Abstract
Distinguishing true antigen stimulated lymphocytes from bystanders activated by the inflammatory milieu has been difficult. Nur77 is an immediate early gene whose expression is rapidly up-regulated by T cell receptor (TCR) signaling in murine T cells and human thymocytes. Nur77-GFP transgenes serve as specific TCR and B cell receptor (BCR) signaling reporters in murine transgenic models. In this study, we demonstrate that endogenous Nur77 protein expression can serve as a reporter of TCR and BCR specific signaling in human PBMCs. Nur77 protein levels were assessed by immunofluorescence and flow cytometry in T and B cells isolated from human PBMCs obtained from healthy donors that had been stimulated by their respective Ag receptors. We demonstrate that endogenous Nur77 is a more specific reporter of antigen-specific signaling events than the commonly used CD69 activation marker in both human T and B cells. This is reflective of the disparity in signaling pathways that regulate the expression of Nur77 and CD69. Assessing endogenous Nur77 protein expression has great potential to identify antigen-activated lymphocytes in human disease.
Introduction
Identification of antigen (Ag)-specific T and B cells is important to understand early pathogenesis and develop therapeutic strategies for immunologically-mediated human diseases such as autoimmunity, cancer, and transplant rejection, particularly in diseases where the inciting Ag is unknown. Infiltrating immune cells at sites of inflammation are heterogeneous. They become activated not only through direct Ag stimulation, but also indirectly by other inflammatory mediators such as type 1 interferons (IFN) and interleukins (IL) (1, 2). Distinguishing true Ag-stimulated lymphocytes in humans from bystanders activated by the inflammatory milieu has been challenging.
Numerous studies have identified the critical role of cytokines and growth factors in promoting chronic inflammation (3, 4). Interest has focussed on downstream transcriptional mediators of these pro-inflammatory signals. The nuclear receptor (NR) superfamily are elusive receptors with no known natural ligand that can directly bind to DNA and regulate gene transcription (5, 6), capable of modulating immune and metabolic pathways (7). The NR4A subfamily of orphan NRs (NR4A1/Nur77, NR4A2/Nurr1, NR4A3/Nor1) have emerged as molecular switches important in cell survival and inflammation. Their diverse and at times paradoxical roles are context and tissue specific and have been associated with carcinogenesis, DNA repair, proliferation, metabolism and inflammatory responses in disease (8–12). In addition to its role as a transcriptional activator, non-genomic pro-apoptotic functions of Nur77 have been described via mitochondrial interactions with Bcl-2 (13, 14). Nurr77’s expression is also rapidly up-regulated by antigen-receptor signaling and is implicated in thymic negative selection (15, 16) and T regulatory cell fate (17).
The expression of Nur77 can serve as a specific TCR signaling reporter as has been demonstrated in human thymic tissue (18) and murine reporter mice (1, 19), as it has been shown not to respond to type I IFN or cytokine stimulation in murine models (1). This coincides with array data that reveals Nur77 is highly up-regulated in the context of Ag driven autoimmune disease (Immunological Genome Project). The induction of Nur77 appears to be spatially and temporally specific. Studies of an in vivo murine model using OTII transgenic mice with TCRs specific for OVA peptide reported the induction of both endogenous Nur77 protein expression and the induction of a Nur77 transgenic reporter in Ag-specific T cells from antigen draining lymph nodes after footpad immunization, but not from their contralateral nodes (20). One shortcoming of using the Nur77-GFP reporter, in contrast to endogenous Nur77, in these studies was its late expression in contralateral lymph node T cells, presumably due to persistent GFP expression in Ag-reactive OTII T cells that had migrated to this node.
Identification of human Ag-specific T and B cells would be of value for understanding autoimmune diseases, immune responses to cancers and infectious disease and for the design and evaluation of targeted therapeutics. We were interested to determine whether the induction of endogenous Nur77 protein can be used to identify antigen specific human T and B cells and whether the degree of induction of Nur77 protein reflects TCR and B cell receptor (BCR) signaling strength respectively. If so, we also wanted to determine whether Nur77 can be used more effectively as a specific marker of Ag-activated human lymphocytes instead of more promiscuous lymphocyte activation markers, such as CD69.
In this study, we demonstrate that induction of Nur77 protein can serve as a reporter of Ag-receptor signaling in peripheral human T and B cells, integrating upstream signalling events. Furthermore, we demonstrate that Nur77 is a more specific reporter of TCR and BCR signaling than CD69 in human T and B cells, respectively. This is reflective of differences in the requirements of upstream specific signaling events between Nur77 and CD69. These results have important implications for identifying antigen specific T and B cells in a variety of human diseases.
Materials and Methods
Collection and processing of human peripheral blood mononuclear cells (PBMCs)
Whole human blood was collected from healthy volunteers in 60 mL syringes containing heparin as the anticoagulant and gently mixed. Samples were immediately diluted with 15 mL PBS. Leukocytes were isolated by density gradient centrifugation (400 × g for 30 min) over Histopaque® 1077 solution (Sigma-Aldrich). PBMCs harvested from the interface were washed with PBS and resuspended in RPMI-1640 medium with 10% FBS. Cell count and viability was determined using Vi-Cell cell counter (XR 2.03, Beckman Coulter). Cells were either prepared for stimulation assays or cryopreserved in complete medium with 10% DMSO.
Mice
Thymocytes from C57Bl/6 mice were used as a positive control for western blot analysis. All mice were housed and bred in specific pathogen–free conditions in the Animal Barrier Facility at UCSF according to the University Animal Care Committee and National Institutes of Health (NIH) guidelines.
Lysate preparation and western blotting
Murine thymocytes and Jurkat cells were stimulated with PMA (20ng mL−1) and ionomycin (1µM) or C305, an anti-Vβ8 mouse mAb (IgM) that reacts with the Jurkat TCR β chain (21) for two hours at 37°C. Cells were lysed directly in SDS-PAGE sample buffer after stimulation. Proteins were separated on SDS-PAGE gels, transferred to Immobilon-P polyvinylidene difluoride membranes (Millipore) by standard immunoblotting techniques. Primary staining was performed with the following antibodies: Nur77 (eBioscience) and GAPDH (Santa Cruz). Horseradish peroxidase (HRP)-conjugated secondary antibodies were from Southern Biotech and visualized with SuperSignal ECL reagent or SuperSignal West Femto maximum sensitivity substrate (Pierce Biotechnology) on Chemi-Doc Image Lab station (Bio-Rad).
PBMC stimulation assays
Fresh or thawed cell suspensions were washed in complete medium, counted and 0.7–1.0 × 106 cells per well were seeded in round bottom 96-well culture plates in RPMI-1640 culture medium supplemented with 10% FBS. Cells were stimulated with indicated antibodies or immunostimulants. Stimuli included Leu4 (IgG1) mouse mAb directed against human CD3ε purified from ascites, goat anti-human IgM Fab’2 (Jackson ImmunoResearch Laboratories, Inc.), Staphylococcal enterotoxin E (SEE; ToxinTechnologies), CpG oligonucleotides, ODN 2216 for T cells and CpG ODN 2006 and 1826 for B cells (InvivoGen), Interferon alpha (gift from PBL Interferon Source), LPS from Escherichia coli O111:B4 (Sigma), human recombinant IL-4 (R&D Systems), ionomycin 1 µM (Calbiochem), PMA (phorbol 12-myristate 13-acetate, 20ng ml−1) (Cell signaling).
Antibodies and other reagents
The following fluorescent conjugated antibodies were used: antibodies to human CD3 (UCHT1; eBioscience and Biolegend), CD4 (RPA-T4), CD45RA (HI100), IgD (IA6-2), CD56 (B159), CD69 (L78) (BD Biosciences), CD8a (HIT8a), CD45RO (UCHL1) (Biolegend), IgM Fab fragment (Jackson ImmunoResearch) were conjugated to fluorescein isothiocyanate (FITC) or Alexa 488, peridinin chlorophyll protein complex (PerCP)-Cy5.5, PE-Cy7, Pacific blue, allophycocyanin (APC) or Alexa647, or Alexa780 for fluorescence-activated cell sorting (FACS) staining; CD20 antibody (clone H1) conjugated to PerCP-Cy5.5 (BD Phosflow), Nur77 (clone 12.14) conjugated to phycoerythrin (PE; eBiosciences), pSTAT1 (Y701; clone 4a) conjugated to PE, and pSTAT6 (Y641; clone 18/P) conjugated to APC (BD Biosciences) for intracellular staining. Human Fc blocking reagent (Miltenyi Biotec) was used in all stains.
Inhibitors were used at the following concentrations: Syk inhibitor Bay 61–3606 10µM (19, 22), cyclosporine A 20µM (23, 24), Go-6983 40nM (19), PP2 20µM (19, 25), purchased from Calbiochem and cycloheximide (CHX) 10µg ml−1 (Sigma); pan-PI(3)K inhibitor GDC-0941 500nM and mTOR inhibitor INK128 200nM (26, 27) (gift of K. Shokat, UCSF); pan-Jak inhibitor tofacitinib (CP-690,550) 1µM (26), Go-6976 40nM (28), MEK inhibitor PD0325901 100nM (29) and AKT inhibitor MK2206 1µM (26) were purchased from Selleck Chemicals (Houston, TX) and Rapamycin 10nM (23) from Gemini Bio-Products. DMSO was used at 0.1% as a vehicle control in both unstimulated and stimulated samples without inhibitor treatment. DMSO treatment did not affect the basal levels or induction of Nur77 or CD69 (data not shown).
Flow cytometry and data analysis
Cells were stained with antibodies of the indicated specificities and analyzed on a BD LSR Fortessa flow cytometer. Flow cytometry plots and analyses were performed using FlowJo (Tree Star). One-way ANOVA followed by Dunnett’s multiple comparisons test or paired two-tailed student’s t test was performed and graphs were generated using GraphPad Prism version 5.0f for Mac (GraphPad Software, La Jolla, California). Differences were considered significant at p < 0.05.
In vitro lymphocyte stimulation (+/− inhibitor)
Single cell suspensions of lymphocytes were plated at a concentration of 1 × 106 cells per ml in complete RPMI-1640 and were incubated in the presence of stimuli as indicated. For inhibitor experiments, cells were pre-incubated with various inhibitors or vehicle control (DMSO) for 30 minutes at the doses indicated above followed by stimulation with Leu4, anti-IgM Fab’2, cytokine (IFNα, IL-4), or TLR ligand (CPG, LPS) at 37°C.
Intracellular Nur77 and pSTAT staining
Live cells were washed and fixed for 10 min with 2% (vol/vol) fresh paraformaldehyde (PFA) at room temperature. Cells were then stained with cell surface markers as indicated. Cells were fixed again with 2% PFA followed by permeabilization with 0.1% TritonX-100 (Calbiochem) for ten min at room temperature. After permeabilization, cells were stained with anti-Nur77 antibody. Cells were washed twice between each step.
For pSTAT staining, live cells were fixed, stained for surface markers and fixed again as per above. They were then permeabilized with 90% ice-cold methanol, vortexed and maintained on ice for 30 minutes. After permeabilization, cells were stained with the appropriate pSTAT antibody.
Results
Endogenous Nur77 protein reflects the strength of TCR signaling in human peripheral T cells
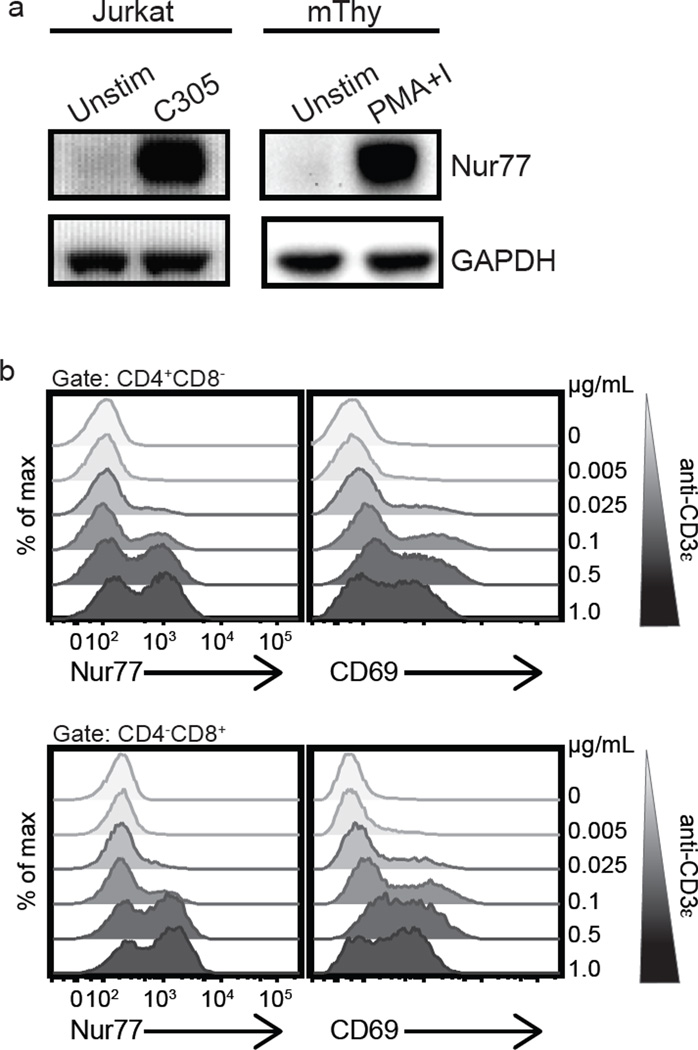
Three independent fluorescent Nur77 reporter lines have been generated in mice and show that Nur77 is a robust marker of specific antigen receptor signaling (1, 19). We wanted to determine whether this held true for endogenous Nur77 induction in human peripheral lymphocytes. Substantial homology exists between the mouse and human NR4A1 genes. Given the 91% shared amino acid identity between the two (30), and limited available anti-human Nur77 reagents for flow cytometry, we determined whether anti-mouse Nur77 antibody could bind human Nur77 protein. To address this, western blotting was performed using lysates from unstimulated Jurkat cells, a human leukemic T cell line, or cells stimulated with the Jurkat specific TCR Vβ8 antibody C305 (21). Nur77 induction in murine thymocytes in response to PMA and ionomycin was used as a positive blotting control. In western blots, a band with appropriate mobility for endogenous Nur77 could be detected in stimulated but not unstimulated Jurkat cells using the anti-mouse Nur77 antibody in response to T cell receptor (TCR) stimulation (Fig. 1a). We confirmed these results in flow cytometry studies in primary human T cells after we adapted the use of this antibody for immunofluorescence and flow cytometry. Polyclonal TCR stimulation of primary human T cells with anti-CD3ε Ab induced Nur77 expression detectable by intracellular staining in a dose dependent manner (Fig. 1b). In vitro TCR stimulation induced endogenous Nur77 in both CD4 and CD8 primary human T cells and the percentages of responding cells correlated with the concentration of stimulating anti-CD3ε mAb (Fig. 1b). Furthermore, this correlated well with CD69 induction.
Figure 1. Endogenous Nur77 expression reflects the strength of antigen receptor signaling of human T cells in vitro.
(a) The Jurkat human T cell line and murine thymocytes (mThy, positive blotting control) were stimulated with C305 or PMA and ionomycin as indicated and were analyzed by immunoblotting for Nur77 and GAPDH (loading control). (b) Histograms represent endogenous intracellular Nur77 and surface CD69 expression of CD4 (top panel) and CD8 (bottom panel) human T cells from a mixed population of PBMCs treated with purified anti-CD3ε mAb for 4 h at the indicated doses (ranging from 0.005 – 1.0 µg ml−1). Data are representative of at least three independent experiments.
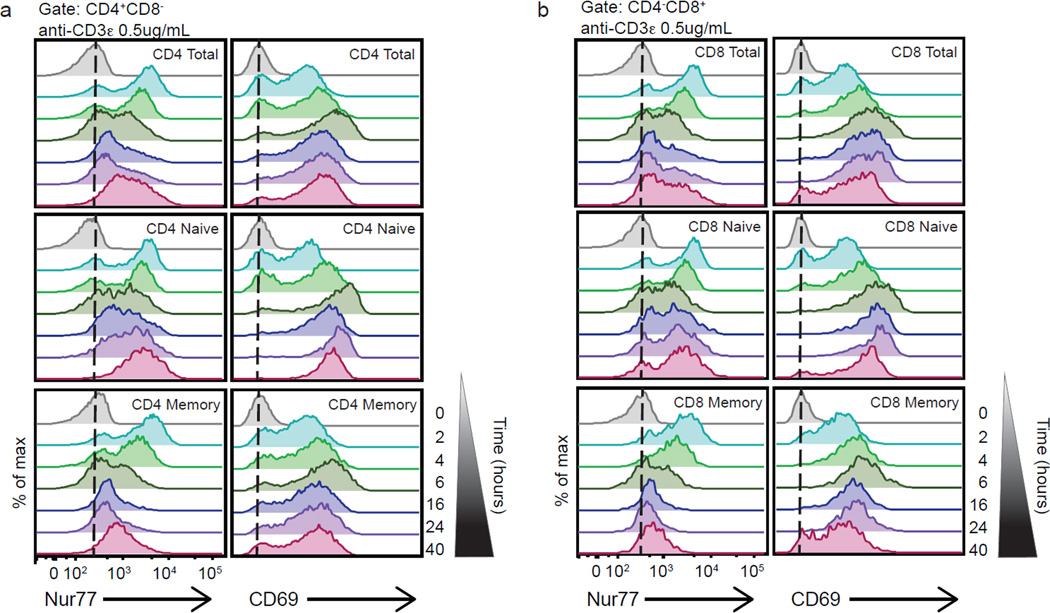
We then examined the temporal characteristics of endogenous Nur77 protein expression in anti-CD3ε stimulated human CD4 and CD8 peripheral blood T cells. Nur77 protein levels peaked between two and four hours after stimulation with anti-CD3ε in both CD4 (Fig. 2a) and CD8 (Fig. 2b) T cells, faster than the peak response of CD69 upregulation. Endogenous Nur77 expression declined after four hours, somewhat faster than the decline of CD69, in the presence of a robust TCR stimulus in vitro, yet expression continued to remain above basal levels at forty hours (Fig. 2a–b). Nur77 induction after TCR stimulation was further characterized in both the naïve (CD45RA+RO−) and memory (CD45RA−RO+) T cell populations. Nur77 production is rapid and robust in both CD4 naïve and memory T cell subsets in response to TCR ligation. Its induction in the memory subset was followed by a more rapid decline after four hours of in vitro stimulation (Fig. 2a). In contrast, the naïve T cells exhibited an initial decline in Nur77 around six hours but had more persistently elevated levels at late time points. These results were mirrored in the CD8 memory and naïve subsets, though the CD8 memory subset exhibited a more rapid decline in intracellular Nur77 (Fig. 2b). Since the CD8+CD45RA+ T cell gate will also capture terminally differentiated CD8 effector memory T cells, CD8 T cells were further distinguished based on CCR7 gating to more accurately resolve the naïve and memory subsets (31–33). There did not appear to be a large difference between the CD8 naïve (RA+CCR7+), central memory (RA−CCR7+) and effector memory (RA−CCR7−) subsets in the dose response and temporal induction of Nur77 in response to TCR stimulation (Supplemental Fig. 1). However, the induction of Nur77 in terminally differentiated (RA+CCR7−) CD8 T cells in response to TCR stimulus appeared to be dampened (Supplemental Fig. 1). Overall, Nur77 was induced rapidly after TCR stimulation, consistent with its known regulation as an immediate early gene. However, its amounts declined more rapidly than CD69, a commonly used marker of early T cell activation, implying that either its transcript is turned off or protein half-life is shorter. Both have been reported in other systems (34, 35). We conclude that elevated intracellular Nur77 protein expression is a sensitive marker for recent antigen encounter in human T cells, as previously demonstrated in murine reporter mice (20).
Figure 2. Endogenous Nur77 levels denote distinct downstream antigen receptor signaling responses after TCR ligation in naïve and memory T cells over time, peaking at early time points.
Histograms represent endogenous intracellular Nur77 and surface CD69 expression of (a) CD4 total T cells (top panels) CD4 naïve T cells (middle panels) and CD4 memory T cells (bottom panels), as well as (b) CD8 total T cells (top panels) CD8 naïve T cells (middle panels) and CD8 memory T cells (bottom panels), from a mixed population of PBMCs treated with anti-CD3ε 0.5 µg ml−1 at the indicated time points (ranging from 2 – 40 hours) (a,b). Data are representative of at least three independent experiments.
Nur77 is a marker of antigen-specific signaling in human T cells
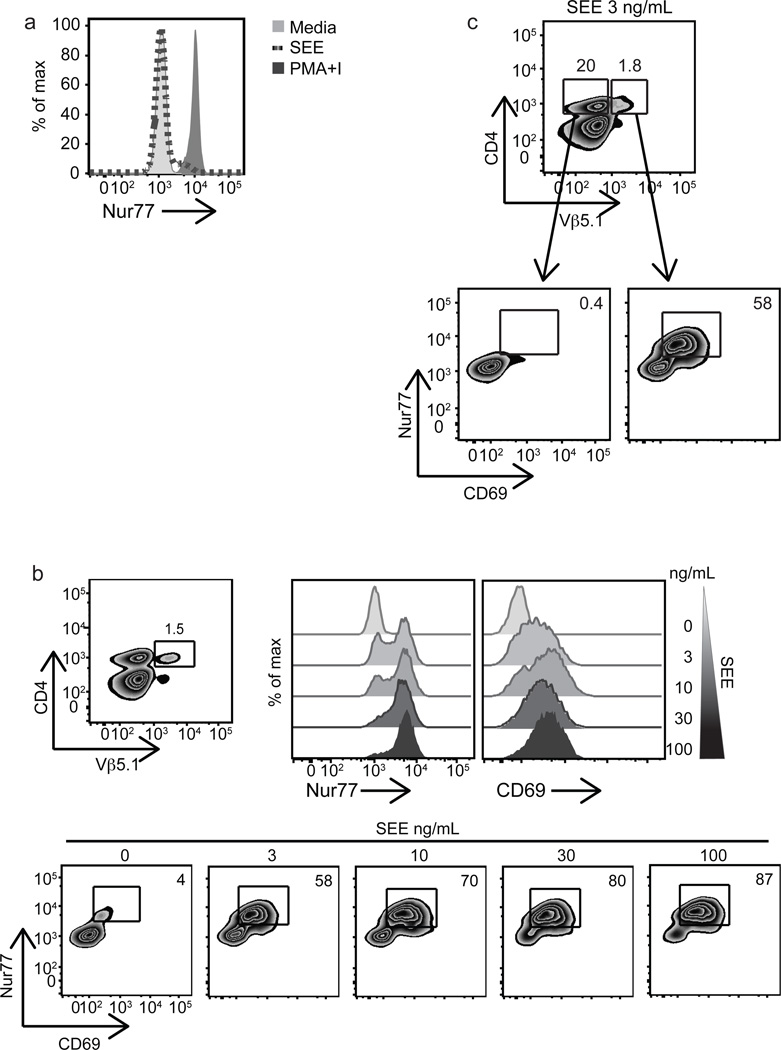
To further validate these results using a more physiologic approach, we stimulated primary human PBMCs with staphylococcal enterotoxin E (SEE), which drives polyclonal activation of Vβ5.1 and Vβ8-expressing T cells through interactions with the TCR in an APC-MHC-dependent manner (36–38). After overnight SEE stimulation, we observed induction of endogenous Nur77 in a small percentage of the unfractionated CD4 T cell population (Fig. 3a). However, when we gated on Vβ5.1 and Vβ8, we observed strong induction of Nur77 and CD69 in a dose dependent manner (Fig. 3b and Supplemental Fig. 2a). Importantly, these responses were specific for the responding Vβ5.1 and Vβ8 populations, as they were not observed in the vast majority of the CD4+Vβ5.1− or CD4+Vβ8− populations (Fig. 3c and Supplemental Fig. 2b). In the superantigen system, we observed a robust and sustained induction of Nur77 at 16 hours in contrast to the rapid decline of Nur77 when using TCR ligation with anti-CD3ε mAb. These results might reflect the retention of super-antigen on the antigen presenting cell (APC)-TCR complex, thereby providing continuous TCR signaling.
Figure 3. Stimulation with SEE validates Nur77 as a marker of antigen specific signaling in human T cells.
Mixed human PBMCs were stimulated with soluble SEE for 16 h and analyzed by FACS. (a) Overlaid histograms are gated on total CD3+CD4+ T cells and represent endogenous Nur77 expression in unstimulated (light grey filled histogram), SEE (dark grey dotted line), or 2 h with PMA and ionomycin (dark grey filled histogram) stimulation. (b) Upper left, plot demonstrates gating strategy used to identify CD4+Vβ5.1+ subset. Upper right, histograms representative of intracellular Nur77 and surface CD69 levels in response to SEE 3 – 100µg ml−1 as indicated in the CD4+Vβ5.1+ subset. Bottom, plots of Nur77 and CD69 dose response of CD4+Vβ5.1+ subset to SEE at indicated doses. (c) Top, CD3+ T cells stained for CD4 and Vβ5.1 expression to identify double positive and CD4+Vβ5.1− subsets. Bottom plots represent Nur77 and CD69 levels in CD4+Vβ5.1− (left) or CD4+Vβ5.1+ (right) subsets stimulated with SEE 3 ng ml−1 overnight. Data are representative of at least two to three independent experiments.
Nur77 is not induced by inflammatory stimuli in human peripheral T cells
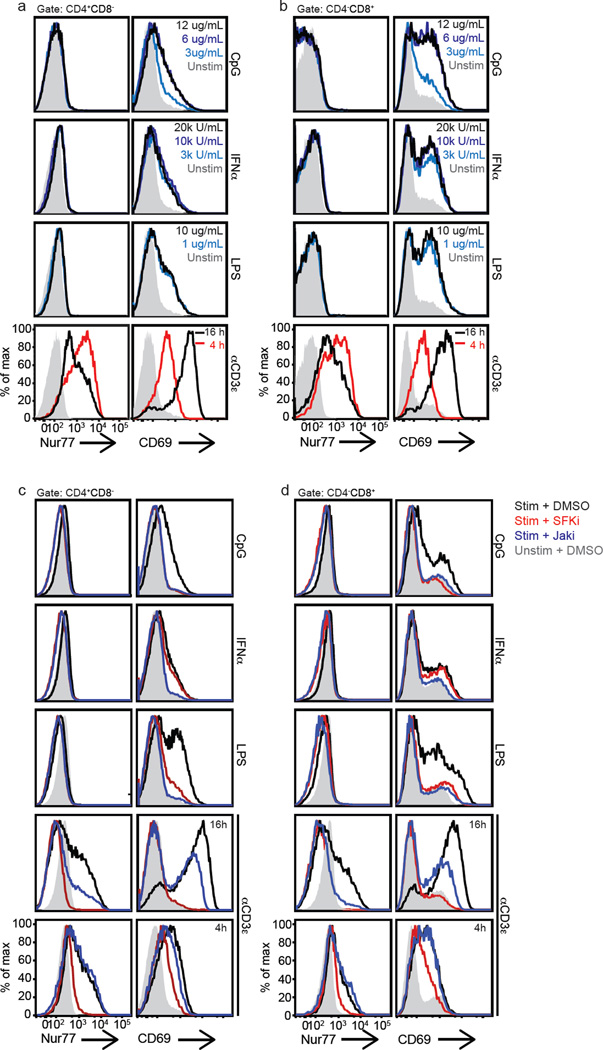
Murine studies previously revealed that Nur77-GFP was not sensitive to mitogenic stimulation, unlike CD69 (1). To address whether Nur77 is a more specific marker of Ag-receptor signaling than CD69 in human T cells, mixed human PBMC populations were stimulated in vitro with various inflammatory stimuli, including CpG ODN, IFNα, LPS, and zymosan, (a fungal cell wall component). Each of these stimuli induced CD69 but not Nur77 expression in human CD4 and CD8 T cells (Fig. 4a–b and data not shown). We also did not observe Nur77 induction in human T cells upon treatment with high doses of IL-6 or IL-2 (data not shown), the latter consistent with published mouse studies (1, 20). These results confirm Nur77’s specificity as a marker of Ag-receptor signaling in T cells and suggest that Nur77 expression may serve as a useful tool to identify Ag-activated T cells in clinical disease settings.
Figure 4. Endogenous Nur77 reflects TCR specific signaling in human T cells, in contrast to CD69, reflective of Jak signaling differences.
Histograms represent endogenous Nur77 and surface CD69 expression of human CD4(a, c) and CD8 (b, d) T cells. (a–b) Mixed human PBMCs were stimulated for 16 h with various immunostimulants: CpG, IFNα, or LPS at the indicated doses, or with anti-CD3ε 1.0 µg ml−1 for 4 or 16 h. Filled in grey histograms represent unstimulated samples treated with media alone. (c–d) Mixed PBMCs were pre-treated in the presence or absence of specific inhibitors and then stimulated for 16 h with CpG 12 µg ml−1, IFNα 20kU ml−1, or LPS 10 µg ml−1, or with anti-CD3ε 1.0 µg ml−1 for 4 or 16 h as indicated. Filled in grey histograms represent unstimulated samples pretreated with vehicle control (DMSO). (SFKi – src family kinase inhibitor, PP2; Jaki – janus kinase inhibitor, tofacitinib). Data are representative of at least three independent experiments and six biologically different donors.
To define the characteristics of the divergent pathways contributing to induction of CD69 via non-TCR mediated pathways, we treated CpG, IFNα, and LPS stimulated human PBMCs with small molecule inhibitors. Since type I IFNs and cytokines activate Janus kinase (JAK) signaling pathways in murine and human T cells (39, 40), cells were pre-incubated with JAK or Src family kinase (SFK) inhibitors — tofacitinib and PP2, respectively. Type I IFN induction of CD69 on CD4 and CD8 T cells proved to be dependent on the JAK signaling pathway with complete inhibition in the presence of tofacitinb, but not PP2 (Fig. 4c–d and Supplemental Fig. 3a). We also found that both the SFKs and JAKs were important in mediating LPS and CpG induction of CD69 on T cells (Fig. 4c–d). The JAK-inhibitor sensitivity suggests that the effects of the TLR ligands were acting indirectly, perhaps through monocyte-derived cytokine release in the mixed PBMC population (41) since TLRs have not been shown to signal through JAKs, consistent with published mouse data (2). The SFK-inhibitor sensitivity coincided with published mouse and human data demonstrating TLR-MyD88-independent LPS and CpG SFK signaling in macrophages resulting in inflammatory cytokine production (42–44).
Interestingly, we observed a late, but not early, effect of JAK-inhibitor on TCR-induced Nur77 induction (Fig. 4c–d and Supplemental Fig. 3b). This demonstrates that direct TCR-mediated Nur77 induction after 4 hours of stimulation is SFK but not JAK dependent as expected. It also suggests that the effects of tofacitinib on the anti-CD3ε induction of Nur77 and CD69 at later time points (16 vs 4 hours) are consistent with undefined secondary effects. We also noted that the late response sensitivity of anti-CD3 stimulation to JAK inhibition varied somewhat between individual donors (Supplemental Fig. 3b).
Nur77 is a specific reporter of B cell receptor signaling strength in human B cells
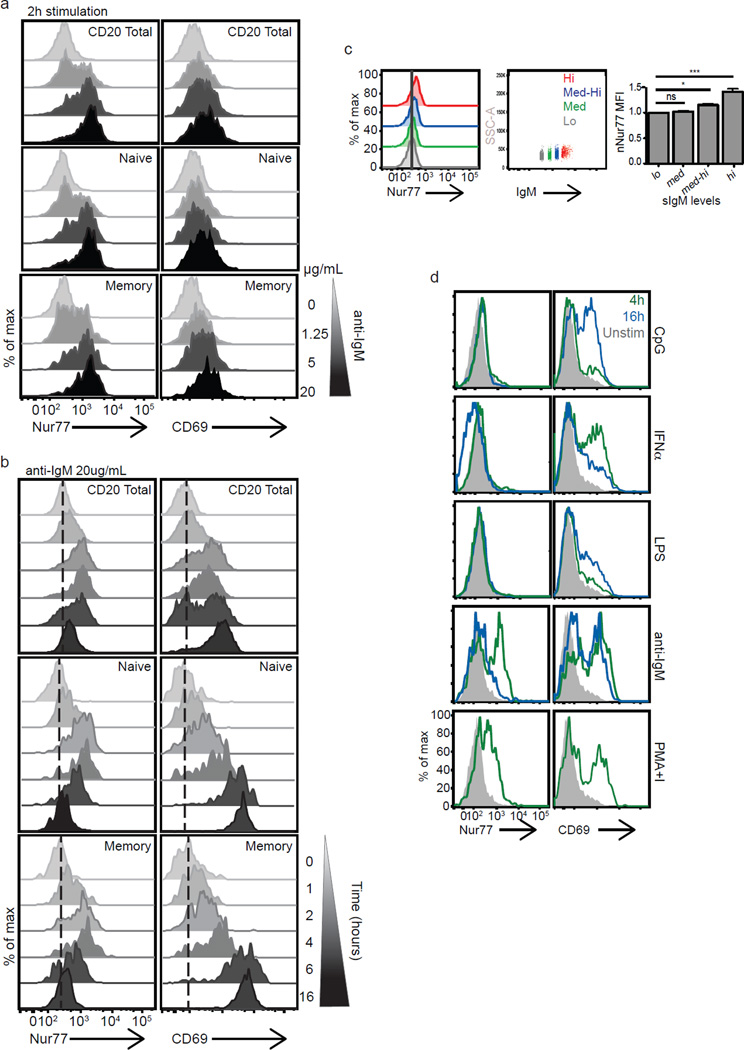
The Nur77-GFP transgene has been used as a reporter of murine B cell signaling events, revealing developmental checkpoints, stages of maturation, and in vivo Ag-receptor signaling in germinal center B cells (19, 45). In humans a majority of newly generated B cells and a significant fraction of mature B cells display some features of autoreactivity (46). Further, this fraction is markedly expanded in patients with autoimmune disease (46–50). Therefore, detection of autoreactive human B cells could be diagnostically important in patients. We asked whether endogenous Nur77 could be stained on a single cell basis in B cells and whether its induction reflected BCR stimulation and signaling strength. We found that Nur77 was strongly induced in human B cells stimulated with anti-IgM in a dose-dependent manner (Fig. 5a). As in T cells, it was robustly induced at early time points, peaking between two and four hours (Fig. 5b). The induction of Nur77 was similar between naïve (CD27−) and memory (CD27+) IgM+ B cell populations, although the memory population appeared to be more sensitive to lower doses of Ag-receptor signaling (Fig. 5a–b), consistent with reports describing comparable early biochemical events despite robust differences in downstream events (51). Additionally, basal Nur77 levels correlated with surface IgM expression in human B cells, which likely reflect differences in tonic B cell signaling (Fig. 5c), consistent with published functional studies in human B cells showing that anergic autoreactive B cells express lower surface IgM (52).
Figure 5. Endogenous Nur77 expression reflects strength of B cell receptor signaling in human B cells.
(a–b) Histograms represent intracellular Nur77 and surface CD69 expression in total human CD20+ B cells, naïve (CD20+IgM+CD27−) and memory (CD20+IgM+CD27+) B cells from mixed PBMC populations (a) treated with varying doses of soluble anti-IgM for 2 h (1.25 – 20 µg ml−1 in a fivefold dilution series) or (b) treated with soluble anti-IgM 20 µg ml−1 from 1–16 hours as indicated. Data are representative of at least three independent experiments and five biologically different donors. (c) Histograms represent basal Nur77 levels in unstimulated primary human CD20+ B cells (left panel) binned on surface IgM expression levels: highest 15% (red), medium-high (blue histogram), medium (green histogram), lowest 15% (grey) (middle panel). Right panel bar graph represents mean Nur77 MFI of B cells based on surface IgM levels and normalized to Nur77 MFI from cells in lowest surface IgM bin +/− SEM. Data are accumulative from two independent experiments and 3–5 biologically different donors. Results are expressed as mean +/− SEM. *p<0.05, ***p<0.001 (d) Mixed human PBMCs were stimulated with various immunostimulants: CpG 12 µg ml−1, IFNα 20kU ml−1, LPS 10 µg ml−1 or with anti-IgM 20 µg ml−1 for 4 h (green histograms) or 16 h (blue histograms) or with PMA and ionomycin for 4 hours. Filled in grey histograms represent unstimulated samples treated with media alone. Data are representative of at least 3 independent experiments.
Interestingly, we find that endogenous Nur77 is a much more specific reporter of Ag-receptor signaling in human B cells than CD69. CD69, but not Nur77 could be efficiently induced by IFNα, TLR-4 (LPS) and TLR-9 (CpG) signaling in a mixed PBMC population (Fig. 5d). One possible explanation for the observed differential induction of Nur77 and CD69 in B cells is that TLR-stimulation of mixed PBMCs may trigger cytokine-dependent CD69 induction while Nur77 induction appears to be independent of JAK-Stat pathways (Supplemental Fig. 3c, ref (1), and data not shown). This is in contrast to what had previously been reported in Nur77-GFP reporter mice (19) and LPS induced Nr4a1 gene expression in murine B cells (53). The delay in CD69 induction by CpG and LPS suggests a delayed or indirect effect on primary human B cells in our assay. The effects of LPS are most likely mediated through TLR4 receptors on monocytes in our cultures since TLR4 receptors are not substantially expressed on human B cells from healthy individuals (54–56). In contrast, the early CD69 induction by IFNα argues for a direct B cell effect, signaling through the JAK-Stat pathway directly (Supplemental Fig. 3c and data not shown).
Biochemical pathways contributing to Nur77 induction reflect T and B cell differences in antigen-receptor signaling
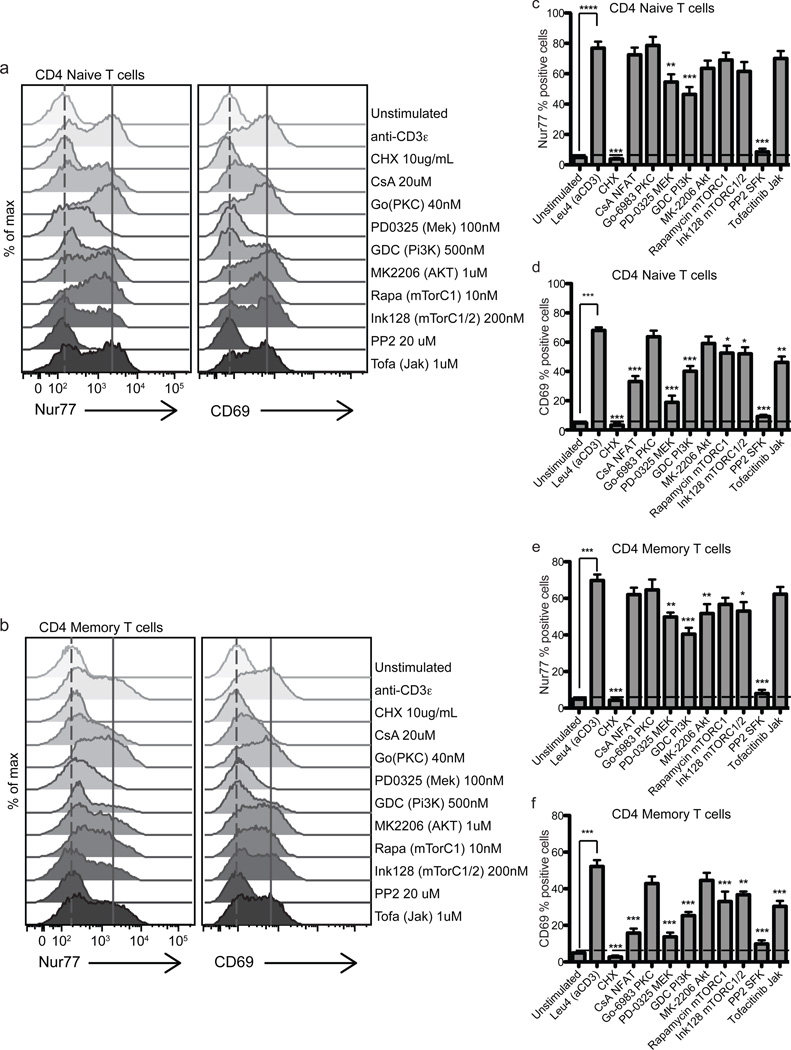
To determine which Ag-receptor induced signaling pathways contribute to Nur77 induction in human T cell subsets and B cells, we treated PBMCs stimulated with anti-CD3ε or anti-IgM with a range of small molecule inhibitors in vitro. These experiments demonstrated a nearly complete dependence on SFKs in T cells and spleen tyrosine kinase (Syk) in B cells, as expected and previously shown (Fig. 6 and 7, (19)). Multiple biochemical pathways are important for Nur77 expression in CD4 T cells. Significant levels of dependency were observed for pathways downstream of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI(3)K) and mitogen-activated protein kinase kinase (MEK) as probed with small molecule inhibitors GDC-0941 and PD0325901, respectively. We also observed significant sensitivity to MK-2206 and INK128 in memory CD4 T cells, inhibitors of Akt and mTORC1/2 (mammalian target of rapamycin complex 1 and 2), respectively, and less sensitivity to rapamycin, inhibitor of mTORC1 alone. Moreover, both memory and naïve T cells’ induction of Nur77 after anti-CD3ε stimulation exhibited similar dependency on the TCR signaling pathways probed (Fig. 6, Supplemental Fig. 4).
Figure 6. Nur77 levels reflect the integration of multiple TCR signaling pathways in human PBMCs.
Histograms represent Nur77 and CD69 induction in CD4+CD8− Naïve (a) and Memory T cells (b) treated with anti-CD3ε in the presence or absence of specific inhibitors for 4 hours. Data in figures 6 a–b are representative of at least 5 biologically different donors. (c–f) Bar graphs represent Nur77 or CD69 percent positive cells in CD4+CD8− Naïve (CD45RA+RO−) (c,d), Memory (CD45RA−RO+) T cells (e,f) treated with anti-CD3ε 1.0 µg ml−1 in the presence or absence of specific inhibitors for 4 hours. Horizontal dashed line in 6c–f marks Nur77 and CD69 % positive of unstimulated cells. Positive gate was set at highest 5% of unstimulated cells. Values in 6c–f are the mean of 5–6 biologically different donors +/− SEM. One-way ANOVA was used to compare unstimulated samples (treated with DMSO vehicle control) and inhibitor treatment groups to Leu4 + inhibitor vehicle control (DMSO). ns p>0.05, *p<0.05, **p<0.01, ***p<0.001
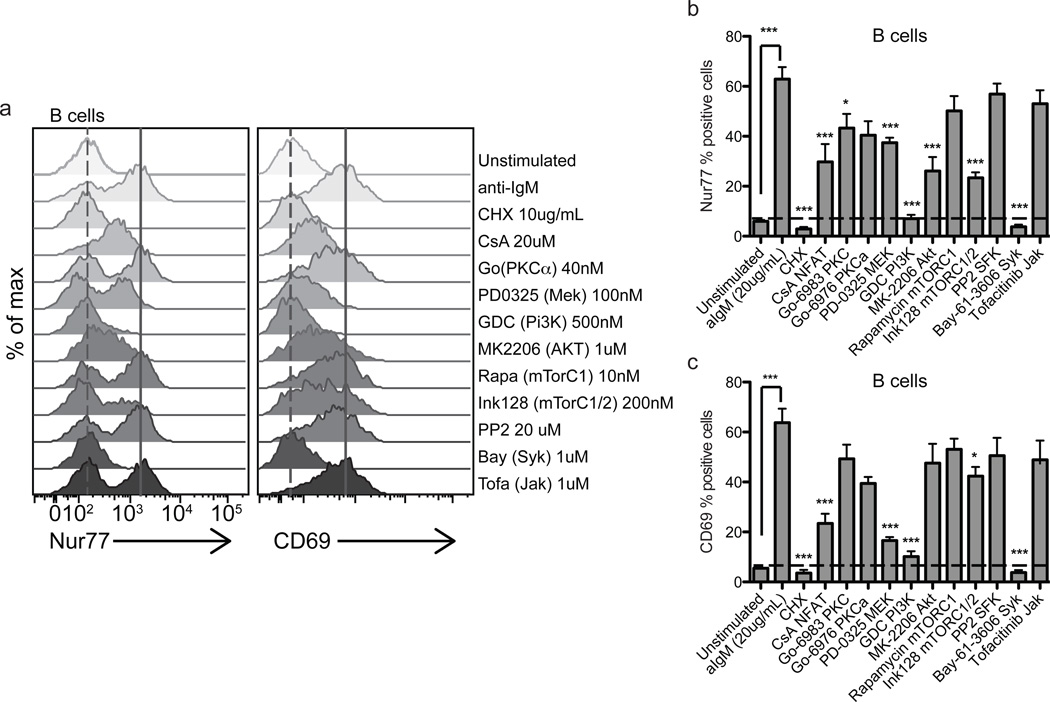
Figure 7. Nur77 levels reflect the integration of multiple BCR signaling pathways in human PBMCs.
Histograms represent Nur77 and CD69 induction in CD20+IgM+ B cells (a) treated with anti-IgM in the presence or absence of specific inhibitors for 4 hours. Histograms are representative of at least 5 biologically different donors. (b,c) Bar graphs represent Nur77 or CD69 % positive cells in CD20+IgM+ B cells treated with anti-IgM in the presence or absence of specific inhibitors for 4 hours. Horizontal dashed lines mark Nur77 or CD69 % positive of unstimulated cells. Positive gate was set at the highest 5% of unstimulated cells. Values in 6b–c are the mean of 5–6 biologically different donors +/− SEM. One-way ANOVA was used to compare unstimulated samples (treated with DMSO vehicle control) and inhibitor treatment groups to anti-IgM + inhibitor vehicle control (DMSO). ns p>0.05, *p<0.05, **p<0.01, ***p<0.001
Interestingly, cyclosporine (CsA), which inhibits calcineurin, a key regulator of the nuclear factor of activated T cell (NFAT) pathway, did not play a significant or substantial role in Nur77 protein expression after TCR signaling, but did in the induction of CD69 in CD4 (Fig. 6c–f) and CD8 (Supplemental Fig. 4) T cells. However, Nur77 protein levels in memory CD4 and CD8 T cells may have been more sensitive to CsA than naïve CD4 T cells, which can be better appreciated in the histograms (Fig. 6a–b, Supplemental Fig. 4a–b), consistent with a published report (57), though this did not reach statistical significance. Interestingly, JAK signaling contributed substantially to CD69 protein levels after TCR stimulation in CD4 naïve and memory T cells (Fig. 6d,f), whereas JAK signaling does not have a significant effect on Nur77 induction in response to four hours of TCR stimulation. These results most likely reflect early cytokine release in these mixed PBMC assays contributing to induction of CD69 and reflecting the relative insensitivity of Nur77 to JAK-Stat signaling (Fig. 4c–d, 5d, and Supplemental Fig. 3a,c (1), and data not shown).
We did not observe a strong dependence of Nur77 induction on PKC signaling using PKC inhibitor Go-6983 (general PKC inhibitor, targeting multiple PKC isoforms) or Go-6976 (classical PKC inhibitor targeting calcium sensitive isoforms, i.e., PKCα and β), coinciding with a published report of calcium dependent, but PKC independent induction of Nur77 (58). This contrasts with what was previously described in murine reporter mice (19). The control of Nur77 transcription can vary depending on cell type (59). Our findings do not rule out a role for PKC signaling in the activation of Nur77 via phosphorylation and translocation as has been previously described in thymocytes (60).
In B cells, Nur77 expression was partially dependent on calcineurin, PKC (Go-6983), MEK, Akt, and mTORC1/2 pathways, and showed a clear dependence on Syk and PI(3)K signaling. The biochemical pathways contributing to the induction of Nur77 and CD69 in B cells after BCR crosslinking appear to be similar, although CD69 protein levels are more sensitive to NFAT and MEK inhibition and are less sensitive to mTORC1/2 inhibition (Fig. 7).
Discussion
Distinguishing true Ag stimulated lymphocytes in humans from bystanders activated by the inflammatory milieu has been difficult. Lymphocytes enriched at sites of inflammation become activated not only through direct Ag stimulation, but also indirectly by other inflammatory mediators. The identification of Ag-specific T and B cell clones has important implications in understanding early events in human diseases. This study describes a novel means of identifying Ag-reactive T and B cells through the detection of endogenous Nur77 protein levels, which serves as a specific reporter of Ag-signaling in both primary human T and B cells.
In CD4 and CD8 T cells, endogenous Nur77 expression directly correlates with the strength of TCR signaling (Fig. 1) consistent with previous reports from Nur77-GFP transgenic mice (1, 19). However, the kinetics of endogenous Nur77 could not be accurately defined in these reporter mice due to the extended in vivo half-life of GFP (61, 62). Nur77 is an immediate early gene and indeed is quickly induced upon Ag-receptor signaling and depends upon new protein synthesis in both T and B cells. Our characterization of Nur77 expression in both memory and naïve T cells demonstrates a rapid induction in both populations with an initial bi-modal response in both subsets. However, after 4–6 hours, Nur77’s expression levels diverge. Nur77 levels rapidly decline in memory T cells and become unimodal, albeit with persistent lower level expression at late time points. In naïve T cells, we observed persistently elevated Nur77 expression.
Temporal differences in Nur77 expression after initial peak induction with anti-CD3ε stimulation in naïve and memory T cell subsets may point toward differences in NR4A1 transcription, protein stability, TCR recycling, cell intrinsic differences in chromatin remodeling (63–65), differences in negative feedback pathways in these cell types, and/or TCR signaling pathways. Disparities in memory and naïve T cell proximal TCR signaling events and effector responses to antigens have been described in both CD4 and CD8 T cells (1, 57, 66–70). Several groups have previously observed greater TCR signaling and calcium mobilization in naïve than in memory T cells from humans and mice (68, 70–73), consistent with our Nur77 results. The distinct Nur77 expression patterns in naïve and memory T cells are reminiscent of calcium flux differences observed in naïve and memory T cells, namely persistent calcium increases in naïve T cells over time versus a rapid decline in memory cells (72).
Despite these differences in Nur77 kinetics, no large disparities were noted in the requirements of the Ag-receptor-induced biochemical pathways between naïve and memory T cells with TCR stimulation at fours hours, although memory T cells appeared to be more sensitive to Akt and mTORC1/2 inhibitors (Fig. 6 and Supplemental Fig. 4). This suggests Nur77 expression is induced by similar TCR signaling events. Downstream TCR signaling events, such as MAPK signaling have been shown to be similar in naïve and memory CD4 T cells (70). How then are the differences in Nur77 expression levels at later time points explained? There are several contributing possibilities. Negative feedback mechanisms may be induced more robustly in memory cell responses to limit immunopathology. The CD45RO isoform expressed in memory T cells is more likely to dimerize and result in dampened TCR signaling compared to the CD45RA isoform expressed in naïve T cells (71). Additionally, the strength of early phosphorylation events are reduced in memory compared to naïve CD4 T cells (70) and may affect later signaling events and gene transcription. Nur77 transcription and protein levels are sensitive to calcium signaling (74, 75) and prolonged calcium increases in naïve T cells may contribute to additional Nur77 transcription. Alternatively, Nur77 may be degraded more rapidly in memory T cells. These mechanisms are not mutually exclusive and can be explored in future studies.
Additionally, we demonstrate that Nur77 levels reflect the strength of BCR signaling in human B cells. The kinetics of Ag-receptor induced Nur77 expression in B cells mirrors that of memory T cells, with a rapid decline in protein levels after four hours of BCR stimulation with anti-IgM. Much as in T cells, multiple Ag-induced BCR signaling pathways contribute to optimal Nur77 protein expression in B cells (Fig. 7). Interestingly, in addition to Syk signaling, Nur77 induction is completely dependent on the PI(3)K signaling pathways, a central part of the BCR-triggered signalsome (76, 77). Cyclosporin A (CsA) appears to interfere more with Nur77 protein expression in response to BCR signaling in B cells, in contrast to what was observed in T cells (Fig. 6, Supplemental Fig. 4). CsA is an immunosuppressive drug widely used in transplant medicine that inhibits calcineurin activity and thereby abrogates NFAT signaling, though there is increasing evidence that CsA has other cellular targets in addition to NFAT (66). CsA has been shown to interfere with TCR-mediated signaling in T cell hybridomas (78–80) by disrupting Nur77 activation without affecting its transcript or protein levels (80). This is consistent with our findings, namely minimal to no effect of CsA on Ag-receptor-induced Nur77 protein levels in T cells (Fig. 6 and Supplemental Fig. 4).
A critically important feature of Nur77 expression in primary human T cells is its specificity for Ag-receptor signaling, in contrast to the commonly used lymphocyte activation marker CD69. CD69 surface expression is induced not only after TCR or BCR signaling, but also via type I IFNs and other immunostimulants (2, 81–83). This appears to be in large part due to JAK-dependent signaling as the pan-JAK inhibitor, tofacitinib, completely abrogates type 1 IFN dependent pSTAT1 and CD69 induction in CD4 and CD8 T cells (Supplemental Fig. 3a and Fig. 4c–d). Nur77 also proves to be a more specific marker of BCR receptor signaling in B cells than CD69, as it is also not induced upon cytokine or TLR stimulation of mixed PBMCs (Fig. 5 and Supplemental Fig. 3c). This differs from what had previously been published in the Nur77-GFP reporter mice (19). We postulate several possibilities may explain this including species differences, tissue specific differences, or prolonged GFP half-life may allow for accumulation of small amounts of Nur77 induction in the setting of immunostimulants that are below the threshold of detection when staining for endogenous Nur77 in human PBMCs.
One limitation for the use of Nur77 in some human studies is the need for detection by intracellular staining, making it difficult to isolate live cells for functional and some forms of genomic analyses. For functional analyses, it would be preferable to identify surrogate surface markers that identify cells enriched in Nur77. However, this has proven to be difficult as many activation markers we have studied can be influenced by inflammatory cytokines. Additionally, surrogate cell surface markers may vary between different diseases. With genomic analyses this may be addressed indirectly by matching NR4A1/NUR77 transcript levels with other response genes of interest. Importantly, recent advancements in genomic methodologies now make it possible to perform gene expression analyses on fixed and permeabilized human cells (84).
In summary, Nur77 can be utilized as a reporter of Ag-specific signaling in primary human T and B cells. Nur77 is an immediate early gene that is rapidly induced after Ag-receptor signaling in both human B and T cells. Elevated levels of Nur77 are likely to represent recent Ag-signaling events, although further studies of in vivo human immune responses are necessary to explore this. Nur77 expression results from integration of multiple Ag-receptor-induced signaling pathways, but is not induced by cytokine or TLR signaling, unlike CD69. These features of Nur77 may prove to be particularly relevant for application to a number of human disease states. Intracellular Nur77 may serve as a reporter to identify bona-fide antigen-specific lymphocytes in cancer, transplant rejection, and autoimmunity.
Supplementary Material
Acknowledgments
The authors thank our donors for their PBMCs. We thank Julie Zikherman for helpful discussions and critical reading of the manuscript. We also thank PBL Interferon Source for providing human IFN-α.
Funding for this project was provided by the Rheumatology Research Foundation Scientist Development Award (to J.A.); the Rosalind Russell Medical Research Foundation Bechtel Award (to J.A.); the UCSF-Stanford Arthritis Center of Excellence (to J.A. and A.W.), which is funded in part by the Northern California Arthritis Foundation; the National Institute of Allergy and Infectious Disease of the National Institutes of Health Awards PO1 AI091580 (to A.W.); and a Rheumatology Research Foundation Within Our Reach grant (to A.W.).
References
- 1.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011;208(6):1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. The Journal of experimental medicine. 1998;188(12):2335–2342. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nature reviews Immunology. 2007;7(6):429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 4.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. The Journal of clinical investigation. 2008;118(11):3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olefsky JM. Nuclear receptor minireview series. The Journal of biological chemistry. 2001;276(40):36863–36864. doi: 10.1074/jbc.R100047200. [DOI] [PubMed] [Google Scholar]
- 6.Evans RM. The steroid and thyroid hormone receptor superfamily. Science (New York, NY) 1988;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bensinger SJ, Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 8.Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter DC, Murphy EP. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18(12):3223–3228. doi: 10.1158/1078-0432.CCR-11-2953. [DOI] [PubMed] [Google Scholar]
- 9.Murphy EP, McEvoy A, Conneely OM, Bresnihan B, FitzGerald O. Involvement of the nuclear orphan receptor NURR1 in the regulation of corticotropin-releasing hormone expression and actions in human inflammatory arthritis. Arthritis and rheumatism. 2001;44(4):782–793. doi: 10.1002/1529-0131(200104)44:4<782::AID-ANR134>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.McMorrow JP, Murphy EP. Inflammation: a role for NR4A orphan nuclear receptors? Biochemical Society transactions. 2011;39(2):688–693. doi: 10.1042/BST0390688. [DOI] [PubMed] [Google Scholar]
- 11.McEvoy AN, Murphy EA, Ponnio T, Conneely OM, Bresnihan B, FitzGerald O, Murphy EP. Activation of nuclear orphan receptor NURR1 transcription by NF-kappa B and cyclic adenosine 5’-monophosphate response element-binding protein in rheumatoid arthritis synovial tissue. J Immunol. 2002;168(6):2979–2987. doi: 10.4049/jimmunol.168.6.2979. [DOI] [PubMed] [Google Scholar]
- 12.Chen HZ, Liu QF, Li L, Wang WJ, Yao LM, Yang M, Liu B, Chen W, Zhan YY, Zhang MQ, et al. The orphan receptor TR3 suppresses intestinal tumorigenesis in mice by downregulating Wnt signalling. Gut. 2012;61(5):714–724. doi: 10.1136/gutjnl-2011-300783. [DOI] [PubMed] [Google Scholar]
- 13.Thompson J, Winoto A. During negative selection, Nur77 family proteins translocate to mitochondria where they associate with Bcl-2 and expose its proapoptotic BH3 domain. The Journal of experimental medicine. 2008;205(5):1029–1036. doi: 10.1084/jem.20080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, Town J, Cao X, Lin F, et al. A short Nur77-derived peptide converts Bcl-2 from a protector to a killer. Cancer cell. 2008;14(4):285–298. doi: 10.1016/j.ccr.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou T, Cheng J, Yang P, Wang Z, Liu C, Su X, Bluethmann H, Mountz JD. Inhibition of Nur77/Nurr1 leads to inefficient clonal deletion of self-reactive T cells. The Journal of experimental medicine. 1996;183(4):1879–1892. doi: 10.1084/jem.183.4.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calnan BJ, Szychowski S, Chan FK, Cado D, Winoto A. A role for the orphan steroid receptor Nur77 in apoptosis accompanying antigen-induced negative selection. Immunity. 1995;3(3):273–282. doi: 10.1016/1074-7613(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 17.Caton AJ, Kropf E, Simons DM, Aitken M, Weissler KA, Jordan MS. Strength of TCR signal from self-peptide modulates autoreactive thymocyte deletion and Foxp3(+) Treg-cell formation. European journal of immunology. 2014;44(3):785–793. doi: 10.1002/eji.201343767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng LE, Chan FK, Cado D, Winoto A. Functional redundancy of the Nur77 and Nor-1 orphan steroid receptors in T-cell apoptosis. The EMBO journal. 1997;16(8):1865–1875. doi: 10.1093/emboj/16.8.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zikherman J, Parameswaran R, Weiss A. Endogenous antigen tunes the responsiveness of naive B cells but not T cells. Nature. 2012;489(7414):160–164. doi: 10.1038/nature11311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au-Yeung BB, Zikherman J, Mueller JL, Ashouri JF, Matloubian M, Cheng DA, Chen Y, Shokat KM, Weiss A. A sharp T-cell antigen receptor signaling threshold for T-cell proliferation. Proc Natl Acad Sci U S A. 2014;111(35):E3679–E3688. doi: 10.1073/pnas.1413726111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weiss A, Stobo JD. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. The Journal of experimental medicine. 1984;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto N, Takeshita K, Shichijo M, Kokubo T, Sato M, Nakashima K, Ishimori M, Nagai H, Li YF, Yura T, et al. The orally available spleen tyrosine kinase inhibitor 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]nicotinamide dihydrochloride (BAY 61-3606) blocks antigen-induced airway inflammation in rodents. The Journal of pharmacology and experimental therapeutics. 2003;306(3):1174–1181. doi: 10.1124/jpet.103.052316. [DOI] [PubMed] [Google Scholar]
- 23.Henderson DJ, Naya I, Bundick RV, Smith GM, Schmidt JA. Comparison of the effects of FK-506, cyclosporin A and rapamycin on IL-2 production. Immunology. 1991;73(3):316–321. [PMC free article] [PubMed] [Google Scholar]
- 24.Flanagan WM, Corthesy B, Bram RJ, Crabtree GR. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- 25.Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. The Journal of biological chemistry. 1996;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 26.Smith GA, Uchida K, Weiss A, Taunton J. Essential biphasic role for JAK3 catalytic activity in IL-2 receptor signaling. Nature chemical biology. 2016;12(5):373–379. doi: 10.1038/nchembio.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. Journal of medicinal chemistry. 2008;51(18):5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 28.Young LH, Balin BJ, Weis MT. Go 6983: a fast acting protein kinase C inhibitor that attenuates myocardial ischemia/reperfusion injury. Cardiovascular drug reviews. 2005;23(3):255–272. doi: 10.1111/j.1527-3466.2005.tb00170.x. [DOI] [PubMed] [Google Scholar]
- 29.Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, Delaney AM, Kaufman M, LePage S, Leopold WR, et al. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorganic & medicinal chemistry letters. 2008;18(24):6501–6504. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 30.Chang C, Kokontis J, Liao SS, Chang Y. Isolation and characterization of human TR3 receptor: a member of steroid receptor superfamily. J Steroid Biochem. 1989;34(1–6):391–395. doi: 10.1016/0022-4731(89)90114-3. [DOI] [PubMed] [Google Scholar]
- 31.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J Immunol. 2005;175(9):5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 33.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology. 2004;22(745–763) doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 34.Santis AG, Lopez-Cabrera M, Sanchez-Madrid F, Proudfoot N. Expression of the early lymphocyte activation antigen CD69, a C-type lectin, is regulated by mRNA degradation associated with AU-rich sequence motifs. European journal of immunology. 1995;25(8):2142–2146. doi: 10.1002/eji.1830250804. [DOI] [PubMed] [Google Scholar]
- 35.Ryseck RP, Macdonald-Bravo H, Mattei MG, Ruppert S, Bravo R. Structure, mapping and expression of a growth factor inducible gene encoding a putative nuclear hormonal binding receptor. The EMBO journal. 1989;8(11):3327–3335. doi: 10.1002/j.1460-2075.1989.tb08494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi JJ, Rath S, Janeway CA., Jr Control of T cell responses to staphylococcal enterotoxins by stimulator cell MHC class II polymorphism. J Immunol. 1991;147(4):1398–1405. [PubMed] [Google Scholar]
- 37.Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, Carrel S, Posnett DN, Choi Y, Marrack P. V beta-specific stimulation of human T cells by staphylococcal toxins. Science (New York, NY) 1989;244(4906):811–813. doi: 10.1126/science.2524876. [DOI] [PubMed] [Google Scholar]
- 38.Fleischer B, Gerardy-Schahn R, Metzroth B, Carrel S, Gerlach D, Kohler W. An evolutionary conserved mechanism of T cell activation by microbial toxins. Evidence for different affinities of T cell receptor-toxin interaction. J Immunol. 1991;146(1):11–17. [PubMed] [Google Scholar]
- 39.Beadling C, Guschin D, Witthuhn BA, Ziemiecki A, Ihle JN, Kerr IM, Cantrell DA. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. The EMBO journal. 1994;13(23):5605–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science (New York, NY) 2002;296(5573):1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- 41.Mattern T, Flad HD, Brade L, Rietschel ET, Ulmer AJ. Stimulation of human T lymphocytes by LPS is MHC unrestricted, but strongly dependent on B7 interactions. J Immunol. 1998;160(7):3412–3418. [PubMed] [Google Scholar]
- 42.Stovall SH, Yi AK, Meals EA, Talati AJ, Godambe SA, English BK. Role of vav1- and src-related tyrosine kinases in macrophage activation by CpG DNA. The Journal of biological chemistry. 2004;279(14):13809–13816. doi: 10.1074/jbc.M311434200. [DOI] [PubMed] [Google Scholar]
- 43.Sanjuan MA, Rao N, Lai KT, Gu Y, Sun S, Fuchs A, Fung-Leung WP, Colonna M, Karlsson L. CpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretion. The Journal of cell biology. 2006;172(7):1057–1068. doi: 10.1083/jcb.200508058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaty CD, Franklin TL, Uehara Y, Wilson CB. Lipopolysaccharide-induced cytokine production in human monocytes: role of tyrosine phosphorylation in transmembrane signal transduction. European journal of immunology. 1994;24(6):1278–1284. doi: 10.1002/eji.1830240606. [DOI] [PubMed] [Google Scholar]
- 45.Mueller J, Matloubian M, Zikherman J. Cutting edge: An in vivo reporter reveals active B cell receptor signaling in the germinal center. J Immunol. 2015;194(7):2993–2997. doi: 10.4049/jimmunol.1403086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science (New York, NY) 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 47.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. The Journal of experimental medicine. 2005;201(5):703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samuels J, Ng YS, Coupillaud C, Paget D, Meffre E. Impaired early B cell tolerance in patients with rheumatoid arthritis. The Journal of experimental medicine. 2005;201(10):1659–1667. doi: 10.1084/jem.20042321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kinnunen T, Chamberlain N, Morbach H, Cantaert T, Lynch M, Preston-Hurlburt P, Herold KC, Hafler DA, O’Connor KC, Meffre E. Specific peripheral B cell tolerance defects in patients with multiple sclerosis. The Journal of clinical investigation. 2013;123(6):2737–2741. doi: 10.1172/JCI68775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gonzalez-Martin A, Adams BD, Lai M, Shepherd J, Salvador-Bernaldez M, Salvador JM, Lu J, Nemazee D, Xiao C. The microRNA miR-148a functions as a critical regulator of B cell tolerance and autoimmunity. Nature immunology. 2016 doi: 10.1038/ni.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moens L, Kane A, Tangye SG. Naive and memory B cells exhibit distinct biochemical responses following BCR engagement. Immunology and cell biology. 2016;94(8):774–786. doi: 10.1038/icb.2016.41. [DOI] [PubMed] [Google Scholar]
- 52.Quach TD, Manjarrez-Orduno N, Adlowitz DG, Silver L, Yang H, Wei C, Milner EC, Sanz I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J Immunol. 2011;186(8):4640–4648. doi: 10.4049/jimmunol.1001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fowler T, Garruss AS, Ghosh A, De S, Becker KG, Wood WH, Weirauch MT, Smale ST, Aronow B, Sen R, et al. Divergence of transcriptional landscape occurs early in B cell activation. Epigenetics & chromatin. 2015;8(20) doi: 10.1186/s13072-015-0012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zarember KA, Godowski PJ. Tissue expression of human Toll-like receptors and differential regulation of Toll-like receptor mRNAs in leukocytes in response to microbes, their products, and cytokines. J Immunol. 2002;168(2):554–561. doi: 10.4049/jimmunol.168.2.554. [DOI] [PubMed] [Google Scholar]
- 55.Ganley-Leal LM, Liang Y, Jagannathan-Bogdan M, Farraye FA, Nikolajczyk BS. Differential regulation of TLR4 expression in human B cells and monocytes. Molecular immunology. 2010;48(1–3):82–88. doi: 10.1016/j.molimm.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bourke E, Bosisio D, Golay J, Polentarutti N, Mantovani A. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells. Blood. 2003;102(3):956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- 57.Adachi K, Davis MM. T-cell receptor ligation induces distinct signaling pathways in naive vs. antigen-experienced T cells. Proc Natl Acad Sci U S A. 2011;108(4):1549–1554. doi: 10.1073/pnas.1017340108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stocco CO, Lau LF, Gibori G. A calcium/calmodulin-dependent activation of ERK1/2 mediates JunD phosphorylation and induction of nur77 and 20alpha-hsd genes by prostaglandin F2alpha in ovarian cells. The Journal of biological chemistry. 2002;277(5):3293–3302. doi: 10.1074/jbc.M110936200. [DOI] [PubMed] [Google Scholar]
- 59.Darragh J, Soloaga A, Beardmore VA, Wingate AD, Wiggin GR, Peggie M, Arthur JS. MSKs are required for the transcription of the nuclear orphan receptors Nur77, Nurr1 and Nor1 downstream of MAPK signalling. The Biochemical journal. 2005;390(Pt 3):749–759. doi: 10.1042/BJ20050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thompson J, Burger ML, Whang H, Winoto A. Protein kinase C regulates mitochondrial targeting of Nur77 and its family member Nor-1 in thymocytes undergoing apoptosis. European journal of immunology. 2010;40(7):2041–2049. doi: 10.1002/eji.200940231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen JB, Sternberg C, Poulsen LK, Bjorn SP, Givskov M, Molin S. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Applied and environmental microbiology. 1998;64(6):2240–2246. doi: 10.1128/aem.64.6.2240-2246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiang CF, Okou DT, Griffin TB, Verret CR, Williams MN. Green fluorescent protein rendered susceptible to proteolysis: positions for protease-sensitive insertions. Archives of biochemistry and biophysics. 2001;394(2):229–235. doi: 10.1006/abbi.2001.2537. [DOI] [PubMed] [Google Scholar]
- 63.Coombs D, Kalergis AM, Nathenson SG, Wofsy C, Goldstein B. Activated TCRs remain marked for internalization after dissociation from pMHC. Nature immunology. 2002;3(10):926–931. doi: 10.1038/ni838. [DOI] [PubMed] [Google Scholar]
- 64.Bevington SL, Cauchy P, Piper J, Bertrand E, Lalli N, Jarvis RC, Gilding LN, Ott S, Bonifer C, Cockerill PN. Inducible chromatin priming is associated with the establishment of immunological memory in T cells. The EMBO journal. 2016;35(5):515–535. doi: 10.15252/embj.201592534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. Developmental regulation of Lck targeting to the CD8 coreceptor controls signaling in naive and memory T cells. The Journal of experimental medicine. 1999;189(10):1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dienz O, Eaton SM, Krahl TJ, Diehl S, Charland C, Dodge J, Swain SL, Budd RC, Haynes L, Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc Natl Acad Sci U S A. 2007;104(17):7175–7180. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Berard M, Tough DF. Qualitative differences between naive and memory T cells. Immunology. 2002;106(2):127–138. doi: 10.1046/j.1365-2567.2002.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farber DL, Acuto O, Bottomly K. Differential T cell receptor-mediated signaling in naive and memory CD4 T cells. European journal of immunology. 1997;27(8):2094–2101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]
- 69.Farber DL. Biochemical signaling pathways for memory T cell recall. Seminars in immunology. 2009;21(2):84–91. doi: 10.1016/j.smim.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hussain SF, Anderson CF, Farber DL. Differential SLP-76 expression and TCR-mediated signaling in effector and memory CD4 T cells. J Immunol. 2002;168(4):1557–1565. doi: 10.4049/jimmunol.168.4.1557. [DOI] [PubMed] [Google Scholar]
- 71.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nature immunology. 2002;3(8):764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 72.Hall SR, Heffernan BM, Thompson NT, Rowan WC. CD4+ CD45RA+ and CD4+ CD45RO+ T cells differ in their TCR-associated signaling responses. European journal of immunology. 1999;29(7):2098–2106. doi: 10.1002/(SICI)1521-4141(199907)29:07<2098::AID-IMMU2098>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 73.Miller RA, Flurkey K, Molloy M, Luby T, Stadecker MJ. Differential sensitivity of virgin and memory T lymphocytes to calcium ionophores suggests a buoyant density separation method and a model for memory cell hyporesponsiveness to Con A. J Immunol. 1991;147(9):3080–3086. [PubMed] [Google Scholar]
- 74.Woronicz JD, Lina A, Calnan BJ, Szychowski S, Cheng L, Winoto A. Regulation of the Nur77 orphan steroid receptor in activation-induced apoptosis. Molecular and cellular biology. 1995;15(11):6364–6376. doi: 10.1128/mcb.15.11.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kasler HG, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Molecular and cellular biology. 2000;20(22):8382–8389. doi: 10.1128/mcb.20.22.8382-8389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Werner M, Hobeika E, Jumaa H. Role of PI3K in the generation and survival of B cells. Immunological reviews. 2010;237(1):55–71. doi: 10.1111/j.1600-065X.2010.00934.x. [DOI] [PubMed] [Google Scholar]
- 77.Fruman DA, Satterthwaite AB, Witte ON. Xid-like phenotypes: a B cell signalosome takes shape. Immunity. 2000;13(1):1–3. doi: 10.1016/s1074-7613(00)00002-9. [DOI] [PubMed] [Google Scholar]
- 78.Shi YF, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339(6226):625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- 79.Fruman DA, Mather PE, Burakoff SJ, Bierer BE. Correlation of calcineurin phosphatase activity and programmed cell death in murine T cell hybridomas. European journal of immunology. 1992;22(10):2513–2517. doi: 10.1002/eji.1830221008. [DOI] [PubMed] [Google Scholar]
- 80.Yazdanbakhsh K, Choi JW, Li Y, Lau LF, Choi Y. Cyclosporin A blocks apoptosis by inhibiting the DNA binding activity of the transcription factor Nur77. Proc Natl Acad Sci U S A. 1995;92(2):437–441. doi: 10.1073/pnas.92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mudd JC, Murphy P, Manion M, Debernardo R, Hardacre J, Ammori J, Hardy GA, Harding CV, Mahabaleshwar GH, Jain MK, et al. Impaired T-cell responses to sphingosine-1-phosphate in HIV-1 infected lymph nodes. Blood. 2013;121(15):2914–2922. doi: 10.1182/blood-2012-07-445783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaya Z, Tretter T, Schlichting J, Leuschner F, Afanasyeva M, Katus HA, Rose NR. Complement receptors regulate lipopolysaccharide-induced T-cell stimulation. Immunology. 2005;114(4):493–498. doi: 10.1111/j.1365-2567.2004.02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kandimalla ER, Bhagat L, Li Y, Yu D, Wang D, Cong YP, Song SS, Tang JX, Sullivan T, Agrawal S. Immunomodulatory oligonucleotides containing a cytosine-phosphate-2’-deoxy-7-deazaguanosine motif as potent toll-like receptor 9 agonists. Proc Natl Acad Sci U S A. 2005;102(19):6925–6930. doi: 10.1073/pnas.0501729102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomsen ER, Mich JK, Yao Z, Hodge RD, Doyle AM, Jang S, Shehata SI, Nelson AM, Shapovalova NV, Levi BP, et al. Fixed single-cell transcriptomic characterization of human radial glial diversity. Nature methods. 2016;13(1):87–93. doi: 10.1038/nmeth.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.