. Contemporary, custom-designed inkjet bioprinters utilize thermal or acoustic forces-based liquid ejection mechanisms to print biological materials at increasing resolution, precision, and speed. The functional principle of thermal inkjet printing is based upon electrical heating of the print head to produce pulses of pressure that force droplets from the nozzle (Figure 3C). Localized heating, ranging from 200°C to 300°C has been demonstrated by several studies to have no detrimental effect on either the viability or function of mammalian cells or stability of biological molecules, such as DNA88, 89. This is because the short duration of the heating pulse (~2 μs) raises the temperature in the printer head by only 4–10°C 90. Another advantage of inkjet printing is the potential to create concentration gradients of cells, materials or growth factors throughout the 3D structure by altering drop density or drop size during the printing process. Despite the fact that thermal inkjet printers are cost effective and offer high speed of bioink deposition along with the cell gradient capability, they pose numerous disadvantages for use in 3D bioprinting. Those include exposure of cells and materials to thermal and mechanical stresses, non-uniform droplet size, low droplet directionality, frequent nozzle clogging, unreliable cell encapsulation and low cell densities (Table 2). Another common drawback of inkjet bioprinting is the requirement for the biological material to be in a liquid form to enable droplet formation. As a result, the printed bioink must subsequently form a solid 3D structure with structural organization and functionality.
Micro-extrusion printing
Micro-extrusion ink deposition technology is commonly used for non-biological 3D printing applications and is becoming more wide-spread for bioprinting applications. The most typical micro-extrusion bioprinters consist of the following components: i) a dispensing system controlled by temperature ii) a computer-controlled three-dimensionally moving stage; iii) a video camera controlling 3D stage movements for precision control; iv) a photoinitiator-activating light source that illuminates the area of ink deposition and v) a piezoelectric humidifier (Figure 3D). The mechanism of micro-extrusion printer function is based on material deposition onto a substrate by robotically-controlled extrusion through nozzles or needles connected to ink-loaded micro-extrusion head (cartridge) by continuous flow (line) of the printed (bio) ink material rather than deposition of liquid droplets. The material is deposited in two-dimensional fashion, directed by the CAD-CAM software, and could occur also in the form of small beads as in the case of bioprinting spheroids illustrated on Figure 4A. Inkjet and extrusion printing are thus the two major printing technologies, fully compatible with 3D printing of cell-laden constructs under physiological conditions. Although bioprinting of viscous bioinks using inkjet printing is relatively challenging, it has been widely used for 3D printing of cell-laden constructs owing to relatively higher cell viability provided by this method as compared to microextrusion printing91.
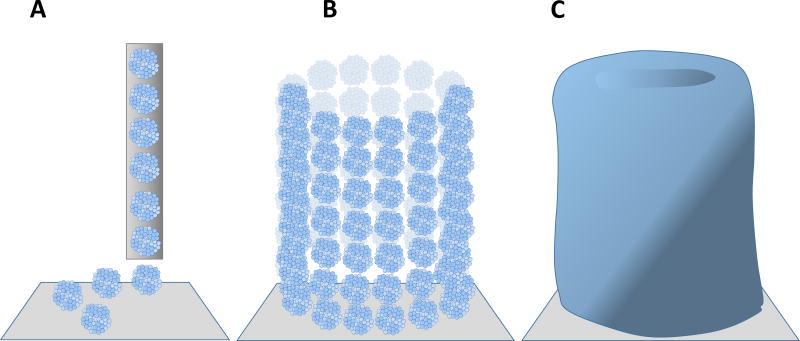
Figure 4.
Scaffold-free bioprinting employs cell spheroids and does not utilize a gel as a carrier. Robotic dispensing of spheroids (A) typically occurs through a nozzle onto a carrier substrate. Cell spheroids can be delivered to form various shapes including blood vessels (B). Cell spheroids will self-organize, fuse, and begin forming their own extracellular matrix (C). Under appropriate mechanical stimulation, fused spheroids can develop enough mechanical integrity to become suitable for implantation as a load-bearing tissue replacement.
The Z-axis material deposition can be achieved by either stage or the micro-extrusion head movements, whereby each deposited layer creates a foundation for the next layer. Materials compatible with micro-extrusion-based printers include biocompatible copolymers, hydrogels and cell spheroids92. The most common forces used in extrusion printers for dispensing of biological materials are pneumatic and mechanical (Figure 3D). Due to the delay associated with the gas volume compression in pneumatic systems, they might provide less direct control over the material flow than printers with mechanical forces-driven dispensing. Mechanical force extrusion printers with screw-based dispensing mechanism are believed to be optimal for printing of highly viscous hydrogels, although pneumatic systems could also be used for printing such materials.
The structure of pneumatic force-driven 3D printers is relatively simple. Force limitations are determined by air pressure capabilities. Printers with mechanically driven mechanisms contain more complex, but compact components and provide better spatial control. However the latter often comes at the price of reduced force capabilities. Micro-extrusion bioprinting technology offers an important advantage over the other types of bioprinting approaches in that it enables depositing cells with very high densities (Table 2). However, the resulting high viscosity of the bioink might be detrimental for cell viability and requires suitable bioink formulation. Therefore, bioinks that allow physiological density of the cells/tissues of interest in engineered tissues and organs are critical for the success of the bioprinting field.
Commercial Bioprinters
Currently, there are several major commercial bioprinter devices. Some of the better known companies, at this writing, are BioFactory by RegenHu (hard and soft tissue bioprinting), BioAssemblyBot by Advanced Solutions Life Science (six-axis robotic arm with up to 8 syringe barrels for dispensing bioink), and Bio3D SYN from Bio3D Technologies (designed to be modifiable for research and scientific purposes). In general, they have each developed their own unique approaches to the theme of building 3D tissue structures. Other examples are the 3D-Bioplotter System developed at the Freiburg Materials Research Centre in Germany93, 94. This bioprinter is compatible with the use of a large variety of biomaterials ranging from soft hydrogels and polymer melts to hard ceramics and metals. 3D CAD models derived from patient-specific computer tomography (CT) data are used in Bioplotter for fabrication of 3D scaffolds with well-defined outer shape and an open inner structure, critical for tissue engineering and controlled drug release. The 3D-Bioplotter is specifically designed to work in the sterile environment of biosafety cabinets which is crucial for biofabrication of scaffolds from alginate cell suspensions. In contrast to other rapid prototyping techniques, the technology used in 3D-Bioplotter is very simple and straightforward. The world’s first commercial 3D bioprinter, NovoGen MMX bioprinter, contains two separate robotically-controlled precision print heads: one for depositing cells and the other for hydrogel scaffold or support matrix. Although the bioprinter was initially designed to fabricate tissues, like blood vessels and nerve conduits, it could potentially be utilized for printing more complex anatomical structures such as heart and its integral tissue components.
Bioprinting Technology Applications
Fabrication of engineered tissue constructs typically involves manual procedures, which impose limitations on the complexity, by which materials of varying properties and dimensions can be interfaced. Three-dimensional printing offers a means to automate the process of fabricating tissue mimics from a variety of compatible materials. The ability to print 3D microenvironments opens new perspectives for the development of new drug screening methods and facilitates fundamental studies in the fields of wound healing, angiogenesis, and stem cell biology. Upon further optimization the existing tissue engineering techniques may lead to the rapid manufacturing of functional 3D tissues and, possibly, even artificial organs. Studies are currently underway to determine the optimal ways to produce fully vascularized, engineered tissue constructs by combining biological self-assembly with 3D printing approaches95.
Strategies for cardiac tissue engineering
The effectiveness of cell therapy applications for cardiac diseases depends on the ability of the implanted cells to survive and properly integrate into the recipient’s heart tissue with the resulting improvement in cardiac function96. Currently, strategies to build thick multi-layer tissues involve integration of vascular structures into the implant prior to transplantation. This has so far resulted in fabrication of artificial tissues with thickness on the order of 100 μm, which still suffer from cell death at the center97. Since viability of printed tissues heavily depends on oxygen supply, thin tissue constructs that can receive oxygen simply by diffusion demonstrate better survival than thick patches with limited accessibility of deep cell layers to freely diffusing oxygen.
In this regard, to generate viable bioprinted tissues the following minimal requirements have to be met: (i) a functional vascular system that can easily be integrated with the recipient/host tissue should be incorporated into the transplant tissue (ii) cells have to be precisely patterned and oriented in the context of a hierarchical structure on microns to millimeters scale and (iii) the engineered tissue structure should incorporate materials that induce and maintain proper phenotype of the cells and do not elicit any adverse reaction from the host, such as inflammation or immune response. However, the advances in technologies to improve the transplanted cell survival for the first 1–3 days, are going to be the most important and useful. This is because if the cells in fabricated tissue can survive for the 1st 3 days after the cardiac transplantation, sprouting of preexisting vessels of the recipient tissue could occur into the grafted tissue to supply it with oxygen and nutrients as heart is an organ with a very robust angiogenesis potential.
Typical materials utilized in cardiovascular bioprinting include synthetic and natural bioactive hydrogels such as gelatin, collagen, fibrin and peptides with cell adhesion-supporting capacity98–100. Microcarriers are another option and offer a highly-specific surface area and bioactive environment for quick cell attachment and proliferation101. Cells can be encapsulated within microcarriers and further incorporated within bioinks for bioprinting. Scaffold-free cell spheroids (Figure 1), generated by biofabrication approaches like hanging drop, micro-molded, microfluidics, and spinner flasks, represent another biological substrate for bioprinting. Spheroids can fuse together and quickly generate mature constructs with heterogeneous cell populations and better biomimicry (Figure 3C). This enables co-culturing different cardiovascular cells types such as cardiomyocytes, endothelial cells, smooth muscle cells and cardiac fibroblasts. However, cell spheroid-based scaffold-free constructs are not stable enough and require a support structure to stabilize the structure initially and several weeks to undergo remodeling and full maturation.
Generation of bioinks from natural decellularized extracellular matrix (dECM) represents a new approach that can be broadly used in the context of extrusion-based 3D printing. Extracellular matrix (ECM) derived from various native tissues represents a new source of bioink with broad utility for bioprinting applications. ECM is considered as an essential structural element of tissue with important role in biochemical signaling, particularly, pertaining to stem cell differentiation and survival102, 103. Bioinks composed exclusively of ECM, simply containing exogenous ECM or producing endogenous ECM as a result of biological activity of spheroids hold enormous potential for 3D printing technology. To produce ECM-based bioink, ECM is first decellularized and then dissolved/concentrated into paste-like material104. In order to make consistent and component controllable bioinks, it is important to standardize the process of ECM decellularization based on tissue sources. To improve the mechanical properties and bioprintability of a tissue scaffold, synthetic hydrogel-based bioinks are often combined with decellularized ECM, which can be performed in the context of a supportive frame to be printed first. Bioinks made of dECM, obtained by decellularization of whole organs, may be capable of better maintaining proper cellular phenotype owing to the ability to provide instructional signals to seeded cells. In support of this concept, dECM bioink induced higher expression of cardiac-specific genes (Myh6 and Actn1) and higher expression of cardiac myosin heavy chain (β-MHC) after 4 days in culture as compared to collagen-based construct104. As an alternative, and more easily standardized method, bottom-up approaches have been used to create ECM formulations from combinations of different types and amounts of individual ECM proteins. By pairing this concept with design of experiments statistical approaches, ECM formulations supportive of cardiac cell types have been developed 103 and could be further optimized in future.
Like many other tissue engineering approaches, 3D printing of myocardial tissue is limited primarily by low resolution of complex structures and availability of appropriate cells for the bioink, i.e. implantable human cardiac cells. Cardiac progenitor cells or iPSCs hold promise for bioprinting tissues and organs as unlimited source of cardiac cells.
3D bioprinting of arteries and microvascular structures
Vascular networks are essential not only for oxygen transport, delivery of nutrients and immune cells or removal of metabolic waste products from cells and tissues, but also for the process of regeneration of cardiovascular and other tissues. Coronary artery disease resulting from deficient blood supply cardiac tissue still accounts for more than 30% of all human deaths and is responsible for approximately 1.2 million hospitalizations each year. Standard of care for this disease includes statins, antiplatelet agents, nitrates, coronary angioplasty, and coronary artery bypass grafting (CABG) surgery.
Vascular tissue engineering holds promise for fabrication of artificial coronary bypass grafts. An ideal tissue-engineered graft should be non-thrombogenic, properly endothelialized and possess biomechanical properties comparable to the native blood vessel. Biomimetic blood vessels can be engineered by using two main approaches: (i) a scaffold-guided method, in which scaffolds using natural, synthetic biomaterials, or de-cellularized ECM are built to support cell attachment, infiltration and proliferation during the in vitro tissue development; (ii) a cell-sheet-based approach, in which a monolayer of 2D-cultured cells is rolled on a mandrel to produce an artery mimicking tubular conduit.105 Despite recent efforts, most small lumen artificial coronary bypass grafts failed to achieve the longevity and the performance of natural autologous grafts and therefore none of them to date has been successfully commercialized for clinical use. Major problems remain to be the loss of the endothelial cell layer and early vessel closure after transplantation.
Three dimensional printing can greatly contribute to the creation of microvascular networks and individual, replacement vessels by enabling generation of scaffolds with patient-specific geometries or direct printing of differentiated endothelial cells, fibroblasts and SMCs, or mesenchymal and hematopoietic stem cells in the context of biocompatible scaffold materials (e.g. hydrogel). However, 3D printing has not yet been employed for fabrication of coronary bypass grafts. Instead, the research efforts have been focused primarily on generation of in-vitro vascular models through lining the inner surface of patterned microchannels with endothelial cells to fabricate microvascular networks for studying angiogenesis and thrombosis or for supplying nutrients and oxygen to engineered tissue.
Three dimensional bioprinting technology enables fabrication of vascular networks with patient-specific patterns and clinically-relevant size of perfusable channels. The following vascularization strategies are used in vascular tissue engineering: 1) generation of vascular constructs by self-assembly of cells; 2) generation of microvasculatures by inkjet based bioprinting; 3) generation of bioprinted constructs with growth factor delivery capabilities; 4) fabrication of vasculature using coaxial nozzle-assisted 3D bioprinting and 5) generation of constructs through channel-based vascularization. Self-assembly of cells utilizes similar adhesive properties of certain cell types to form spontaneous and stable aggregates or structures without external stimuli. The examples of such structures are spheroids and self-assembling tubular structures65, 106.
Spheroids generated from cell suspensions were used in a recent study as building blocks to fuse into vasculature-like constructs52 (Figure 3). Tubular structures with controllable channel diameter, wall thickness and branching pattern could be fabricated by fusion of multicellular spheroids on agarose rods as templates. Vascular construct with double-layered wall and specific branching pattern could recently be obtained by depositing multicellular cylinders composed of human smooth muscle cells (HSMC) and human skin fibroblasts (HSF).54
Kucukgul et al. 3D-bioprinted aggregates of mouse embryonic fibroblast (MEF) instead of spheroids, to form an arterial (aortic) tissue construct107. Simultaneous deposition of human microvascular endothelial cells (HMEC) and fibrin as a scaffold material allowed Cui at al. to bioprint microvascular constructs by using a modified commercial inkjet printer. The scaffold of the fabricated construct retained proper shape after printing, while endothelial cells spontaneously formed tubular structures upon proliferation.108
Hollow calcium alginate filaments (channels) were fabricated by using a coaxial nozzle-assisted 3D bioprinting system, dispensing sodium alginate solution (with or without cells) that was crosslinked after coming into contact with calcium chloride solution in the inner chamber of the coaxial nozzle. The hollow filaments were further used as building blocks for bioprinting vascular constructs109. A similar strategy was used in yet another study by Yu et al.110. This strategy allowed bioprinting of vasculature of defined geometry, length, and orientation. Coaxial nozzle-assisted 3D bioprinting technology is limited by the availability of bioink. Only alginate-based bioink is currently used with this technology owing to its fast crosslinking capacity.
Besides direct printing of vascular channels, many research teams generate vascular networks by using sacrificial materials within engineered tissue constructs, which involves 3D printing of water soluble material-based filament networks into a supportive matrix material (typically cell-laden hydrogels) with subsequent dissolving of the filaments with special solvents or altering temperature. A new 3D printing-based approach for creating vascularized, heterogeneous tissue constructs was reported by Kolesky et al. The authors initially fabricated a multilayer tissue construct by co-printing two inks at 20–22°C: the fugitive Pluronic F127 ink and a cell-laden GelMA ink with green fluorescent protein-expressing human neonatal dermal fibroblasts (HNDFs). Then they deposited pure GelMA ink at 37°C to fully encapsulate the printed features, followed by photopolymerization of the GelMA matrix by cross-linking. The fugitive ink was subsequently liquefied and removed from the 3D construct so that the evacuated channels could be endothelialized. The authors clearly observed both the GFP-expressing HNDFs in GelMA and the red-HUVECs lining the embedded 3D vasculature by confocal microscopy.95 Thus the 3D printing platform allows fabrication of artificial tissue constructs by programmed deposition of multiple cell types along with vascular structures within extracellular matrices.
3D Bioprinting of the Myocardium
Myocardial infarction (MI) accounts for nearly half of the 7.3 million heart disease-related deaths each year111. If the coronary blood supply is not recovered quickly within 60 minutes, a large number of cardiac cells, including cardiomyocytes (CM), within the blood-deprived myocardium are lost. A prolonged vigorous inflammatory response and postinfarction LV remodeling ultimately lead to heart failure. Because cardiomyocytes, being the main building blocks of the heart tissue112, have very limited capacity to proliferate and replace damaged cells, the ischemia damaged heart fails to recover after MI113. The native population of cardiac progenitor cells (CPCs) is very limited and decreases significantly upon aging, thereby compromising the myocardial repair potential.114
Although pre-clinical and clinical studies of cell therapy demonstrated promising results,115–117 this approach is limited by very low long term grafts.118 Heart transplantation, as the last therapeutic option for severe heart failure, is limited by shortage of organ donors on the one hand, and allogeneic transplant rejection, on the other.119 A myocardial tissue regeneration strategy, as a cutting edge treatment option for MI, relies on tissue engineering technologies including 3D printing. A successful fabrication of myocardial tissue from chick embryonic cardiomyocytes mixed with collagen solution was performed back in 1997 by Eschenhagen et al. leading to establishment of the first coherently contracting 3D model of heart tissue that allowed direct measurement of isometric contractile force. 120 Fabrication of human myocardial tissue equivalent (hMTE) using human induced pluripotent stem cells (hiPSC) holds potential for replacing some of the conventional therapies in the future. 121, 122 Using high fidelity cMRI information to guide the engineering of hMTE with regards to the shape and size of the artificial tissue implants that would mimic the essential characteristics of the natural myocardium is the major goal of 3D printing of hMTE.
Fabrication of cardiac tissue implants, in addition to proper vascularization and efficient oxygen exchange, requires proper density of cardiomyocytes and various supporting cells.4 These conditions can be achieved by various tissue bioprinting techniques providing unique capabilities for patterning and assembling cells with defined density and spatial distribution. Gaebel et al.123 used LIFT cell printing approach to fabricate a cardiac patch seeded with human umbilical vein endothelial cells (HUVEC) and human mesenchymal stem cells (hMSC) in a defined pattern. The authors’ incentive for co-printing hMSC with HUVEC was based on the recent finding that MSC could inhibit apoptosis of endothelial cells under hypoxic condition, thereby increasing their survival, and stimulate angiogenesis. Specific vascular patterns were successfully generated by LIFT printing of fluorescently-labeled HUVEC (green) and hMSC (red) arranged in a capillary-like pattern on a polyester urethane urea (PEUU) cardiac patch, whereas control patches were generated without LIFT by random seeding of equal amounts of each cell type. Patches with LIFT-patterned cells were cultivated further and transplanted onto infarcted zones of rat hearts following left anterior descending (LAD)-ligation. Cardiac performance was assessed 8 weeks post infarction and showed that the LIFT-generated cell patterning stimulated growth of co-cultured HUVECs and hMSCs, leading to significant improvement of functional characteristics of the infarcted hearts. This study also demonstrated an increased capillary density as well as integration of transplanted cells into the recipient’s vascular system by functional connection to its blood vessels, suggesting functional benefit of LIFT-generated cardiac patch for wound healing and functional preservation during MI treatment.
Recently Gaetani and co-workers demonstrated that microstructure tissue printing using a combination of alginate scaffold with human fetal cardiomyocyte progenitor cells (hCMPCs) can be used to fabricate a cardiogenic patch with defined pore size and improved viability124. To further improve this technology of myocardial tissue engineering, a new cardiogenic scaffold consisting of hCMPCs and a hyaluronic acid/gelatin (HA/gel) based biomaterial was created. This advanced bioink enhanced attachment and survival of hCMPCs without affecting their growth and differentiation potential. Besides this, the cells’ commitment for the cardiac lineage was maintained as evidenced by upregulation of early cardiac transcription factors and expression of sarcomeric protein Troponin T. The bioprinted tissue patch demonstrated excellent cell survival and engraftment when tested in a murine model of MI. Hearts received the hCMPCs scaffold transplantation showed improved cardiac function after MI.125
Fabrication of heart valves by 3D printing
Three dimensional printing also holds strong potential for fabrication of engineered heart valves. Currently heart valve replacement surgery involves implantation of either mechanical or chemically cross-linked tissue heart valves126. The advantage of synthetic valves over biological valves is that they are mechanically robust and typically have a longer lifetime127. However, patients with prosthetic valves are required to permanently take anticoagulants. Biological valves, made from either an allogeneic or xenogeneic source, do not require the patient to take anticoagulants128. In addition, neither of the current valve replacement types are able to grow and remodel with the patient. 3D bioprinting could in principle address all of the current limitations of valve replacements.
To date the engineered heart valves have been fabricated by using an extrusion-based 3D printer from two types of photocrosslinkable hydrogels: one rigid (~75 kPa) hydrogel for the root and the other soft (~5 kPa) hydrogel for the leaflets129.The ability to simultaneously print two materials with distinct mechanical properties provided by the 3D printer, allowed better mimicry of the differential stiffness of the native heart valve tissues such as the leaflets and the root. Another study from the same group showed that interstitial cells (PAVIC) from porcine aortic valve can survive for up to three weeks in the context of 3D printed artificial heart valves.
In yet another study aortic root sinus smooth muscle cells (SMCs) and aortic valve leaflet interstitial cells (VICs) encapsulated into the root and leaflet portions of the valve, respectively, remained viable for 7 days in culture. The VIC cells could remodel the printed hydrogels by depositing their own collagen- and glycosaminoglycan-based ECM. Encapsulated SMC and VIC showed elevated expression of alpha-smooth muscle actin and vimentin, respectively, demonstrating that anatomically complex and heterogeneously encapsulated aortic valve hydrogel conduits can be successfully fabricated using the 3D bioprinting approach130. Despite the progress in heart valve tissue engineering, no functional testing of any of the printed valves has been performed to date.
Whole heart bioprinting
Heart is mainly composed of three different types of cardiac tissues: myocardium, endocardium, and pericardium. The main cell types that make up the cardiac tissues are cardiomyocytes, cardiac fibroblasts, and endothelial cells. It has been reported that myocytes constitute up to 30–40% of the entire cell population of normal adult heart and the rest of it is represented by non-myocytes with the majority being fibroblasts112. However, a recent study by Pinto et al. demonstrated that endothelial cells make up the largest portion of non-myocytes in the heart.131
A group of specialized pacemaker cells located in the right atrium and called the sinoatrial node (SAN), can autonomously generate electrical impulses to set off contractions of the myocardium. Anisotropic self-alignment and contractile synchronization of cardiomyocytes in the myocardium promotes the electrical activation of the cardiac muscles. In addition to the intrinsic automaticity of SAN, its pacemaker activity is normally controlled by opposing input from the parasympathetic and sympathetic nerves of the peripheral nervous system (PNS).
Endocardium, the innermost layer of the heart chambers and heart valves, is primarily made of endothelial cells that form overlapping regions to seal the heart chambers and connect the surrounding blood vessels. Apart from preventing blood leakage, it also plays important role of a blood-heart barrier controlling entry and exit of certain types of molecules. Pericardium is represented by a double-wall fibroserous sac, enclosing the whole heart and the root of the blood vessels. The space between the two membranes of the pericardium, known as the pericardial cavity, contains pericardial fluid that serves as a lubricant facilitating membranes sliding over each other. In addition to the three major cardiac tissues, an important role in the heart structure and function also belongs to ECM, which controls cell fate and differentiation, and regulates protein expression. In normal myocardium, viscoelasticity of the collagen-enriched ECM and cardiomyocytes must be matched to generate actomyosin forces and pump of the heart.132
Recent advances in cardiovascular tissue engineering led to the ability to fabricate various cardiac tissues and heart components by employing state-of-the-art 3D bioprinting technologies. Despite demonstrated functionality and structural similarity of the engineered heart tissues to the native counterparts, their full structural and functional integration in the diseased organ may still be problematic, making successful implantation of myocardial patch, vascular (coronary or aortic) grafts or heart valves quite challenging. The importance of the structural integrity of the heart is predicated primarily by the electrophysiological coupling of cardiomyocytes, determining their highly synchronized ability to respond to the pacemaker activity of the SAN. Furthermore, repairing/curing the primary cause of cardiac malfunction may not always be sufficient to fully restore normal heart function since global anatomical and physiological changes to the whole organ may occur as a result the original, single cause. In light of this consideration, fabrication of the whole organ as opposed to its individual components (tissue implants) may become a more advantageous approach.
In fact, recent attempts in whole organ bioprinting demonstrated that the general structure of the whole heart can be fabricated. Hinton et al. developed a 3D bioprinting technology, suitable for fabrication of complex biological structures that was termed “freeform reversible embedding of suspended hydrogels”. This technology enabled 3D printing of hydrated materials such as alginate, collagen, and fibrin with an elastic modulus of <500 kPa. This method relied on direct bioprinting of the bioinks into a support bath of gelatin microparticles, which allowed depositing the supporting hydrogel under room temperature to construct large-scale volumetric objects that was impossible to achieve before. The support bath could then be liquefied at elevated temperature to release the bioprinted structures. In this study, the authors utilized CAD models of 3D optical, computed tomography, and magnetic resonance imaging data. This technology of whole heart bioprinting enabled fabrication of embryonic hearts with complex internal and external anatomical architecture at ~200 μm resolution133.
Despite the remarkable advances in bioprinting of whole organ models for educational and surgical guide purposes, to date bioprinting technology has not successfully printed any therapeutically relevant tissue constructs. One of the reasons is that each individual bioprinting technique has its own intrinsic disadvantages. In this regard, combining two or multiple bioprinting techniques or using bioprinting in conjunction with other tissue engineering technologies appears to be a more reasonable strategy for overcoming the above constraints.
Spheroid Bioprinting without Exogenous Structural Biomaterial
Thus far we have discussed cell and tissue engineering where the structural integrity of the cell-seeded system depends on the integrity of the substrate material to which the cells are attached. The underlying assumption is that engineered tissues require a scaffold (natural or synthetic) at the outset to support mechanical loading, particularly of vascular structures, as they become integrated into the host. However, formation of a tissue can occur in vitro without providing cells a supporting matrix in a process akin to embryonic development where morphogenesis of tissues is linked to cell-cell contact of originally isolated groups of cells134 followed by formation of ECM. This approach is developing favor in tissue engineering and capitalizes on the idea that the ideal ECM is formed by the cells themselves as an adaptation to the in vitro or in vivo environment. Cell spheroids, collections of thousands of a single cell type, are ideal because no ECM is required and they will self-assemble by forming cell-cell junctions135. Spheroids are easily generated by centrifugation to form pellets, hanging drops or micro-molds136.
Spheroids consisting of human dermal microvascular endothelial cells have also been shown in vitro to form dense tubular vessel-like networks within 72 hours and exhibit a significantly decreased rate of apoptotic cell death when compared to mono-culture HDMEC spheroids. After transplantation, these networks interconnected to the host microvasculature by external inosculation.137 In fact, spheroids have been shown to be more resistant against hypoxia and apoptotic cell death.138 Moreover, they secrete higher levels of pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2).138
Cell spheroids can be loaded into ‘bioink’ cartridges and dispensed using a bioprinter into a pre-determined 3D configuration typically in a layer-by-layer deposition135. This technique frequently relies upon a gel material or ‘biopaper’ to support the spheroids. Tissue or organ printing using self-assembled cell spheroids as a possible alternative to classic, solid, biodegradable, scaffold-based approaches could dramatically enhance and transform the field of tissue engineering by enabling large-scale industrial robotic biofabrication of living human organ constructs with “built-in” perfusable intraorgan branched vascular trees52, 139. It was recently demonstrated that a combination of human umbilical vein endothelial cells (40%), human aortic smooth muscle cells (10%), and normal human dermal fibroblasts (50%) could form multi-cellular spheroids (MCS) of approximately 25,000 cells140. These spheroids were robotically delivered to an array of needles and ‘skewered’ in a tube-like configuration (1.5 mm ID × 7 mm length) where they fused together after 4 days. After removal from the array, the tube was perfused for 4 additional days in preparation for implantation as a rat aorta vascular graft. After 5 days in vivo, the graft showed collagen production and some cellular rearrangement where endothelial cells were found in the lumen. This study was a clear demonstration of the capacity of cell spheroids to self-assemble and produce their own extracellular matrix (Figure 3C).
Scaffold-free fabrication of cardiac patches was performed with contractile cardiac spheroids by plating a mixture of rat neonatal ventricular cardiomyocytes, human dermal fibroblasts, and human coronary microartery endothelial cells in ultra-low attachment plates141. Approximately 14,000 spheroids containing 1,000 cells each were fused into a patch-like construct grafted into rat hearts. The patches were adherent to the surface of the heart and contractile after 7 days. Histologic results showed that after 2 days a microvascular network began forming in the spheroid. Further, spheroids can be manipulated with robotic control to form a variety of physiologically relevant cardiovascular geometries composed of multiple cell types without the use of any ECM material.
Future Perspective
The technologies reported in this paper comprise fundamental advances in the fabrication of engineered cardiovascular structures using variations of 3D printing. Bioprinting of soft tissues of the cardiovascular system relative to hard tissues (i.e., bone and cartilage) necessitates special design criteria and the technology is quickly advancing to address these criteria. Most critical is the need to match cell density and mechanical properties with the native structure while supporting cell viability and cell organization throughout the tissue. In addition, the speed of printing and fidelity with which printing can reproducibly replicate a native structure must be improved. When these criteria are realized, the concept of printing needed components for cardiovascular repair, including intact organs, in the operating suite may be within reach.
Supplementary Material
Acknowledgments
The authors thank Mr. Wesley Labarge and Dr. Saidulu Mattapally for helpful discussion of the manuscript.
SOURCES OF FUNDING
This work was supported by US Public Health Service grants NIH RO1s HL95077, HL114120, HL 131017, and UO1 HL134764.
Abbreviations and acronyms
- AM
additive manufacturing
- β-MHC
beta myosin heavy chain
- Bioink
building blocks containing cells/biomaterials mixture or spheroids
- Biomimetic
denotes synthetic methods, systems or elements of nature that mimic biochemical processes
- CABG
coronary artery bypass grafting
- CAD
computer-aided design
- CAD-CAM
computer-aided design and computer-aided manufacturing
- CM
cardiomyocytes
- CPCs
cardiac progenitor cells
- CT
computer tomography
- dECM
decellularized extracellular matrix
- FDM
fused deposition modeling
- GelMA
gelatin methacryloyl
- hCMPCs
human fetal cardiomyocyte progenitor cells
- (h)iPSCs
(human) induced pluripotent stem cells
- HMEC
human microvascular endothelial cells
- hMSC
human mesenchymal stem cells
- hMTE
human myocardial tissue equivalent
- HNDFs
human neonatal dermal fibroblasts
- HSF
human skin fibroblasts
- (H)SMC
(human) smooth muscle cells
- HUVEC
human umbilical vein endothelial cells
- kHz
kilohertz, a unit of frequency
- LAB
laser-assisted bioprinting
- LAD-ligation
left anterior descending ligation
- LIFT
laser-induced forward transfer
- LV remodeling
left ventricular remodeling
- MEF
mouse embryonic fibroblast
- MI
myocardial infarction
- MPE
multiphoton excitation
- MPLSM
multiphoton laser scanning microscopy
- MSC
multi-cellular spheroids
- PAVIC
porcine aortic valve interstitial cells
- PBE
pressure-based extrusion
- PDMS
polydimethylsiloxane
- PEUU
polyester urethane urea
- PNS
peripheral nervous system
- SAN
sinoatrial node
- SLA
stereo lithography
- SLS
selective laser sintering
- TNF-α
tumor necrosis factor alpha
Footnotes
CONFLICT OF INTEREST DISCLOSURES
None.
References
- 1.Plunkett N, O’Brien FJ. Bioreactors in tissue engineering. Technol Health Care. 2011;19:55–69. doi: 10.3233/THC-2011-0605. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Dutton JR, Su L, Zhang J, Ye L. The influence of a spatiotemporal 3D environment on endothelial cell differentiation of human induced pluripotent stem cells. Biomaterials. 2014;35:3786–93. doi: 10.1016/j.biomaterials.2014.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. J Exp Zool. 1959;142:5–73. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- 4.Hirt MN, Hansen A, Eschenhagen T. Cardiac tissue engineering: state of the art. Circulation research. 2014;114:354–67. doi: 10.1161/CIRCRESAHA.114.300522. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Lee H, Chung M, Jeon NL. Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip. 2013;13:1489–500. doi: 10.1039/c3lc41320a. [DOI] [PubMed] [Google Scholar]
- 6.Gao X, Zhang X, Tong H, Lin B, Qin J. A simple elastic membrane-based microfluidic chip for the proliferation and differentiation of mesenchymal stem cells under tensile stress. Electrophoresis. 2011;32:3431–6. doi: 10.1002/elps.201100237. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu K, Araki H, Sakata K, Tonomura W, Hashida M, Konishi S. Microfluidic devices for construction of contractile skeletal muscle microtissues. J Biosci Bioeng. 2015;119:212–6. doi: 10.1016/j.jbiosc.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Berthier E, Young EW, Beebe D. Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip. 2012;12:1224–37. doi: 10.1039/c2lc20982a. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal A, Goss JA, Cho A, McCain ML, Parker KK. Microfluidic heart on a chip for higher throughput pharmacological studies. Lab Chip. 2013;13:3599–608. doi: 10.1039/c3lc50350j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang KJ, Suh KY. A multi-layer microfluidic device for efficient culture and analysis of renal tubular cells. Lab Chip. 2010;10:36–42. doi: 10.1039/b907515a. [DOI] [PubMed] [Google Scholar]
- 11.Baudoin R, Griscom L, Monge M, Legallais C, Leclerc E. Development of a renal microchip for in vitro distal tubule models. Biotechnol Prog. 2007;23:1245–53. doi: 10.1021/bp0603513. [DOI] [PubMed] [Google Scholar]
- 12.Snouber LC, Letourneur F, Chafey P, Broussard C, Monge M, Legallais C, Leclerc E. Analysis of transcriptomic and proteomic profiles demonstrates improved Madin-Darby canine kidney cell function in a renal microfluidic biochip. Biotechnol Prog. 2012;28:474–84. doi: 10.1002/btpr.743. [DOI] [PubMed] [Google Scholar]
- 13.Jang KJ, Mehr AP, Hamilton GA, McPartlin LA, Chung S, Suh KY, Ingber DE. Human kidney proximal tubule-on-a-chip for drug transport and nephrotoxicity assessment. Integr Biol (Camb) 2013;5:1119–29. doi: 10.1039/c3ib40049b. [DOI] [PubMed] [Google Scholar]
- 14.Mahler GJ, Esch MB, Glahn RP, Shuler ML. Characterization of a gastrointestinal tract microscale cell culture analog used to predict drug toxicity. Biotechnol Bioeng. 2009;104:193–205. doi: 10.1002/bit.22366. [DOI] [PubMed] [Google Scholar]
- 15.Kim HJ, Ingber DE. Gut-on-a-Chip microenvironment induces human intestinal cells to undergo villus differentiation. Integr Biol (Camb) 2013;5:1130–40. doi: 10.1039/c3ib40126j. [DOI] [PubMed] [Google Scholar]
- 16.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328:1662–8. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fritsche CS, Simsch O, Weinberg EJ, Orrick B, Stamm C, Kaazempur-Mofrad MR, Borenstein JT, Hetzer R, Vacanti JP. Pulmonary tissue engineering using dual-compartment polymer scaffolds with integrated vascular tree. Int J Artif Organs. 2009;32:701–10. doi: 10.1177/039139880903201001. [DOI] [PubMed] [Google Scholar]
- 18.Tavana H, Zamankhan P, Christensen PJ, Grotberg JB, Takayama S. Epithelium damage and protection during reopening of occluded airways in a physiologic microfluidic pulmonary airway model. Biomed Microdevices. 2011;13:731–42. doi: 10.1007/s10544-011-9543-5. [DOI] [PubMed] [Google Scholar]
- 19.Chao P, Maguire T, Novik E, Cheng KC, Yarmush ML. Evaluation of a microfluidic based cell culture platform with primary human hepatocytes for the prediction of hepatic clearance in human. Biochem Pharmacol. 2009;78:625–32. doi: 10.1016/j.bcp.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Legendre A, Baudoin R, Alberto G, Paullier P, Naudot M, Bricks T, Brocheton J, Jacques S, Cotton J, Leclerc E. Metabolic characterization of primary rat hepatocytes cultivated in parallel microfluidic biochips. J Pharm Sci. 2013;102:3264–76. doi: 10.1002/jps.23466. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S, Prot JM, Leclerc E, Bois FY. Zonation related function and ubiquitination regulation in human hepatocellular carcinoma cells in dynamic vs. static culture conditions. BMC Genomics. 2012;13:54. doi: 10.1186/1471-2164-13-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen JW, Bhatia SN. Formation of steady-state oxygen gradients in vitro: application to liver zonation. Biotechnol Bioeng. 2003;82:253–62. doi: 10.1002/bit.10569. [DOI] [PubMed] [Google Scholar]
- 23.Lee PJ, Hung PJ, Lee LP. An artificial liver sinusoid with a microfluidic endothelial-like barrier for primary hepatocyte culture. Biotechnol Bioeng. 2007;97:1340–6. doi: 10.1002/bit.21360. [DOI] [PubMed] [Google Scholar]
- 24.Khanal G, Chung K, Solis-Wever X, Johnson B, Pappas D. Ischemia/reperfusion injury of primary porcine cardiomyocytes in a low-shear microfluidic culture and analysis device. Analyst. 2011;136:3519–26. doi: 10.1039/c0an00845a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giridharan GA, Nguyen MD, Estrada R, Parichehreh V, Hamid T, Ismahil MA, Prabhu SD, Sethu P. Microfluidic cardiac cell culture model (muCCCM) Anal Chem. 2010;82:7581–7. doi: 10.1021/ac1012893. [DOI] [PubMed] [Google Scholar]
- 26.Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, Parker KK. Muscle on a chip: in vitro contractility assays for smooth and striated muscle. J Pharmacol Toxicol Methods. 2012;65:126–35. doi: 10.1016/j.vascn.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sin A, Chin KC, Jamil MF, Kostov Y, Rao G, Shuler ML. The design and fabrication of three-chamber microscale cell culture analog devices with integrated dissolved oxygen sensors. Biotechnol Prog. 2004;20:338–45. doi: 10.1021/bp034077d. [DOI] [PubMed] [Google Scholar]
- 28.Viravaidya K, Shuler ML. Incorporation of 3T3-L1 cells to mimic bioaccumulation in a microscale cell culture analog device for toxicity studies. Biotechnol Prog. 2004;20:590–7. doi: 10.1021/bp034238d. [DOI] [PubMed] [Google Scholar]
- 29.Park SH, Sim WY, Min BH, Yang SS, Khademhosseini A, Kaplan DL. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PLoS One. 2012;7:e46689. doi: 10.1371/journal.pone.0046689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Gazit Z, Pelled G, Gazit D, Vunjak-Novakovic G. Patterning osteogenesis by inducible gene expression in microfluidic culture systems. Integr Biol (Camb) 2011;3:39–47. doi: 10.1039/c0ib00053a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Torisawa YS, Spina CS, Mammoto T, Mammoto A, Weaver JC, Tat T, Collins JJ, Ingber DE. Bone marrow-on-a-chip replicates hematopoietic niche physiology in vitro. Nat Methods. 2014;11:663–9. doi: 10.1038/nmeth.2938. [DOI] [PubMed] [Google Scholar]
- 32.Puleo CM, McIntosh Ambrose W, Takezawa T, Elisseeff J, Wang TH. Integration and application of vitrified collagen in multilayered microfluidic devices for corneal microtissue culture. Lab Chip. 2009;9:3221–7. doi: 10.1039/b908332d. [DOI] [PubMed] [Google Scholar]
- 33.O’Neill AT, Monteiro-Riviere NA, Walker GM. Characterization of microfluidic human epidermal keratinocyte culture. Cytotechnology. 2008;56:197–207. doi: 10.1007/s10616-008-9149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shin M, Matsuda K, Ishii O, Terai H, Kaazempur-Mofrad M, Borenstein J, Detmar M, Vacanti JP. Endothelialized networks with a vascular geometry in microfabricated poly(dimethyl siloxane) Biomed Microdevices. 2004;6:269–78. doi: 10.1023/B:BMMD.0000048559.29932.27. [DOI] [PubMed] [Google Scholar]
- 35.van der Meer AD, Orlova VV, ten Dijke P, van den Berg A, Mummery CL. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip. 2013;13:3562–8. doi: 10.1039/c3lc50435b. [DOI] [PubMed] [Google Scholar]
- 36.Liu MC, Shih HC, Wu JG, Weng TW, Wu CY, Lu JC, Tung YC. Electrofluidic pressure sensor embedded microfluidic device: a study of endothelial cells under hydrostatic pressure and shear stress combinations. Lab Chip. 2013;13:1743–53. doi: 10.1039/c3lc41414k. [DOI] [PubMed] [Google Scholar]
- 37.Shi M, Majumdar D, Gao Y, Brewer BM, Goodwin CR, McLean JA, Li D, Webb DJ. Glia co-culture with neurons in microfluidic platforms promotes the formation and stabilization of synaptic contacts. Lab Chip. 2013;13:3008–21. doi: 10.1039/c3lc50249j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HS, Liu S, McDonald J, Thakor N, Yang IH. Neuromuscular junction in a microfluidic device. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:2833–5. doi: 10.1109/EMBC.2013.6610130. [DOI] [PubMed] [Google Scholar]
- 39.Shayan G, Shuler ML, Lee KH. The effect of astrocytes on the induction of barrier properties in aortic endothelial cells. Biotechnol Prog. 2011;27:1137–45. doi: 10.1002/btpr.620. [DOI] [PubMed] [Google Scholar]
- 40.Booth R, Kim H. Characterization of a microfluidic in vitro model of the blood-brain barrier (muBBB) Lab Chip. 2012;12:1784–92. doi: 10.1039/c2lc40094d. [DOI] [PubMed] [Google Scholar]
- 41.Wood DK, Soriano A, Mahadevan L, Higgins JM, Bhatia SN. A biophysical indicator of vaso-occlusive risk in sickle cell disease. Sci Transl Med. 2012;4:123ra26. doi: 10.1126/scitranslmed.3002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhatia SN, Ingber DE. Microfluidic organs-on-chips. Nat Biotechnol. 2014;32:760–72. doi: 10.1038/nbt.2989. [DOI] [PubMed] [Google Scholar]
- 43.Cheah LT, Dou YH, Seymour AM, Dyer CE, Haswell SJ, Wadhawan JD, Greenman J. Microfluidic perfusion system for maintaining viable heart tissue with real-time electrochemical monitoring of reactive oxygen species. Lab Chip. 2010;10:2720–6. doi: 10.1039/c004910g. [DOI] [PubMed] [Google Scholar]
- 44.Thomas PC, Halter M, Tona A, Raghavan SR, Plant AL, Forry SP. A noninvasive thin film sensor for monitoring oxygen tension during in vitro cell culture. Anal Chem. 2009;81:9239–46. doi: 10.1021/ac9013379. [DOI] [PubMed] [Google Scholar]
- 45.Baker BM, Trappmann B, Stapleton SC, Toro E, Chen CS. Microfluidics embedded within extracellular matrix to define vascular architectures and pattern diffusive gradients. Lab Chip. 2013;13:3246–52. doi: 10.1039/c3lc50493j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen DH, Stapleton SC, Yang MT, Cha SS, Choi CK, Galie PA, Chen CS. Biomimetic model to reconstitute angiogenic sprouting morphogenesis in vitro. Proc Natl Acad Sci U S A. 2013;110:6712–7. doi: 10.1073/pnas.1221526110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bischel LL, Young EW, Mader BR, Beebe DJ. Tubeless microfluidic angiogenesis assay with three-dimensional endothelial-lined microvessels. Biomaterials. 2013;34:1471–7. doi: 10.1016/j.biomaterials.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu Y-H, Moya ML, Hughes CCW, Georgea SC, Lee AP. A microfluidic platform for generating large-scale nearly identical human microphysiological system arrays. Lab on a chip. 2013;13:2990–2998. doi: 10.1039/c3lc50424g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Achyuta AK, Conway AJ, Crouse RB, Bannister EC, Lee RN, Katnik CP, Behensky AA, Cuevas J, Sundaram SS. A modular approach to create a neurovascular unit-on-a-chip. Lab Chip. 2013;13:542–53. doi: 10.1039/c2lc41033h. [DOI] [PubMed] [Google Scholar]
- 50.Reiffel AJ, Kafka C, Hernandez KA, Popa S, Perez JL, Zhou S, Pramanik S, Brown BN, Ryu WS, Bonassar LJ, Spector JA. High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities. PLoS One. 2013;8:e56506. doi: 10.1371/journal.pone.0056506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cohen DL, Malone E, Lipson H, Bonassar LJ. Direct freeform fabrication of seeded hydrogels in arbitrary geometries. Tissue Eng. 2006;12:1325–35. doi: 10.1089/ten.2006.12.1325. [DOI] [PubMed] [Google Scholar]
- 52.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: tissue spheroids as building blocks. Biomaterials. 2009;30:2164–74. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC. 3D printed bionic ears. Nano Lett. 2013;13:2634–9. doi: 10.1021/nl4007744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials. 2009;30:5910–7. doi: 10.1016/j.biomaterials.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harvey Lodish AB, Lawrence Zipursky S, Matsudaira Paul, Baltimore David, Darnell James. Molecular Cell Biology. 4. 2000. [Google Scholar]
- 56.Ripley B, Kelil T, Cheezum MK, Goncalves A, Di Carli MF, Rybicki FJ, Steigner M, Mitsouras D, Blankstein R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. Journal of cardiovascular computed tomography. 2016;10:28–36. doi: 10.1016/j.jcct.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG, in het Panhuis M. Bio-ink for on-demand printing of living cells. Biomaterials Science. 2013;1:224–230. doi: 10.1039/c2bm00114d. [DOI] [PubMed] [Google Scholar]
- 58.Chung JHY, Naficy S, Yue Z, Kapsa R, Quigley A, Moulton SE, Wallace GG. Bio-ink properties and printability for extrusion printing living cells. Biomaterials Science. 2013;1:763–773. doi: 10.1039/c3bm00012e. [DOI] [PubMed] [Google Scholar]
- 59.Yeong WY, Sudarmadji N, Yu HY, Chua CK, Leong KF, Venkatraman SS, Boey YC, Tan LP. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010;6:2028–34. doi: 10.1016/j.actbio.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 60.Melchels FP, Feijen J, Grijpma DW. A review on stereolithography and its applications in biomedical engineering. Biomaterials. 2010;31:6121–30. doi: 10.1016/j.biomaterials.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 61.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J Biol Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Cui R, Sun L, Aifantis KE, Fan Y, Feng Q, Cui F, Watari F. 3D-Printed Biopolymers for Tissue Engineering Application. International Journal of Polymer Science. 2014;2014:13. [Google Scholar]
- 63.Richards DJ, Tan Y, Jia J, Yao H, Mei Y. 3D Printing for Tissue Engineering. Isr J Chem. 2013;53:805–814. [PMC free article] [PubMed] [Google Scholar]
- 64.Seliktar D, Dikovsky D, Napadensky E. Bioprinting and Tissue Engineering: Recent Advances and Future Perspectives. Israel Journal of Chemistry. 2013;53:795–804. [Google Scholar]
- 65.Mosadegh B, Xiong G, Dunham S, Min JK. Current progress in 3D printing for cardiovascular tissue engineering. Biomed Mater. 2015;10:034002. doi: 10.1088/1748-6041/10/3/034002. [DOI] [PubMed] [Google Scholar]
- 66.Cui Y, Kim SN, Naik RR, McAlpine MC. Biomimetic peptide nanosensors. Acc Chem Res. 2012;45:696–704. doi: 10.1021/ar2002057. [DOI] [PubMed] [Google Scholar]
- 67.Nguyen TD, Deshmukh N, Nagarah JM, Kramer T, Purohit PK, Berry MJ, McAlpine MC. Piezoelectric nanoribbons for monitoring cellular deformations. Nat Nanotechnol. 2012;7:587–93. doi: 10.1038/nnano.2012.112. [DOI] [PubMed] [Google Scholar]
- 68.Feiner R, Engel L, Fleischer S, Malki M, Gal I, Shapira A, Shacham-Diamand Y, Dvir T. Engineered hybrid cardiac patches with multifunctional electronics for online monitoring and regulation of tissue function. Nat Mater. 2016;15:679–85. doi: 10.1038/nmat4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014;32:773–85. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 70.Bohandy J, Kim BF, Adrian FJ. Metal deposition from a supported metal film using an excimer laser. Journal of Applied Physics. 1986;60:1538–1539. [Google Scholar]
- 71.Barron JA, Ringeisen BR, Kim H, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films. 2004;453–454:383–387. [Google Scholar]
- 72.Campagnola PJD, DM, Epling GA, Hoffacker KD, Howell AR, Pitts JD, Goodman SL. 3-Dimensional Submicron Polymerization of Acrylamide by Multiphoton Excitation of Xanthene Dyes. Macromolecules. 2000;33:1511–1513. [Google Scholar]
- 73.Pitts JDC, PJ, Epling GA, Goodman SL. Submicron Multiphoton Free-Form Fabrication of Proteins and Polymers: Studies of Reaction Efficiencies and Applications in Sustained Release. Macromolecules. 2000;33:1514–1523. [Google Scholar]
- 74.Lee SH, Moon JJ, West JL. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials. 2008;29:2962–8. doi: 10.1016/j.biomaterials.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maruo S, Nakamura O, Kawata S. Three-dimensional microfabrication with two-photon-absorbed photopolymerization. Optics letters. 1997;22:132–4. doi: 10.1364/ol.22.000132. [DOI] [PubMed] [Google Scholar]
- 76.LaFratta CN, Baldacchini T, Farrer RA, Fourkas JT, Teich MC, Saleh BEA, Naughton MJ. Replication of two-photon-polymerized structures with extremely high aspect ratios and large overhangs. J Phys Chem B. 2004;108:11256–11258. [Google Scholar]
- 77.Allen R, Nielson R, Wise DD, Shear JB. Catalytic three-dimensional protein architectures. Analytical Chemistry. 2005;77:5089–5095. doi: 10.1021/ac0507892. [DOI] [PubMed] [Google Scholar]
- 78.Hoffmann JC, West JL. Three-dimensional photolithographic patterning of multiple bioactive ligands in poly(ethylene glycol) hydrogels. Soft matter. 2010;6:5056–5063. [Google Scholar]
- 79.Tayalia P, Mazur E, Mooney DJ. Controlled architectural and chemotactic studies of 3D cell migration. Biomaterials. 2011;32:2634–2641. doi: 10.1016/j.biomaterials.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wylie RG, Ahsan S, Aizawa Y, Maxwell KL, Morshead CM, Shoichet MS. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nature Materials. 2011;10:799–806. doi: 10.1038/nmat3101. [DOI] [PubMed] [Google Scholar]
- 81.Pitts JDH, AR, Taboada R, Banerjee I, Wang J, Goodman SL, Campagnola PJ. New Photoactivators for Multiphoton Excited Three-dimensional Submicron Cross-linking of Proteins: Bovine Serum Albumin and Type I Collagen. Photochemistry and Photobiology. 2002;76:135–144. doi: 10.1562/0031-8655(2002)076<0135:npfmet>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 82.Basu S, Cunningham LP, Pins GD, Bush KA, Taboada R, Howell AR, Wang J, Campagnola PJ. Multiphoton excited fabrication of collagen matrixes cross-linked by a modified benzophenone dimer: bioactivity and enzymatic degradation. Biomacromolecules. 2005;6:1465–74. doi: 10.1021/bm049258y. [DOI] [PubMed] [Google Scholar]
- 83.Basu S, Wolgemuth CW, Campagnola PJ. Measurement of normal and anomalous diffusion of dyes within protein structures fabricated via multiphoton excited cross-linking. Biomacromolecules. 2004;5:2347–57. doi: 10.1021/bm049707u. [DOI] [PubMed] [Google Scholar]
- 84.Basu S, Campagnola PJ. Properties of crosslinked protein matrices for tissue engineering applications synthesized by multiphoton excitation. J Biomed Mater Res A. 2004;71:359–68. doi: 10.1002/jbm.a.30175. [DOI] [PubMed] [Google Scholar]
- 85.Pins GD, Bush KA, Cunningham LP, Campagnola PJ. Multiphoton excited fabricated nano and micro patterned extracellular matrix proteins direct cellular morphology. J Biomed Mater Res A. 2006;78:194–204. doi: 10.1002/jbm.a.30680. [DOI] [PubMed] [Google Scholar]
- 86.Cunningham LP, Veilleux MP, Campagnola PJ. Freeform multiphoton excited microfabrication for biological applications using a rapid prototyping CAD-based approach. Optics express. 2006;14:8613–21. doi: 10.1364/oe.14.008613. [DOI] [PubMed] [Google Scholar]
- 87.Xu T, Kincaid H, Atala A, Yoo JJ. High-Throughput Production of Single-Cell Microparticles Using an Inkjet Printing Technology. Journal of Manufacturing Science and Engineering. 2008;130:021017–021017. [Google Scholar]
- 88.Goldmann T, Gonzalez JS. DNA-printing: utilization of a standard inkjet printer for the transfer of nucleic acids to solid supports. J Biochem Biophys Methods. 2000;42:105–10. doi: 10.1016/s0165-022x(99)00049-4. [DOI] [PubMed] [Google Scholar]
- 89.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27:3580–8. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 90.Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal inkjet printed Chinese hamster ovary cells. Biotechnol Bioeng. 2010;106:963–9. doi: 10.1002/bit.22762. [DOI] [PubMed] [Google Scholar]
- 91.Kirchmajer DM, Gorkin R, Iii, in het Panhuis M. An overview of the suitability of hydrogel-forming polymers for extrusion-based 3D-printing. Journal of Materials Chemistry B. 2015;3:4105–4117. doi: 10.1039/c5tb00393h. [DOI] [PubMed] [Google Scholar]
- 92.Peltola SM, Melchels FP, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008;40:268–80. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 93.Van Rie J, Declercq H, Van Hoorick J, Dierick M, Van Hoorebeke L, Cornelissen R, Thienpont H, Dubruel P, Van Vlierberghe S. Cryogel-PCL combination scaffolds for bone tissue repair. J Mater Sci Mater Med. 2015;26:123. doi: 10.1007/s10856-015-5465-8. [DOI] [PubMed] [Google Scholar]
- 94.Chien KB, Makridakis E, Shah RN. Three-dimensional printing of soy protein scaffolds for tissue regeneration. Tissue Eng Part C Methods. 2013;19:417–26. doi: 10.1089/ten.TEC.2012.0383. [DOI] [PubMed] [Google Scholar]
- 95.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater. 2014;26:3124–30. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 96.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–7. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, Okano T. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, Zorlutuna P, Vrana NE, Ghaemmaghami AM, Dokmeci MR, Khademhosseini A. Direct-write Bioprinting of Cell-laden Methacrylated Gelatin Hydrogels. Biofabrication. 2014;6:024105–024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35:49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 100.Loo Y, Lakshmanan A, Ni M, Toh LL, Wang S, Hauser CA. Peptide Bioink: Self-Assembling Nanofibrous Scaffolds for Three-Dimensional Organotypic Cultures. Nano Lett. 2015;15:6919–25. doi: 10.1021/acs.nanolett.5b02859. [DOI] [PubMed] [Google Scholar]
- 101.Levato R, Visser J, Planell JA, Engel E, Malda J, Mateos-Timoneda MA. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6:035020. doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 102.Hanson KP, Jung JP, Tran QA, Hsu SP, Iida R, Ajeti V, Campagnola PJ, Eliceiri KW, Squirrell JM, Lyons GE, Ogle BM. Spatial and temporal analysis of extracellular matrix proteins in the developing murine heart: a blueprint for regeneration. Tissue Eng Part A. 2013;19:1132–43. doi: 10.1089/ten.tea.2012.0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jung JP, Hu D, Domian IJ, Ogle BM. An integrated statistical model for enhanced murine cardiomyocyte differentiation via optimized engagement of 3D extracellular matrices. Scientific reports. 2015;5:18705. doi: 10.1038/srep18705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pati F, Jang J, Ha DH, Won Kim S, Rhie JW, Shim JH, Kim DH, Cho DW. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat Commun. 2014;5:3935. doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seifu DG, Purnama A, Mequanint K, Mantovani D. Small-diameter vascular tissue engineering. Nature reviews Cardiology. 2013;10:410–21. doi: 10.1038/nrcardio.2013.77. [DOI] [PubMed] [Google Scholar]
- 106.Duan B. State-of-the-Art Review of 3D Bioprinting for Cardiovascular Tissue Engineering. Ann Biomed Eng. 2016 doi: 10.1007/s10439-016-1607-5. [DOI] [PubMed] [Google Scholar]
- 107.Kucukgul C, Ozler Sb, Inci I, Karakas E, Irmak S, Gozuacik D, Taralp A, Koc B, Koc B. 3D bioprinting of biomimetic aortic vascular constructs with self-supporting cells. doi: 10.1002/bit.25493. [DOI] [PubMed] [Google Scholar]
- 108.Cui X, Boland T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials. 2009;30:6221–6227. doi: 10.1016/j.biomaterials.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 109.Gao Q, He Y, Fu JZ, Liu A, Ma L. Coaxial nozzle-assisted 3D bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 2015;61:203–15. doi: 10.1016/j.biomaterials.2015.05.031. [DOI] [PubMed] [Google Scholar]
- 110.Yu Y, Zhang Y, Ozbolat IT. A Hybrid Bioprinting Approach for Scale-Up Tissue Fabrication. Journal of Manufacturing Science and Engineering. 2014;136:061013–061013. [Google Scholar]
- 111.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 113.Etzion S, Kedes LH, Kloner RA, Leor J. Myocardial regeneration: present and future trends. American journal of cardiovascular drugs: drugs, devices, and other interventions. 2001;1:233–44. doi: 10.2165/00129784-200101040-00002. [DOI] [PubMed] [Google Scholar]
- 114.Valiente-Alandi I, Albo-Castellanos C, Herrero D, Sanchez I, Bernad A. Bmi1 (+) cardiac progenitor cells contribute to myocardial repair following acute injury. Stem cell research & therapy. 2016;7:100. doi: 10.1186/s13287-016-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–57. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair--lessons from clinical trials. Nat Rev Cardiol. 2014;11:232–46. doi: 10.1038/nrcardio.2014.9. [DOI] [PubMed] [Google Scholar]
- 118.Reffelmann T, Kloner RA. Cellular cardiomyoplasty--cardiomyocytes, skeletal myoblasts, or stem cells for regenerating myocardium and treatment of heart failure? Cardiovasc Res. 2003;58:358–68. doi: 10.1016/s0008-6363(02)00739-3. [DOI] [PubMed] [Google Scholar]
- 119.Santoso MR, Yang PC. Magnetic Nanoparticles for Targeting and Imaging of Stem Cells in Myocardial Infarction. Stem Cells Int. 2016;2016:4198790. doi: 10.1155/2016/4198790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1997;11:683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 121.Wendel JS, Ye L, Tao R, Zhang J, Zhang J, Kamp TJ, Tranquillo RT. Functional Effects of a Tissue-Engineered Cardiac Patch From Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes in a Rat Infarct Model. Stem cells translational medicine. 2015;4:1324–32. doi: 10.5966/sctm.2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wendel JS, Ye L, Zhang P, Tranquillo RT, Zhang JJ. Functional consequences of a tissue-engineered myocardial patch for cardiac repair in a rat infarct model. Tissue engineering Part A. 2014;20:1325–35. doi: 10.1089/ten.tea.2013.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gaebel R, Ma N, Liu J, Guan J, Koch L, Klopsch C, Gruene M, Toelk A, Wang W, Mark P, Wang F, Chichkov B, Li W, Steinhoff G. Patterning human stem cells and endothelial cells with laser printing for cardiac regeneration. Biomaterials. 2011;32:9218–30. doi: 10.1016/j.biomaterials.2011.08.071. [DOI] [PubMed] [Google Scholar]
- 124.Gaetani R, Doevendans PA, Metz CH, Alblas J, Messina E, Giacomello A, Sluijter JP. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33:1782–90. doi: 10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 125.Gaetani R, Feyen DA, Verhage V, Slaats R, Messina E, Christman KL, Giacomello A, Doevendans PA, Sluijter JP. Epicardial application of cardiac progenitor cells in a 3D-printed gelatin/hyaluronic acid patch preserves cardiac function after myocardial infarction. Biomaterials. 2015;61:339–48. doi: 10.1016/j.biomaterials.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 126.Jana S, Tefft BJ, Spoon DB, Simari RD. Scaffolds for tissue engineering of cardiac valves. Acta Biomater. 2014;10:2877–93. doi: 10.1016/j.actbio.2014.03.014. [DOI] [PubMed] [Google Scholar]
- 127.Schoen FJ. Heart valve tissue engineering: quo vadis? Curr Opin Biotechnol. 2011;22:698–705. doi: 10.1016/j.copbio.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 128.Chambers J. Prosthetic heart valves. Int J Clin Pract. 2014;68:1227–30. doi: 10.1111/ijcp.12309. [DOI] [PubMed] [Google Scholar]
- 129.Hockaday LA, Kang KH, Colangelo NW, Cheung PY, Duan B, Malone E, Wu J, Girardi LN, Bonassar LJ, Lipson H, Chu CC, Butcher JT. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4:035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Duan B, Hockaday LA, Kang KH, Butcher JT. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res A. 2013;101:1255–64. doi: 10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting Cardiac Cellular Composition. Circulation research. 2016;118:400–9. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, Bakht SM, Yang J, Jia W, Dell’Erba V, Assawes P, Shin SR, Dokmeci MR, Oklu R, Khademhosseini A. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng. 2016 doi: 10.1007/s10439-016-1612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci Adv. 2015;1:e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perez-Pomares JM, Foty RA. Tissue fusion and cell sorting in embryonic development and disease: biomedical implications. Bioessays. 2006;28:809–21. doi: 10.1002/bies.20442. [DOI] [PubMed] [Google Scholar]
- 135.Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication. 2010;2:022001. doi: 10.1088/1758-5082/2/2/022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Olsen TR. Bioprocessing of Tissues using Cellular Spheroids. Journal of Bioprocessing & Biotechniques. 2014:04. [Google Scholar]
- 137.Walser R, Metzger W, Gorg A, Pohlemann T, Menger MD, Laschke MW. Generation of co-culture spheroids as vascularisation units for bone tissue engineering. European cells & materials. 2013;26:222–33. doi: 10.22203/ecm.v026a16. [DOI] [PubMed] [Google Scholar]
- 138.Bhang SH, Cho SW, La WG, Lee TJ, Yang HS, Sun AY, Baek SH, Rhie JW, Kim BS. Angiogenesis in ischemic tissue produced by spheroid grafting of human adipose-derived stromal cells. Biomaterials. 2011;32:2734–47. doi: 10.1016/j.biomaterials.2010.12.035. [DOI] [PubMed] [Google Scholar]
- 139.Ehrmann RL, Gey GO. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956;16:1375–403. [PubMed] [Google Scholar]
- 140.Itoh M, Nakayama K, Noguchi R, Kamohara K, Furukawa K, Uchihashi K, Toda S, Oyama J, Node K, Morita S. Scaffold-Free Tubular Tissues Created by a Bio-3D Printer Undergo Remodeling and Endothelialization when Implanted in Rat Aortae. PLoS One. 2015;10:e0136681. doi: 10.1371/journal.pone.0136681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Noguchi R, Nakayama K, Itoh M, Kamohara K, Furukawa K, Oyama J, Node K, Morita S. Development of a three-dimensional pre-vascularized scaffold-free contractile cardiac patch for treating heart disease. J Heart Lung Transplant. 2016;35:137–45. doi: 10.1016/j.healun.2015.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.